Abstract
For decades, the selection of chemotherapeutic regimens for the treatment of recurrent or metastatic nasopharyngeal carcinoma has been mainly empirical. To our knowledge, there is no phase 3 trial that has been conducted to determine the optimal treatment for these patients before our publication. Recently, we published an article in The Lancet entitled “Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial.” The results of our study indicate that gemcitabine plus cisplatin could improve the survival of patients with recurrent or metastatic nasopharyngeal carcinoma compared with conventional fluorouracil plus cisplatin.
Similar content being viewed by others
Background
Nasopharyngeal carcinoma (NPC) is an endemic cancer, with the highest incidence in Southeast Asia [1, 2]. Radiotherapy or chemoradiotherapy has become the primary treatment of early or locoregionally advanced NPC [3, 4]. However, about one-third of these patients have treatment failure due to distant metastasis [5]. The median overall survival (OS) of recurrent or metastatic NPC patients is only approximately 20 months [6]. Fluorouracil plus cisplatin is generally regarded as the standard first-line chemotherapy regimen for these patients, although it has never been directly compared with best supportive care. However, the fluorouracil regimen is limited by the requirement for deep-vein catheterization and the short duration of response. Therefore, using carefully designed, large clinical trials to find novel agents for the treatment of metastatic NPC is of critical importance. As such, in our recent Lancet article entitled “Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial,” [7] we presented the preliminary results of our study of the efficacy and safety of two competing cisplatin-based combinations—gemcitabine plus cisplatin versus fluorouracil plus cisplatin—in patients with recurrent or metastatic NPC.
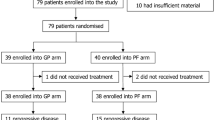
The study was conducted in 22 centers in China. In these centers, Epstein-Barr virus is the major etiology of NPC. A total of 362 patients were randomized in a 1:1 ratio to receive either gemcitabine plus cisplatin or fluorouracil plus cisplatin for a maximum of six 21-day treatment cycles. This was an open-labelled study. The primary endpoint was progression-free survival (PFS). The key findings were the statistically and clinically significant survival improvement of patients who received gemcitabine plus cisplatin compared with patients who received fluorouracil plus cisplatin, in both PFS [median: 7.0 vs. 5.6 months; hazard ratio (HR) 0.55; P < 0.001] and OS (median 29.1 vs. 20.9 months; HR 0.62; P = 0.003). Although the follow-up time for OS was relatively short, the substantial gain in OS might be explained by the low crossover rate of 8% to gemcitabine in the fluorouracil-plus-cisplatin group. Final OS results with more in-depth analyses are being generated.
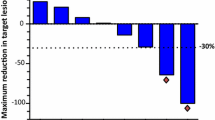
Our exploratory analyses further suggest that the improvement of PFS for patients who received gemcitabine plus cisplatin was consistent across most subgroups. However, it should be noted that the enrolled patients were from endemic areas, where the primary NPC histological classifications are non-keratinizing undifferentiated (type III) and non-keratinizing differentiated (type II) diseases. For keratinizing subtype (type I), which is more prevalent in Western countries, whether gemcitabine is superior to fluorouracil needs more investigation. Overall, gemcitabine demonstrated more remarkable anti-tumor activity compared with fluorouracil, as seen by the significant improvement in objective response rate (64% vs. 42%, P < 0.001). Disease control rates were similarly high in both arms (90% vs. 86%).
The adverse events of both regimens were as expected. Patients who received gemcitabine had increased risks of grade ≥3 leukopenia, neutropenia, and thrombocytopenia, whereas those who received fluorouracil had an increased risk of grade ≥3 mucosal inflammation. The occurrence rates of serious adverse events were similar between the two arms (4% in the gemcitabine-plus-cisplatin group vs. 6% in the fluorouracil-plus-cisplatin group).
The next frontier in the treatment of NPC patients will entail improved prognosis stratification with existing and novel biomarkers and the development of novel drugs beyond conventional chemotherapeutics [8].
Conclusions
Our data indicate that, for patients with recurrent or metastatic NPC, gemcitabine is superior to fluorouracil in terms of OS and PFS. These results could establish gemcitabine plus cisplatin as the new standard first-line treatment regimen for this patient population.
References
Zhang LF, Li YH, Xie SH, Ling W, Chen SH, Liu Q, et al. Incidence trend of nasopharyngeal carcinoma from 1987 to 2011 in Sihui County, Guangdong Province, South China: an age-period-cohort analysis. Chin J Cancer. 2015;34(8):350–7.
Wei KR, Zheng RS, Zhang SW, Liang ZH, Ou ZX, Chen WQ. Nasopharyngeal carcinoma incidence and mortality in china in 2010. Chin J Cancer. 2014;33(8):381–7.
Guan Y, Liu S, Wang HY, Guo Y, Xiao WW, Chen CY, et al. Long-term outcomes of a phase II randomized controlled trial comparing intensity-modulated radiotherapy with or without weekly cisplatin for the treatment of locally recurrent nasopharyngeal carcinoma. Chin J Cancer. 2016;35:20.
Lee N, Harris J, Garden AS, Straube W, Glisson B, Xia P, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. 2009;27(22):3684–90.
Yang L, Hong S, Wang Y, Chen H, Liang S, Peng P, et al. Development and external validation of nomograms for predicting survival in nasopharyngeal carcinoma patients after definitive radiotherapy. Sci Rep. 2015;5:15638.
Jin Y, Shi YX, Cai XY, Xia XY, Cai YC, Cao Y, et al. Comparison of five cisplatin-based regimens frequently used as the first-line protocols in metastatic nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2012;138(10):1717–25.
Zhang L, Huang Y, Hong S, Yang Y, Yu G, Jia J, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388(10054):1883–92.
Chua ML, Wee JT, Hui EP, Chan AT. Nasopharyngeal carcinoma. Lancet. 2016;387(10022):1012–24.
Authors’ contributions
SDH drafted the manuscript. LZ reviewed and revised the manuscript. Both authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Hong, S., Zhang, L. Gemcitabine improves survival in patients with recurrent or metastatic nasopharyngeal carcinoma. Chin J Cancer 35, 100 (2016). https://doi.org/10.1186/s40880-016-0163-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40880-016-0163-6