Abstract
Background
Planarians are non-parasitic Platyhelminthes (flatworms) famous for their regeneration ability and for having a well-organized brain. Dugesia japonica is a typical planarian species that is widely distributed in the East Asia. Extensive cellular and molecular experimental methods have been developed to identify the functions of thousands of genes in this species, making this planarian a good experimental model for regeneration biology and neurobiology. However, no genome-level information is available for D. japonica, and few gene regulatory networks have been identified thus far.
Results
To obtain whole-genome information on this species and to study its gene regulatory networks, we extracted genomic DNA from 200 planarians derived from a laboratory-bred asexual clonal strain, and sequenced 476 Gb of data by second-generation sequencing. Kmer frequency graphing and fosmid sequence analysis indicated a complex genome that would be difficult to assemble using second-generation sequencing short reads. To address this challenge, we developed a new assembly strategy and improved the de novo genome assembly, producing a 1.56 Gb genome sequence (DjGenome ver1.0, including 202,925 scaffolds and N50 length 27,741 bp) that covers 99.4% of all 19,543 genes in the assembled transcriptome, although the genome is fragmented as 80% of the genome consists of repeated sequences (genomic frequency ≥ 2). By genome comparison between two planarian genera, we identified conserved non-coding elements (CNEs), which are indicative of gene regulatory elements. Transgenic experiments using Xenopus laevis indicated that one of the CNEs in the Djndk gene may be a regulatory element, suggesting that the regulation of the ndk gene and the brain formation mechanism may be conserved between vertebrates and invertebrates.
Conclusion
This draft genome and CNE analysis will contribute to resolving gene regulatory networks in planarians. The genome database is available at: http://www.planarian.jp.
Similar content being viewed by others
Background
Planarian is a common name applied to species of non-parasitic Platyhelminthes (flatworms) of the turbellaria class. Planarians have attracted great interest in the field of regeneration biology for many years [1]. Dugesia japonica (D. japonica) is a typical freshwater planarian species that is widely distributed in East Asia [2]. This planarian is able to regenerate a complete individual from a tiny excised part of its body, which makes it a good model for regeneration biology and regenerative medicine research [3]. D. japonica also has a well-organized brain and shows decision-making behavior [4,5,6], and is rapidly becoming a model animal used in the study of neurobiology [7,8,9,10].
Advances in cellular and molecular biology experimental methods, as well as nucleic acid sequencing technologies, have helped increase our ability to examine the biology of planarians at the molecular level. Using PCR, cDNA libraries, RNA in situ hybridization and immunoscreening, D. japonica cell-type-specific genes have been isolated [11]. Highly sensitive in situ hybridization methods were developed for identifying mRNA locations and expression in particular cell types [5]. Loss-of-function assays, including RNA interference, were also devised to characterize gene functions [12]. RNA microarrays have been generated to identify genes important for regeneration, and head-specific genes [13]. Fluorescence-activated cell sorting (FACS) has been used to isolate discrete cell populations, which could then be used for single-cell gene profiling, functional transplantation studies, and neurobiology studies [14,15,16,17]. In addition, a transcriptome resource (EST data) is also available [18, 19]. All of these modern research methods and resources have enabled us to link the phenomena of D. japonica’s robust regeneration and brain formation to underlying genes. To achieve a complete understanding of those genes and their regulatory networks, genome-level information on D. japonica is required. However, the unusual number of SNPs that accumulate during asexual reproduction has confounded our efforts to obtain long contigs, although we used the laboratory clonal strain of D. japonica [19]. Here we present the first draft genome from only second-generation sequencing data, made possible by overcoming heterogenesis by using a newly developed assembly strategy, and analysis of conserved non-coding elements (CNEs) of D. japonica. These results will contribute knowledge essential for further research into gene regulatory networks in planarian.
Methods
Animal culture and DNA extraction
The asexual D. japonica strain SSP-9 T-5 was derived from a single individual and maintained in the Agata lab since 2005 [19]. The planarians were kept in autoclaved tap water at 22–24 °C in dim light, fed with chicken liver twice a week, and starved for at least 1 week before experiments. Genomic DNA was extracted using the Wizard® SV Genomic DNA Purification System from Promega.
Kmer analysis and genome size estimation
Kmers were counted in the Illumina Hiseq sequencing data using Jellyfish with the “–C” parameter [20]. Genome size was estimated by the formula G = Nbase/Cbase = NKmer/CKmer (G is genome size, Nbase and NKmer are the numbers of bases and Kmers, and Cbase and CKmer are expected sequencing depth of bases and Kmers).
Genome assembly and annotation
The preliminary genome de novo assembly was performed by de Brujin graph-based method, Allpth-lg [21], SOAPdenovo [22], and Velvet [23]. To improve the assembly, a new strategy was used, containing four steps. At first, all pair-end sequencing reads were locally assembled into precise pseudo-long reads by AnyTag [24]. Secondly, all Sanger long reads, Roche 454 long reads, and newly produced pseudo-long reads were assembled into contigs using an overlap-layout algorithm, Newbler [25]. Furthermore, Illumina mate-pair sequencing reads were used to link the contigs and generate scaffolds by SSPASE [26]. Thirdly, all pair-end and mate-pair information from the sequencing was utilized for gap closure by GapFiller [27]. Finally, super-scaffolds were generated by L_RNA_Scaffolder [28] based on RNA evidence. Genome annotation was performed using MAKER [29].
Transcriptome assembly and annotation
The independent transcriptome de novo assembly was done by Trinity [30]. The transcriptome annotation was performed using Trinotate [31].
Discovery of CNEs
Only scaffolds with mRNA evidence were selected by BLAT [32]. Coding regions were masked out by the letter “N” from the selected scaffolds using bedtools [33]. Repeated sequences were subsequently masked out by the letter “N” from the previously selected scaffolds using RepeatMasker (http://www.repeatmasker.org/). One-to-one matched scaffold pairs between the two planarian genomes were identified using NUCmer [34]. For specific genes of interest, additional Blastn procedures (both local Blastn [35] and online Blastn at the website of Schmidtea mediterranea (S. mediterranea) genome database [36]) were performed to refine the results, and finally, CNEs on the scaffolds were located.
Enhancer activity assay using Xenopus embryos
The reporter plasmid, actGFP, carrying a chicken β-actin basal promoter (− 55 to + 53), was previously described as βGFP [37]. Non-coding sequences conserved between D. japonica and S. mediterranea ndk genomic regions were cloned in the actGFP from D. japonica genomic DNA by polymerase chain reaction (PCR) and verified by sequencing. Searches for putative transcription-factor-binding motifs were performed with transcription-factor-binding sites collected from the TRANSFAC and JASPAR databases [38, 39]. Transgenic Xenopus embryos were generated with a sperm nuclear transplantation method, as described [40]. The fraction of embryos that developed normally until scoring stages (stages 13–14) was subjected to in situ hybridization for detecting GFP expression with maximum sensitivity.
Results
Genome sequencing
For genome sequencing, we used a pool of around 200 planarian individuals from an asexually propagating clonal strain of D. japonica [19]. After extracting genomic DNA, we prepared short insert (~ 180 bp, 200 bp, 250 bp, and 450 bp) pair-end DNA libraries and large insert (~ 3 kb, ~ 8 kb, ~ 20 kb) mate-pair libraries. Three second-generation sequencing (SGS) platforms (Illumina GAIIx, Illumina Hiseq2000 and Roche 454 sequencing) were used. We also constructed a genomic DNA fosmid library of D. japonica with insert length ~ 35 kb, and 172 fosmids were sequenced by end-sequencing using a first-generation (Sanger) sequencing platform. Totally, 476.48 Gb data were generated (Table 1).
Kmer frequency analysis and genome size estimation
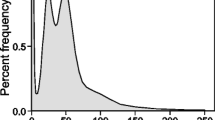
After quality control of the raw sequencing data, the average Q value of bases of sequencing reads was greater than 30 (Additional file 1), which indicated clean data with low sequencing error. Probably, due to the lack of sequencing data and the sampling algorithm of Kmergenie [41], previous kmer frequency analysis using only Illumina GAIIx data did not give a clean kmer species distribution peak [19]. After using more Hiseq 2000 pair-end sequencing data (from insert library 250 bp (2nd) and insert library 450 bp, Table 1), we calculated the kmer (k = 17) species and kmer individuals frequency graphs (Fig. 1) using the Jellyfish software [20], and generated 51.6G correct kmers in total. Although relatively clear peaks were shown, these graphs appeared unusual. The kmer species and individual graphs should be very similar after analyzing sequencing data from an ideal genome that has little heterozygosity and few repeated sequences [42], but these two graphs derived from D. japonica genome sequencing data were clearly different. In the kmer species frequency graph, the conspicuous high peak and the large number of kmer species before the unapparent low homozygous peak indicated D. japonica genome indicates a highly heterogeneous genome (Fig. 1a). The kmer individuals frequency graph (Fig. 1b) has a main peak at 34 (genomic frequency = 1), and depth of more than 80% of the kmers are higher than 68 (genomic frequency ≥ 2), indicating that the D. japonica genome is a highly repetitious genome in which about 80% of the genome comprises repeated sequences [42]. Accordingly, D. japonica may have a highly complex genome structure.
Kmer (k = 17) frequency analysis. a Kmer species frequency graph. The horizontal axis shows the depth of kmer species, and the vertical axis shows the percentage of each kmer species value (blue curve). b. Kmer individuals frequency graph. The horizontal axis shows depth of kmer individuals, the left vertical axis shows the percentage of each kmer individual’s value (blue curve), and the right vertical axis shows the accumulative frequency of the kmer individuals (red curve)
The D. japonica genome is normally a diploid genome that contains eight pairs of chromosomes (2n = 16). According to these kmer frequency graphs, the estimated D. japonica genome size is ~ 1.52 Gb. The D. japonica genome commonly has 16 chromosomes, twice the number of another planarian species, S. mediterranea (2n = 8, with an estimated genome size 769.5 Mb from Spencer Johnston’s unpublished data). In addition, our previous flow cytometry results also showed that the D. japonica genome size is ~ 1.9 times that of the S. mediterranea genome [19]. Thus, the deduced D. japonica genome size should be around 1.46 Gb, similar to the genome size estimated from the kmer frequency graph.
Fosmid sequences revealed some features of the D. japonica genome
To display genome features visually, we screened out several individual fosmid clones of certain genes by a colony multiplex qPCR-based 3S3DBC method [43] from the D. japonica genomic DNA fosmid library and sequenced them by the Sanger method. We then aligned next-generation sequencing DNA and RNA reads to those fosmid sequences.
In one representative example, the alignment between DNA sequencing reads and one fosmid (named DJF-016O13) insert sequence of the gene Djth (Fig. 2) showed that only the sequences around the coding region aligned closely (58 reads mapped on coding regions, 55 of which matched perfectly), but sequences outside of coding regions are highly variable, which is caused by repetitive sequences (41% of the sequence were marked as repeated sequences, as shown by red bars in Fig. 2). Alignment between RNA sequencing reads and the fosmid insert sequence also indicated that a large fraction of the repeated sequences are transcribed, suggesting that these repeats are consist of retrotransposons, which iswas also in accord with the Repbase [44] annotation of this fosmid (Additional file 2). Furthermore, we found several SNPs even in the coding region, as previously reported by Nishimura et al. [19].
One fosmid insert sequence of the gene Djth (DJF-016O13). This figure shows the alignment of genomic DNA and RNA sequencing reads to one fosmid insert sequence of the gene Djth (DJF-016O13) and the repeated elements annotation of this fosmid sequence by Repbase. Green arrowheads show the10th and 11th exon of the Djth gene on the fosmid insert sequence. Grey spots show matches between the sequencing reads and the reference fosmid sequence, and black spots show mismatches. Red bars represent repetitive sequences
Preliminary genome assembly using SGS short reads
Because most of our genome sequencing data are short pair-end reads obtained from second-generation sequencers, De Bruijn graph-based genome methods could be used to assemble the genome. We used Allpath-lg [21] (not completed due to superabundant memory requirement), SOAPdenovo [22] and Velvet [23] to assemble the clean genome sequencing reads after quality control. However, there is a strong possibility that the complexity of the D. japonica genome would severely interfere with genome assembly. Not surprisingly, the de novo assembled results were fragmented (scaffold N50 < 1000 bp, Additional file 3) irrespective of the assembler used, parameters set, or the stringency with which the input data were trimmed. Accordingly, the combination of heterogenesis and repeated sequences of the genome disturbed de novo genome assembly using only short SGS data. To address this challenge, a new assembly strategy was required.
Genome de novo assembly using merged pseudo-long reads
The result from de Brujin graph-based de novo assembly using short sequencing reads was not satisfactory. Thus, we developed a more convenient pseudo-long read strategy (Fig. 3), taking advantage of the numerous pre-existing short sequencing data. In brief, all short pair-end sequencing reads from small insert libraries (~ 180 bp, 200 bp, 250 bp, 450 bp) were, at first, locally assembled into precise pseudo-long reads [24] with average length ~ 452 bp, which is as long as common Roche 454 sequencing reads. Secondly, genome contigs were generated from all Sanger long reads, Roche 454 long reads, and the newly produced pseudo-long reads by using an overlap-layout algorithm. Mate-pair sequencing reads from long-insert libraries (~ 3 kb, ~ 8 kb and 20 kb) were used to link the contigs and generate scaffolds of the D. japonica genome [45]. Finally, all pair-end and mate-pair information from the sequencing was utilized for gap closure of the previously assembled scaffolds [46]. An optional super-scaffold generation step could also be performed by further linking between scaffolds [28] using mRNA evidence, which contains the orientation and order information of the exon sequences, which could be exploited to help us determine the orientation and order of previously obtained scaffolds containing those exon sequences. Although the distance information between the RNA-guided scaffolds is ambiguous, these scaffolds are of great value for the subsequent genome annotation.
Finally, by assembling the new pseudo-long reads, we obtained an assembled genome size 1.56 Gb, with a contig N50 size of 1.4 kb, a scaffold N50 size of 23.2 kb, and an RNA-guided super-scaffold N50 size of 27.7 kb (Table 2). We called this assembly result DjGenome ver 1.0. Over 75% of the assembly was covered by scaffolds ≥10 kb. Although genome complexity hampered the assembly, this new strategy yielded a much-improved genome assembly, whose scaffold N50 length was more than 20 times longer than the previous short read-based assembly.
Assessment of the D. japonica genome
The completeness of the assembled scaffolds was assessed by gVolante [47]. In total, 91.13% of the 248 core conserved eukaryotic genes were covered by the assembled scaffolds, indicating a high level of completeness of the D. japonica genome assembly.
We also assessed the genome assembly results by using fosmid and RNA sequencing data. After sequencing using first-generation Sanger technology and quality control, 89,721 fosmid end sequences (FESs) were generated. All of these sequences were mapped back to the assembled reference genome scaffolds using Bowtie [48]; 80.6% could be mapped back to the reference genome. Some complete fosmid insert sequences were also obtained by Sanger sequencing and assembled by CAP3 [49]. After aligning them to the assembled genome scaffolds, a representative example showing the alignment between the Djth fosmid sequence (DJF-016O13) and its corresponding genome scaffold matched well, although some mismatches existed and large gaps in the scaffold caused by repeated sequences could not align to the fosmid (Fig. 4).
Alignment between the Djth fosmid insert sequence and its corresponding genome scaffold. In the illustration of the Djth fosmid structure, green arrowheads show the 10th and 11th exons of the Djth gene in the fosmid (see Fig. 2). In the illustration of the Djth fosmid repeated sequences, red bars represent repetitive sequences. In the illustration of the alignment between Djth fosmid and scaffold, pink blocks show matched sequences between the fosmid and the corresponding scaffold, while white blocks show mismatches, which were mainly caused by gaps in the scaffold
Moreover, the D. japonica transcriptome was obtained by assembling Roche 454 RNA sequencing reads derived from our previous results [19]. The assembled transcriptome was ~ 34.78 Mb in size, and it contained 19,543 genes (with 25,566 transcripts). All the transcripts were aligned to the assembled genome scaffolds using BLAT [32] with identity ≥95%. In the results, ~ 99.4% of all transcripts could find their corresponding genome scaffolds, and ~ 97.8% of all transcripts’ bases were covered by the genome, which also indicated that this assembled genome covered nearly all coding gene regions (Table 3).
Thus, assessment of our D. japonica genome assembly by comparison with FESs and the transcriptome showed the relative completeness of the draft genome, especially of the coding region. However, due to the heterogenous and repetitive sequences, some gaps and chimeric scaffolds may still exist.
Repeated sequences and genome annotation
Repeated sequences were identified by using both RepeatModeler and RepeatMasker (http://www.repeatmasker.org/). RepeatModeler was used to build the consensus models of putative interspersed repeats as a new repeated sequence library based on the genome. RepeatMasker was used to search the planarian genome against the combined library Repbase. The two results were integrated to gain a comprehensive analysis of repeats in the D. japonica genome. Approximately 39.7% of the assembled genome sequences were marked as repetitive elements (Table 4). This rate is lower than that estimated by kmer frequency analysis. Ends of contigs were also often occupied by tandem repeats. This suggests that repetitive sequences interrupt genome assembly and make it difficult to assemble a draft genome. Moreover, except for unclassified repeats in the assembled genome, the majority of repeated elements were retrotransposons and DNA transposons, which agreed with our observations from the fosmid survey.
To predict coding genes in the D. japonica genome after masking repeated elements, we used evidence-based prediction followed by de novo prediction. In the evidence-based method, 2,857,787 long RNA sequences (including ESTs, 454 sequences and assembled transcripts) were aligned against the D. japonica genome with BLAT (identity > 95%) [32]. The best alignment output result of each RNA sequence was taken as evidence of a coding region in the genome, and the information was further used by employing Augustus [50] to help de novo gene prediction. Finally, we identified a total of 23,997 coding genes in the D. japonica genome and annotated them by Blast2GO annotation [51]. 15,601 genes were assigned a gene ontology (GO) annotation (Fig. 5 & Additional file 4).
The comprehensive gene and protein prediction were performed by MAKER genome annotation program [29], which employs several algorithms for annotating genomic regions such as repeats and homologous regions to genes and proteins of other organisms. Maker reported 8,958,273 hits within 179,194 (88%) unique scaffolds of our genome assembly. There were homologous regions to 138,931 unique proteins in Swiss-Prot database from 5572 organisms (Table 5).
So far there are only three experimentally confirmed protein sequences from S. mediterranea in Swiss-Prot database, all of which had homologous hits in our D. japonica reference assembly. We separately run Blastx on the unconfirmed 1366 protein sequences of S. mediterranea in Uniprot database and found homologous hits to 1129 (82%) of them. Moreover, from our D. japonica’s genome, we found several conserved critical protein coding genes which were recently reported missing from the genome of S. mediterranea in the report by Grohme et al. [52], including MAD1L1, BUB1, ANAPC7, NCAPD3, LIG3, NFE2L1, ACADM, et al. Thus, the completeness of our assembled genome and the conserved genes it contains make D. japonica a good model animal for further research.
Database of DjGenome ver 1.0
For easy access to the assembly and annotation data, we prepared a D. japonica online genome database (http://www.planarian.jp/). It allows users to download the genome and the transcriptome results, and features a graphical interface for browsing the genome using the JBrowse platform [53, 54]. In addition to the genome assembly, annotated regions by MAKER, Augustus, and Blastx hits to Swiss-Prot are available different tracks.
Discovery of conserved non-coding elements in planarian
Because of protein functional constraints, coding regions are expected to exhibit sequence conservation between related species. In addition, some non-coding elements also show functional constraints, and such conservation outside of exons can be detected by cross-species comparison. In vertebrates, conserved non-coding elements (CNEs) were found to include transcription factor binding sites [55], and they are accepted as beacons of gene regulatory elements [56]. Planarians have been used as a model animal for regeneration and stem cell research for many years. Although the planarians D. japonica and S. mediterranea exhibit strong morphological, physiological and functional similarity, their evolutionary positions are distant (even further than the distance between human and chicken [57]) and their gene sequences also have large differences. Genome sequence comparison between these two planarian genera could help us to find common CNEs among planarians, which would light the way to studying molecular regulatory networks in planarian. By combining the results from NUCmer [34] and Blastn procedures (both local Blastn [35] and online Blastn at the website of S. mediterranea genome database [36]), we identified CNEs from 33,924 D. japonica genome scaffolds that have mRNA evidence matched on 9738 S. mediterranea genome scaffolds.
A CNE is a regulatory element of the Djndk gene
In 2002, the planarian gene Djndk (a homolog of the vertebrate fgfrl1 gene), was shown to play a crucial role in brain formation during planarian regeneration [58, 59]. However, although more than 10 years have passed since then, the regulatory elements that restrict the expression of Djndk to the brain region are still unknown. To test whether the CNEs we identified by genome comparison are indeed regulatory elements, and with the hope of finding regulators of the Djndk gene, we took the gene Djndk as an example for CNE functional analysis.
There is only one corresponding scaffold (77.80 kbp) in our assembled genome of D. japonica, which contains all 8 exons of the gene ndk and its flanking sequences on both 5′ upstream and 3′ downstream. Two scaffolds, v31.003636 (53.55 kbp, contains exons 1–5 of ndk) and v31.014514 (21.89 kbp, contains exons 6–exon 8 of ndk), were found from the genome of S. mediterranea (Smed Sexual v31 in SmedGD [36]). After genome comparison between those full scaffolds, five CNEs were distinguished (Fig. 6). To examine whether CNEs of the Djndk gene exhibit regulatory activity, we focused on CNE3 (140 bp) as a representative example for transgenic expression experiments (Fig. 7), since we found the similar conserved non-coding sequences among vertebrate FGFRL-1 (vertebrate nou-darake homolog) loci. The 140 bp CNE3 was inserted upstream of the promoter of a β-actin promoter-driven GFP expression vector to form a reporter construct called CNE-actGFP. When this expression vector was injected into Xenopus laevis embryos, the GFP expression pattern was localized in the neural-plate-forming region of the embryo, which was especially evident in the anterior region at the end of gastrulation (Fig. 7a). Such expression pattern was reproducibly observed in the normally-developed injected embryos (30%, n = 40/132), confirming the discovery of this reporter-gene-expression regulation by CNE3.
Transgenic experiments suggested that Djndk CNE3 might be a regulatory element. a The arrowhead shows CNE3 inserted actGFP vector, CNE (140)-actGFP driven GFP to express in the anterior region at the end of gastrulation in transgenic Xenopus embryos (n = 132). b Putative transcription factor-binding motifs are boxed in different colors; those subjected to mutation analysis are indicated by asterisks. The detailed point mutation design of three transcription factor-binding motifs are shown by red colored words. c Mutation analysis of CNE3 (the 140 bp element). actGFP is an empty reporter construct that contains the β-actin basal promoter; wt (140) is the construct of CNE (140)-actGFP used in (a); mt1, m2 and mt3 are point mutations (Msx (M), Tcf/Lef (T), and Jun/Fos (J)) generated from wt (140), and the detailed mutation design is shown in (b). The bar chart shows the percentage of the embryos that showed GFP expression in the neural plate among total developed embryos injected with the vector constructs. Actual numbers of GFP-positive cases and total numbers of scored embryos are indicated in parentheses. The chi-square test showed that the percentage of positive cases in the wt (140) and the Jun/Fos mutant constructs are significantly different (P < 0.0001), whereas the differences observed in other cases were not significant (P > 0.05)
By aligning the CNE sequences between D. japonica and S. mediterranea, putative transcription factor-binding motifs were identified from perfectly or almost matched sequences (Fig. 7b). We made three point mutations in the expression vector in putative Msx(M), Tcf/lef1(T) and Jun/Fos(J) transcription factor binding sites, respectively (Fig. 7b). Statistical analysis by the chi-square test showed that the percentage of positive cases driven by the Jun/Fos mutant binding site constructs were significantly reduced compared to the wild-type CNE “wt” (140 bp) (P < 0.0001). These results suggest that the 140 bp CNE3 might be a regulatory element of the Djndk gene, and that the Jun/Fos-related transcription factor(s) regulates the expression through this region. Accordingly, CNEs are good candidates of regulatory elements in the planarian genome, and further analysis should be done in the future to thoroughly dissect their roles in gene regulatory networks in planarian.
Discussion
In this study, we assembled the D. japonica draft genome. Our kmer frequency graphs, analysis of fosmid sequences, and our further genome analysis after assembly, taken together with previous heterogenesis observations, indicated that D. japonica possesses a hyper-varying genome structure. Although, the planarian clonal line we used for genomic DNA extraction was derived from a re-cloned planarian individual, we recently found accumulation of large number of mutations during planarian asexual proliferation, suggesting that planarian has a property to avoid complete genome stability to adapt changing environment [19], which may account for the broad distribution of D. japonica across Russia, China, Korea, Japan, and other East Asian countries [2]. One possibility is that, in the kmer frequency graphs, those kmers came from differences between the genome sets of different cells even within one individual. Recently, in planarians we reported that activation of retrotransposons occurs in the course of cell differentiation from the pluripotent stem cell state [60]. In addition, the genomic DNA used for sequencing was pooled from ~ 200 individuals, which would add more complexity in the samples using for the D. japonica’s genome project and make the genome assembly much more challenging.
During genome assembly, simply using the SGS short sequencing data and assembling by the de Bruijn graph-based method did not produce good assembly results. Alternative solutions for this problem, such as bacterial artificial chromosome (BAC)-to-BAC sequencing and fosmid-pooling sequencing [61] are difficult and expensive. It is well known that long sequencing reads have great advantages over short reads in assembling complicated genomes (long reads can solve the assembly problems caused by repetitive sequences and, to a large extent, relieve the interference caused by heterozygosity) [52], so we executed a new strategy by locally merging short pair-end reads into long reads, and used those long reads to de novo assemble the genome by an overlap-layout-based method. The final assembly result was significantly improved. This strategy could be further used for assembling other complicated genomes that present similar difficulties when only short pair-end reads are available. Besides the strategy of merging short pair-end reads into pseudo-long reads, much longer sequencing reads from third generation sequencing technology (e.g., Pacbio) could facilitate improvement of the genome assembly of D. japonica in the future. Moreover, because genomes extracted from different planarian cells even within one individual are possibly heterogenetic, which increased the complexity of the genome structure of D. japonica analyzed here and hampered the genome assembly, the usage of single-cell or single chromosome sequencing technology will help reduce the complexity and improve the genome assembly in future research.
CNEs are well accepted as locations of gene expression regulatory elements. In this research, we also proved that one CNE (CNE3) in the Djndk gene is a regulatory element that has a binding site for the Jun/Fos-related transcription factors. Previous result showed that Xenopus Djndk homologue (FGFRL-1) was expressed in the anterior part of the neural plate of Xenopus embryos, which will form the brain during development [62]. However, in the present transgenic expression experiment, CNE3 could only restrain the reporter gene expression to within the whole neural plate, but not to within the anterior part of the neural plate. This suggests that some other regulatory factor(s) are present in addition to CNE3. We found five CNEs, and thus some other CNE(s) could also be regulatory elements for transcription factors, or possibly other regulators such as miRNA may also play a part in regulating Djndk gene expression. In addition, CNE3 in planarians has a similar counterpart in the human, mouse, and frog ndk-homologous genes, and thus we expect that the regulation of the ndk gene and even ndk-related aspects of the brain formation mechanism might be conserved between vertebrates and invertebrates.
Conclusions
We presented the first draft genome of the planarian D. japonica. Although the draft genome is still fragmented, it covers nearly all gene coding regions and is useful for helping to identify CNEs in the planarians, which will facilitate research on the gene regulatory networks of planarians.
References
Pallas P S. Spicilegia zoologica quibus novae imprimis et obscurae animaliu speciosiconibus atque conamentariis illustratur. Fasc X, Berolini. 1774.
Kawakatsu M, Oki I, Tamura S. Taxonomy and geographical-distribution of Dugesia-japonica and D-Ryukyuensis in the far-east. Hydrobiologia. 1995;305:55–61.
Agata K. Regeneration and gene regulation in planarians. Curr Opin Genet Dev. 2003;13:492–6.
Pagán OR. The first brain: the neuroscience of planarians. 1st edition: Oxford University Press; 2014.
Agata K, Soejima Y, Kato K, Kobayashi C, Umesono Y, Watanabe K. Structure of the planarian central nervous system (CNS) revealed by neuronal cell markers. Zool Sci. 1998;15:433–40.
Inoue T, Hoshino H, Yamashita T, Shimoyama S, Agata K. Planarian shows decision-making behavior in response to multiple stimuli by integrative brain function. Zoological Lett. 2015;1:7.
Okamoto K, Takeuchi K, Agata K. Neural projections in planarian brain revealed by fluorescent dye tracing. Zool Sci. 2005;22:535–46.
Inoue T, Kumamoto H, Okamoto K, Umesono Y, Sakai M, Sanchez AA, Agata K. Morphological and functional recovery of the planarian photosensing system during head regeneration. Zool Sci. 2004;21:275–83.
Nishimura K, Inoue T, Yoshimoto K, Taniguchi T, Kitamura Y, Agata K. Regeneration of dopaminergic neurons after 6-hydroxydopamine-induced lesion in planarian brain. J Neurochem. 2011;119:1217–31.
Shimoyama S, Inoue T, Kashima M, Agata K. Multiple neuropeptide-coding genes involved in planarian pharynx extension. Zool Sci. 2016;33:311–9.
Agata K, Watanabe K. Molecular and cellular aspects of planarian regeneration. Semin Cell Dev Biol. 1999;10:377–83.
Sanchez AA, Newmark PA. Double-stranded RNA specifically disrupts gene expression during planarian regeneration. Proc Natl Acad Sci U S A. 1999;96:5049–54.
Nakazawa M. Search for the evolutionary origin of a brain: planarian brain characterized by microarray. Mol Biol Evol. 2003;20:784–91.
Hayashi T, Asami M, Higuchi S, Shibata N, Agata K. Isolation of planarian X-ray-sensitive stem cells by fluorescence-activated cell sorting. Develop Growth Differ. 2006;48:371–80.
Inoue T, Hayashi T, Takechi K, Agata K. Clathrin-mediated endocytic signals are required for the regeneration of, as well as homeostasis in the planarian CNS. Development. 2007;134:1679–89.
Hayashi T, Shibata N, Okumura R, Kudome T, Nishimura O, Tarui H, Agata K. Single-cell gene profiling of planarian stem cells using fluorescent activated cell sorting and its “index sorting” function for stem cell research. Develop Growth Differ. 2010;52:131–44.
Asami M, Nakatsuka T, Hayashi T, Kou K, Kagawa H, Agata K. Cultivation and characterization of planarian neuronal cells isolated by fluorescence activated cell sorting (FACS). Zool Sci. 2002;19:1257–65.
Nishimura O, Hirao Y, Tarui H, Agata K. Comparative transcriptome analysis between planarian Dugesia japonica and other platyhelminth species. BMC Genomics. 2012;13:289.
Nishimura O, Hosoda K, Kawaguchi E, Yazawa S, Hayashi T, Inoue T, Umesono Y, Agata K. Unusually large number of mutations in asexually reproducing clonal planarian dugesia japonica. PLoS One. 2015;10:e143525.
Marcais G, Kingsford C. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics. 2011;27:764–70.
Gnerre S, Maccallum I, Przybylski D, Ribeiro FJ, Burton JN, Walker BJ, Sharpe T, Hall G, Shea TP, Sykes S, Berlin AM, Aird D, Costello M, Daza R, Williams L, Nicol R, Gnirke A, Nusbaum C, Lander ES, Jaffe DB. High-quality draft assemblies of mammalian genomes from massively parallel sequence data. Proc Natl Acad Sci U S A. 2011;108:1513–8.
Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, He G, Chen Y, Pan Q, Liu Y, Tang J, Wu G, Zhang H, Shi Y, Liu Y, Yu C, Wang B, Lu Y, Han C, Cheung DW, Yiu SM, Peng S, Xiaoqian Z, Liu G, Liao X, Li Y, Yang H, Wang J, Lam TW, Wang J. SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience. 2012;1:18.
Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18:821–9.
Ruan J, Jiang L, Chong Z, Gong Q, Li H, Li C, Tao Y, Zheng C, Zhai W, Turissini D, Cannon CH, Lu X, Wu CI. Pseudo-sanger sequencing: massively parallel production of long and near error-free reads using NGS technology. BMC Genomics. 2013;14:711.
Margulies M, Egholm M, Altman WE, Attiya S, Bader JS, Bemben LA, et al. Genome sequencing in microfabricated high-density picolitre reactors. Nature. 2005;437:376–80.
Boetzer M, Pirovano W. SSPACE-LongRead: scaffolding bacterial draft genomes using long read sequence information. BMC Bioinformatics. 2014;15:211.
Nadalin F, Vezzi F, Policriti A. GapFiller: a de novo assembly approach to fill the gap within paired reads. BMC Bioinformatics. 2012;13(Suppl 14):S8.
Xue W, Li JT, Zhu YP, Hou GY, Kong XF, Kuang YY, Sun XW. L_RNA_scaffolder: scaffolding genomes with transcripts. BMC Genomics. 2013;14:604.
Cantarel BL, Korf I, Robb SM, Parra G, Ross E, Moore B, Holt C, Sanchez AA, Yandell M. MAKER: an easy-to-use annotation pipeline designed for emerging model organism genomes. Genome Res. 2008;18:188–96.
Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29:644–52.
Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, et al. De novo transcript sequence reconstruction from RNA-seq using the trinity platform for reference generation and analysis. Nat Protoc. 2013;8:1494–512.
Kent WJ. BLAT--the BLAST-like alignment tool. Genome Res. 2002;12:656–64.
Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–2.
Kurtz S, Phillippy A, Delcher AL, Smoot M, Shumway M, Antonescu C, Salzberg SL. Versatile and open software for comparing large genomes. Genome Biol. 2004;5:R12.
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10.
Robb SM, Ross E, Sanchez AA. SmedGD: the Schmidtea mediterranea genome database. Nucleic Acids Res. 2008;36:D599–606.
Ogino H, Fisher M, Grainger RM. Convergence of a head-field selector Otx2 and notch signaling: a mechanism for lens specification. Development. 2008;135:249–58.
Wingender E. The TRANSFAC project as an example of framework technology that supports the analysis of genomic regulation. Brief Bioinform. 2008;9:326–32.
Portales-Casamar E, Thongjuea S, Kwon AT, Arenillas D, Zhao X, Valen E, et al. JASPAR 2010: the greatly expanded open-access database of transcription factor binding profiles. Nucleic Acids Res. 2010;38:D105–10.
Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–83.
Chikhi R., Medvedev P. Informed and automated k-mer size selection for genome assembly. arXiv: 2013.
Liu B, Shi Y, Yuan J, Hu X, Zhang H, Li N, Li Z, Chen Y, Mu D, Fan W: Estimation of genomic characteristics by analyzing k-mer frequency in de novo genome projects. arXiv:1308.2012[q-bio.GN].
An Y, Toyoda A, Zhao C, Fujiyama A, Agata K. A colony multiplex quantitative PCR-based 3S3DBC method and variations of it for screening DNA libraries. PLoS One. 2015;10:e116997.
Jurka J, Kapitonov VV, Pavlicek A, Klonowski P, Kohany O, Walichiewicz J. Repbase update, a database of eukaryotic repetitive elements. Cytogenet Genome Res. 2005;110:462–7.
Boetzer M, Henkel CV, Jansen HJ, Butler D, Pirovano W. Scaffolding pre-assembled contigs using SSPACE. Bioinformatics. 2011;27:578–9.
Boetzer M, Pirovano W. Toward almost closed genomes with GapFiller. Genome Biol. 2012;13:R56.
Nishimura O, Hara Y, Kuraku S. gVolante for standardizing completeness assessment of genome and transcriptome assemblies. Bioinformatics. 2017;33:3635–7.
Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25.
Huang X, Madan A. CAP3: a DNA sequence assembly program. Genome Res. 1999;9:868–77.
Stanke M, Keller O, Gunduz I, Hayes A, Waack S, Morgenstern B. AUGUSTUS: ab initio prediction of alternative transcripts. Nucleic Acids Res. 2006;34:W435–9.
Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–6.
Grohme M A, Schloissnig S, Rozanski A, Pippel Martin, Young G R, Winkler S, et al. The genome of Schmidtea mediterranea and the evolution of core cellular mechanisms. Nature 2018; 554:56–61.
Skinner ME, Uzilov AV, Stein LD, Mungall CJ, Holmes IH. JBrowse: a next-generation genome browser. Genome Res. 2009;19:1630–8.
Buels R, Yao E, Diesh CM, Hayes RD, Munoz-Torres M, Helt G, et al. JBrowse: a dynamic web platform for genome visualization and analysis. Genome Biol. 2016;17:66.
Levy S, Hannenhalli S, Workman C. Enrichment of regulatory signals in conserved non-coding genomic sequence. Bioinformatics. 2001;17:871–7.
Hardison RC. Conserved noncoding sequences are reliable guides to regulatory elements. Trends Genet. 2000;16:369–72.
Egger B, Hobmayer B, Hooge M, Hrouda M, Ishida S, Kobayashi C, et al. To be or not to be a flatworm: the acoel controversy. PLoS One. 2009;4:e5502.
Cebria F, Kobayashi C, Umesono Y, Nakazawa M, Mineta K, Ikeo K, et al. FGFR-related gene nou-darake restricts brain tissues to the head region of planarians. Nature. 2002;419:620–4.
Agata K, Umesono Y. Brain regeneration from pluripotent stem cells in planarian. Philos Trans R Soc Lond Ser B Biol Sci. 2008;363:2071–8.
Shibata N, Kashima M, Ishiko T, Nishimura O, Rouhana L, Misaki K, et al. Inheritance of a nuclear PIWI from pluripotent stem cells by somatic descendants ensures differentiation by silencing transposons in planarian. Dev Cell. 2016;37:226–37.
Zhang G, Fang X, Guo X, Li L, Luo R, Xu F. et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 2012; 490:49–54.
Hayashi S, Itoh M, Taira S, Agata K, Taira M. Expression patterns of Xenopus FGF receptor-like 1/nou-darake in early Xenopus development resemble those of planarian nou-darake and Xenopus FGF8. Dev Dyn. 2004;230:700–7.
Acknowledgements
We thank Osamu Nishimura for his preliminary genome sequencing work, and thank him and Makoto Kashima for fruitful discussions, and Elizabeth Nakajima for reviewing and proofreading the manuscript.
Ethics approvals
Not applicable.
Funding
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas to K. A. (22124001), a Grant-in-Aid for Creative Scientific Research to K. A. (17GS0318), Global COE Program A06 of Kyoto University, Joint Research of National Institute of Genetics (NIG-JOINT, A67), and a Japan Society for the Promotion of Science (JSPS) Research Fellowship to Y. A. (13 J01078).
Availability of data and materials
All transcriptome sequencing data and genome Illumina GAIIx pair-end sequencing data (insert size 180, 200 and 250 bp) were obtained from online recourses [19]. Illumina Hiseq pair-end data (insert size 250, 250, and 450 bp) were sequenced and provided by the Comparative Genomics Laboratory of NIG in Japan. Another Illumina Hiseq pair-end data (insert size 450 bp) and mate-pair data (insert size 3 kbp, 8kbp and 20 kbp) were sequenced by Hokkaido System Science Co., Ltd. Roche 454 pair-end (insert size 3 kbp and 8 kbp) and shotgun (600 and 800 bp) data were sequenced by Agata Lab. D. japonica genomic DNA fosmid library was constructed and also sequenced by Genomics Laboratory of NIG in Japan. All sequencing data is available in DDBJ database (BioProject Accession number: PRJDB6148). The final genome and transcriptome assembly results is available in the genome database, and could be download from http://www.planarian.jp.
Author information
Authors and Affiliations
Contributions
YA and KA conceived and designed the project; YA, TI, AT, and AF performed DNA preparation and sequencing. YA and CZ performed the genome assembly and annotation. AS, SAM, RB and HC constructed the browser of genome database. AK and HO carried out the transgenic experiments. YA wrote the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Quality control of sequencing reads. (PDF 255 kb)
Additional file 2:
Repbase annotation of repeat sequences on the fosmid DJF-016O13. (XLSX 15 kb)
Additional file 3:
De novo assembly by De Bruijn Graph algorithm. (XLSX 10 kb)
Additional file 4:
Gene ontology annotation. (XLSX 11 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
An, Y., Kawaguchi, A., Zhao, C. et al. Draft genome of Dugesia japonica provides insights into conserved regulatory elements of the brain restriction gene nou-darake in planarians. Zoological Lett 4, 24 (2018). https://doi.org/10.1186/s40851-018-0102-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40851-018-0102-2