Abstract
The present study was conducted to investigate the ecological aspects of sand flies in southwestern North Khorasan, in which cutaneous leishmaniasis caused by Leishmania major has been reported with the highest annual incidence in Iran. Sampling was carried out in four localities including: Khorasha (natural), Ghamiteh (natural), Jorbat (semi urban) and Brick kilns (urban), twice a month using 105 sticky paper traps from indoors and outdoors dwellings during May-December 2017. Specimens were removed from sticky papers, washed in acetone, preserved in 80% ethanol, mounted on microscopic slides by Puri’s medium, and identified using valid morphological keys. Simpson (D), richness (S), Menhinick (DMg), Margalef (DMn), Shannon-Weiner (H′), evenness (J’) were calculated for species diversity. The synanthropic index was determined for the first time in the area. Totally 517 specimens were collected, 47% in outdoors and 30.4% in human indoor dwellings and 22.6% in animal. Eight species of sand flies including 5 species of the genus Phlebotomus and 3 species of the genus Sergentomyia were identified. Phlebotomus papatasi and Sergentomyia sintoni were the most common and Eudominant species, active in all months, collected in the maximum number and percentage in September and August, respectively, and showed the highest abundance in outdoors. The synanthropic index ranged from 6.25 to 38.9 in the study area. The Shannon-Wiener index was estimated to be up to 1.4 and 1.37 in Khorasha and November, respectively, which showed the highest diversity due to maximal richness and evenness compared to other areas. High abundance of Ph. papatasi, as the main vector of cutaneous leishmaniasis, can enhance the potential risk of emerging CL in new areas, the data can be equally important when vector control measures are considered.
Similar content being viewed by others
Introduction
Phlebotomine sand flies (Diptera: Psychodidae) are tiny dipteran insects which may have wide variety of hosts for blood feeding. They are vectors of several agents of leishmaniasis [1, 2]. Leishmaniasis occurs in most parts of the world, is found in about 89 countries, and is native to Asia, Africa, the Americas, and the Mediterranean region [3]. About 350 million people live in areas at risk for leishmaniasis, and about 2 million new cases are reported worldwide [3]. About 87% of new cases of cutaneous leishmaniasis were reported from Afghanistan, Algeria, Brazil, Colombia, Syria, Libya, Tunisia, Pakistan, Iraq and Iran. Iran is one of the top ten countries in this regard [4].
There are three main form of the disease: visceral leishmaniasis (VL), cutaneous leishmaniasis (CL), and mucocutaneous leishmaniasis (MCL) [4]. Zoonotic Cutaneous Leishmaniasis (ZCL) and Anthroponotic Cutaneous Leishmaniasis (ACL) are common types of leishmaniasis in Iran [5]. Zoonotic Cutaneous Leishmaniasis has a high prevalence in 17 out of 31 provinces of Iran. The main vector of ZCL in Iran is Phlebotomus papatasi and its causative agent is Leishmania major [6]. Anthroponotic Cutaneous Leishmaniasis is spread in at least 8 provinces of Iran. Phlebotomus sergenti is the main vector of ACL in Iran and its agent is Leishmania tropica [6, 7]. Cutaneous leishmaniasis has been reported from all counties of North Khorasan province [8, 9].
Approximately 1000 species of Phlebotomine sand flies have been identified worldwide [10, 11]. The first comprehensive study on Phlebotomine sand flies of Iran was conducted in 1964 by Theodor and Mesghali. They reported 34 species of Phlebotomine sand flies, including 20 species of the genus Phlebotomus and 14 species of the genus Sergentomyia [12, 13]. This list has been increased to 53 species of Phlebotomine sand flies, including 34 species of the genus Phlebotomus and 19 species of the genus Sergentomyia due to recent studies [14, 15].
In North Khorasan, the first entomological studies began in 1975 in the city of Esfarayen, where L. major was detected as the dominant parasite in Ph. papatasi [16]. Phlebotomus kandelakii was confirmed to be infected with Leishmania infantum by molecular methods in Shirvan [17]. Recently, the richness and diversity of Phlebotomine sand flies have been studied in some rural areas of North Khorasan [18].
Species diversity is one of the most important aspects of insect ecology [19]. Species diversity is studied at three levels: Alpha (α), Beta (β) and Gamma (γ). Alpha is the study of biodiversity in one community, has two components - species richness and evenness, while Beta is the comparing of biodiversity between two or more communities. Gama is the study of all types of diversity in an area [20]. There are plenty of indices to study for biodiversity, but the most common are Simpson and Shannon-Wienner [20]. Biodiversity of Phlebotomine sand flies has been done in some provinces of Iran including Qom [21], Khuzestan [22] and West Azerbayjan Province [23].
In general, the behavioral and environmental patterns of sand flies vary in different climates. Knowledge about the ecological aspects of Phlebotomine sand flies can be mentioned as an additional feature for better finding of dynamism of vector and disease between human and reservoirs [20, 24]. Knowledge on the ecology of sand flies is an approach for a proper vector control program. High incidence of CL in Jajarm County, North Khorasan Province give enough importance for doing this study. The annual incidence rate of CL in Jajarm County was in average 237.8/100000 people [25], which was several times higher than the incidence rate of CL in Iran, which was reported averagely 32 /100000 people over 30 year period study in Iran [26]. The aim of this study was to determine ecological aspects of sand flies (Diptera: Psychodidae, Phlebotominae) in an endemic regions of cutaneous leishmaniasis in southwestern North Khorasan, Iran.
Materials and methods
Study area
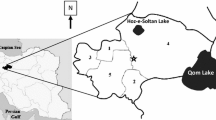
This study was conducted in Jajarm County which is located in southwest of North Khorasan Province between latitude 36°57′00″N, longitude 56°22′48″E and an altitude of 1000 m. It is the most attractive city with several historical and ancient sites, a population of approximately 39,580 and an area of 35,000 km2 (according Census Report in 2016). It is located on the border of the central desert of Iran and has unique vegetation. Geographically, the county is surrounded from the north to Maneh and Samolghan cities, from the west to Garmeh city, from the south to Semnan Province, from the southeast to Razavi Khorasan Province, from the east to Esfarayen city and from the northeast to the city of Bojnurd. The climate is temperate and dry with a mean annual rainfall of 150 mm, relative humidity of 50%, and annual temperature of 16.5 °C. People are mostly occupied in animal husbandry and agriculture.
Sand fly collection
Sampling was implemented in four localities including: Khorasha (natural), Ghamiteh (natural), Jorbat (semi urban) and Brick kilns (urban) in Jajarm County (Fig. 1), where the cases of ZCL have been confirmed. In each locality, two fixed sites were selected based on their topographic conditions. Specimens were collected twice a month using 105 sticky paper traps from indoors (corners of rooms, storage, bathrooms and toilets, human dwelling and stables) and outdoors (in the cracks of clay walls, yards, around rodents’ nests or animal shelters) from May to December 2017 from fixed places. The traps were installed before sunset and collected the next day before sunrise. The sand flies were separated from sticky papers by needle or brush, washed in acetone, preserved in small vials in 80% ethanol, mounted by Puri’s medium on microscopy slides, and then identified using the morphological-based valid keys [27, 28]. The abbreviations of genera and subgenera of sand flies followed by Galati et al. [29].
Synanthropic index, dominance structure and sex ratio
Synanthropic index was computed for all species by the following formula:
Where ‘a’ is the percentage of caught species in the urban area, ‘b’ is the percentage of collected species in the Semi-urban area and ‘c’ is the percentage of collected species in the natural area. The index is ranged between + 100 to − 100; the value of + 100 shows the strong preference of species for anthropogenic settlements and − 100 indicates the lack of preference of species to human dwelling. The average value represents variation degrees of synanthropy [30, 31].
Dominance structure and sex ratio were also calculated for each species in the area. Five categories of dominance structure were considered: Eudominant (ED) (> 10%), Dominant (5-10%), subdominant (SD) (2-5%), recedent (R) (1–2%), and subrecedent (SR) species (< 1%) followed by the following formula:
Where “i” is the total number of specimens of a species and t = total samples [32, 33].
Biodiversity and rarefaction analysis
Indices of species richness, evenness, dominance and diversity were calculated using the Margalef (\(DMg=\frac{S-1}{{\mathit{\ln}}_N}\)), Menhinick’s (\(DMn=\frac{S}{\sqrt{N}}\)), Simpson’s dominance (D = λ = \({\sum}_{i=1}^S{P_i}^2\)), Evenness (J or E or Pielou’s index) (\(\mathrm{J}=\frac{H^{\prime }}{H^{\prime}\max }=\frac{H^{\prime }}{\log (S)}\Big)\) and Shannon indices (H ′ = − Σpi × lnpi) at spatial and temporal scales, where N represents the total number of individuals in the sample, S represents the number of species in the sample, Pi = \(\frac{n_i}{N}\); which Pi is the proportion of individuals observed in ith species, ni is the number of individuals in taxon ith and H′ is the Shannon-Wiener function [34]. To estimate the sand fly richness and the adequacy of sampling efforts, rarefaction curves have been used, which is shown by the following formula:
\(E(Sn)={\sum}_{i=1}^S\left[1-\frac{\left(\genfrac{}{}{0pt}{}{N- Ni}{n}\right)}{\left(\genfrac{}{}{0pt}{}{N}{n}\right)}\right]\), where N = total number of individuals in the sample, S = total number of species and Ni = number of individuals of species number i [35].
Geoghraphic distribution of sand flies
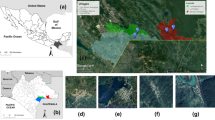
To map the distribution of the dominant species in this study, the latitude and longitude of each region were recorded with Global Positioning System (GPS), and after identifying the samples, the frequency of this species was added to the table in the Arc map, as the main component of ArcGIS software of geospatial processing program. Then its spatial distribution at different times of the sampling was prepared as a map.
Result
A total of 517 specimens were collected by sticky paper traps and identified during sampling efforts, of which 5 species belonged to genus Phlebotomus from two subgenera: Phlebotomus and Paraphlebotomus. Three species were classified in the genus and subgenus Sergentomyia. Most sand flies were caught from outdoor environments (47%), followed by indoor human (30.4%) and animal (22.6%) dwellings. Phlebotomus papatasi and Se. sintoni showed the highest abundance in outdoor environments and were Eudominant species up to 46 and 40.6%, respectively. The sex ratio was calculated up to 0.55 for all samples. These values were up to 2.4 and 1.39 for Phlebotomus and Sergentomyia, respectively. In general, the number of collected females was 64.2% of the total samples (Table 1, Fig. 2).
Seasonal activity of sand flies began in early May and peaked in August and ended in late December in the study area. The highest abundance of phlebotomine sand flies was observed in August (29.2%), whereas the lowest abundance was in December (1.5%). Phlebotomus papatasi and Se. sintoni were active in all months and were collected with maximum number and percentage in September and August, respectively. The highest prevalence of sand flies was recorded in summer, followed by autumn and spring. The predominant species, Ph. papatasi and Se. sintoni, were found with the highest number and percentage (n: 147, 61.8% and n: 136, 64.8%) in the warm season. Population fluctuations of other species by season and month are shown in Tables 2 and 3.
The synanthropic index for phlebotomine sand flies was in the range of 6.25–38.9 in the study area. The index was up to 26.2 and 7.56 for Se. sintoni and Ph. papatasi, respectively. The highest and lowest synanthropic indices were estimated for Ph. jacusiele (38.9) and Ph. sergenti (6.25), respectively. The preference status of other species for compatibility or incompatibility with human settlements is shown in Table 4.
Biodiversity indices of sand flies at spatial and temporal scales are shown in Tables 5 and 6. The Shannon-Wiener index was estimated to be up to 1.4 and 1.37 in the Khorasha area and November, respectively. Maximum richness (S) was revealed in Brick kilns (S = 9) and August, September and November (S = 7). Menhinick (DMg) and Margalef (DMn), as indices of species richness, did not show the highest common numerical values in an area, while they were jointly high in November. The highest values of evenness (J’) index were recorded in Khorasha (J’ = 0.50) and December (J’ = 0.98). While the highest Simpson’s diversity index was observed in Ghamiteh (D: 0.41), followed by Brick kilns, Jorbat (D: 0.38) and Khorasha (D: 0.29), indicating the strong influence of Eudominant species, Ph. papatasi on other species in the area.
Comparison of biodiversity indices showed that there is a significant difference between these indices in spatial and temporal scales (p < 0.05) (Figs. 3 and 4). The highest species diversity was found in Khorasha and November due to maximal richness, diversity and also relatively high evenness (Tables 5 and 6).
The rarefaction curves show the stability of the number of species in each sample (horizontal axis shows the number of individuals and vertical axis shows the number of expected species yielded from the method), almost all rarefaction curves (at the spatial scale) indicates reaching the asymptotic line. This curve tends to stabilize with nine species in Brick kilns and November. In May, the curve did not reach the asymptotic line. More sampling efforts are likely to be required to increase the richness (Fig. 5).
Spatial and temporal distribution of Ph. papatasi, as the most abundant specie in the study area, is mapped in Fig. 6. It showed Brick kilns and Jorbat as urban and semi urban areas had more frequency of the main vector of zoonotic cutaneous leishmaniasis in 4 months.
Discussion
Leishmaniasis is a global health problem due to the significant gap in our understanding of sand fly ecology and unsuccessful control measures [36]. The behavior, biology and ecology of sand flies vary in areas with diverse ecosystems that can prevent control measures. It seems that the best way to reduce the effects on human health is to avoid contact between humans and vectors. Given the high incidence of CL in different cities of North Khorasan Province (average 237.8/100000 people), which is 7 times the annual incidence rate of CL in Iran [25], the establishing reemergence of ZCL in the northeastern provinces of Iran strengthens. Therefore, a basic understanding of the ecological aspects of the native species of the area helps us to properly control the vectors and reduce the burden of disease. The study is considered as the first research on diversity, dominance, seasonal activity, distribution and synanthropic index of sand flies in Jajarm County, southwest of North Khorasan.
In the present study, 5 species of Phlebotomus and 3 species of Sergentomyia were collected and identified. Phlebotomus papatasi and Se. sintoni are in the Eudominant class and are collected both indoors and outdoors. These species were mostly trapped from outdoor environments, indicating the exophilic and exophagic behavior in the region. Phlebotomus papatasi is associated with the epidemiology of leishmaniasis [25], Se. sintoni is believed to prefer feeding on lizards [37]. In accordance with the present study, these species have been introduced as the most abundant species in other studies in Iran [18, 22, 38, 39].
Phlebotomus papatasi is known as the main vector of native and non-native foci of ZCL in Iran [18, 40] and neighboring countries [41], incriminated to prefer semi-arid regions [38], which is consistent with the present study and the findings of other researchers who believe that high precipitation is a limiting factor in species distribution [42, 43]. This may be the reason for the abundance of species in the area.
The results indicated that phlebotomine sand flies are mostly present in the warm season, their monthly activity begins in early May and reaches its peak in August and ends in late December in the study area. In contrast, monthly population fluctuations of sand flies were reported to start in late May and finish in late October with two peaks of activity in early July and another in early August by Aghaie-Afshar et al. in Kerman Province [44] and Mawloudi et al. in Paveh County, west of Iran [45]. This difference in the onset of activity may be due to various climatic factors, milder climate can increase the duration of the activity season as mentioned in a study in Bushehr County, southern Iran, where the activity of sand flies started in early April and ended in early January, with their highest activity occurring in early July [46]. However the most prevalent species, Ph. papatasi and Se. sintoni, in the present study showed a mono-modal trend with a major peak in September and August, respectively, which is in agreement with other researchers’ findings [39]. Other species had lower monthly population densities, which may be due to the specific ecological niches of these species.
There is a significant difference between the sex ratio (Male/female) of sand flies in our research (p < 0.005). The sex ratio was up to 0.77 and 0.37 in Yazd Province [39] and was 5.95 and 1.04 in Ilam Province [47] for Ph. papatasi and Se. sintoni as the dominant species, respectively. In the present study, it was 2.26 and 0.06 for these species, respectively. In general, females were predominated over males. In contrast, in other studies in different parts of Iran, the number of males was always higher than females [48,49,50]. It seems to be due to differences in sampling methods.
The synanthropic index of sand flies was between + 6.25 and + 38.9 in the study area, which strengthens the tendencies of species to reproduce or grow in the human environment. This index was between − 91.18 and − 69.84 in Khuzestan Province [22]. Barata et al. reported a range of 0.4 to 100 for the synanthropic index of sand flies in Brazil [51]. In general, the index ranges from − 100 to + 100 [52]. Sergentomyia sintoni showed higher synanthropic behavior than ph. papatasi, possibly due to changes in the ecological needs and behavioral habits of the species, which require further studies in the future. The synanthropic index was not debatable for other species due to low population density.
There was a significant difference in diversity indices in spatial and temporal scales in the study area (Figs. 3 and 4, p < 0.05). The highest levels of richness were observed in Brick kilns in August, September and November. Commonly, diversity is positively correlated to species richness [20]. The fact is that although the richness was high, higher diversity was observed in Khorasha and November. This is because the biodiversity index is influenced by two other factors, including species evenness and dominance. The low or high rates of these factors can influence the biodiversity index [53,54,55]. Since species richness is influenced by sampling intensity, a standard rarefaction curve is used to confirm adequacy in sampling efforts at temporal and spatial scales by reaching the asymptotic line. The curve indicated that further sampling efforts may be needed to assess satisfaction with species richness in May. It may also reflect the fact that some species would start to emerge a little later in spring. There is little data about the biodiversity of Iranian sand flies, indices for other species of insects were studied in northern Iran [54, 55]. Findings of this study showed higher biodiversity (Mean: H ‘=1.21, J’ = 0.29) in the area compared to the northeast (H’ = 0.527–1.033, J’ = 0.345–0.380) (18) and northwest (H’ = 0.4131, J’ = 0.5309) [42] as well as lower in East Azerbaijan Province (H’ = 1.413–1.918) [23], and the Provinces of East and West Azerbaijan and Ardabil (H’ = 1.68–2.30, J’ = 0.73–0.88) [38]. Decreased species diversity in urban and semi-urban areas compared to natural (Khorasha) can also be due to the increase in population of dominant species such as Ph. papatasi and Se. sintoni, which are well adapted to human environments (Table 4). These findings are consistent with other studies that typically identify urban areas with species richness and diversity less than natural ecosystems [56, 57].
Conclusion
This study provides useful data on the ecological aspects of sand flies in endemic foci of CL in Jajarm, North Khorasan, and can be helpful in a species-specific control analysis of sand flies to evaluate the risk of Leishmania transmission. Occurrence of Ph. papatasi, as Eudominant species, has potential implications for human health in our study area [25], highlighting the need for regular health education programs, along with systematic surveillance of sand flies, human and rodent communities.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Bates PA. Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. Int J Parasitol. 2007;37(10):1097–106.
Tripet F, Clegg S, Elnaiem D-E, Ward RD. Cooperative blood-feeding and the function and implications of feeding aggregations in the sand fly, Lutzomyia longipalpis (Diptera: Psychodidae). PLoS Negl Trop Dis. 2009;3(8):e503.
Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J, Arenas R. Leishmaniasis: a review. F1000Res. 2017;6:750:1–15.
WHO. World Health Organization, 2021. Leishmaniasis. Geneva: WHO; 2021. Available at: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. Accessed 20 May 2021
Nezamzadeh-Ezhiyeh H, Mirhendi H, Jafari R, Veysi A, Rassi Y, Oshaghi MA, et al. An eco-epidemiological study on zoonotic cutaneous Leishmaniasis in Central Iran. Iran J Public Health. 2021;50(2):350.
Yaghoobi-Ershadi M. Phlebotomine sand flies (Diptera: Psychodidae) in Iran and their role on Leishmania transmission. J Arthropod Borne Dis. 2012;6(1):1.
Veysi A, Yaghoobi-Ershadi MR, Rassi Y, Hosseini-Vasoukolaei N, Jeddi-Tehrani M, Rezaee-Node A, et al. Rearing and biology of Phlebotomus sergenti, the main vector of anthroponotic cutaneous leishmaniasis in Iran. J Arthropod Borne Dis. 2017;11(4):504.
Rajabzadeh R, Arzamani K, Shoraka HR, Riyhani H. Epidemiological survey and geographical distribution of cutaneous Leishmaniasis in North Khorasan province, 2006-2013. Int J Epidemiol Res. 2015;2(4):197–203.
Shirzadi MR, Javanbakht M, Vatandoost H, Jesri N, Saghafipour A, Fouladi-Fard R, et al. Impact of environmental and climate factors on spatial distribution of cutaneous Leishmaniasis in northeastern Iran: utilizing remote sensing. J Arthropod Borne Dis. 2020;14(1):56.
Shimabukuro PHF, de Andrade AJ, Galati EAB. Checklist of American sand flies (Diptera, Psychodidae, Phlebotominae): genera, species, and their distribution. ZooKeys. 2017;660:67.
Galati EAB. Phlebotominae (Diptera, Psychodidae): classification, morphology and terminology of adults and identification of American taxa. In: Rangel EF, Shaw JJ, editors. Brazilian sand flies. Cham: International Publishing AG; : Springer; 2018. p. 9–212.
Adler S, Theodor O, Lourie E. On sandflies from Persia and Palestine. Bull Entomol Res. 1930;21(4):529–39.
Theodor O, Mesghali A. On the phlebotominae of Iran. J Med Entomol. 1964;1(3):285–300.
Zahraei-Ramazani A, Kumar D, Mirhendi H, Sundar S, Mishra R, Moin-Vaziri V, et al. Morphological and genotypic variations among the species of the subgenus Adlerius (Diptera: Psychodidae, Phlebotomus) in Iran. J Arthropod Borne Dis. 2015;9(1):84.
Saghafipour A, Vatandoost H, Zahraei-Ramazani AR, Yaghoobi-Ershadi MR, Rassi Y, Shirzadi MR, et al. Spatial distribution of phlebotomine sand fly species (Diptera: Psychodidae) in Qom Province, Central Iran. J Med Entomol. 2017;54(1):35–43.
Javadian E, Nadim A, Tahvildare-Bidruni G, Assefi V. Epidemiology of cutaneous leishmaniasis in Iran: B. Khorassan part V: report on a focus of zoonotic cutaneous leishmaniasis in Esferayen. Bull Soc Pathol Exot Filiales. 1976;69(2):140–3.
Rassi Y, Abai M, Oshaghi M, Javadian E, Sanei A, Rafidzadeh S, et al. First detection of Leishmania infantum in Phlebotomus kandelakii using molecular methods in North-Eastern Islamic Republic of Iran. East Mediterr Health J. 2012;18(4):387–92.
Arzamani K, Vatandoost H, Rassi Y, Akhavan AA, Abai MR, Alavinia M, et al. Richness and diversity of phlebotomine sand flies (Diptera: Psychodidae) in North Khorasan Province, northeast of Iran. J Arthropod Borne Dis. 2018;12(3):232.
Gaggiotti OE, Chao A, Peres-Neto P, Chiu CH, Edwards C, Fortin MJ, et al. Diversity from genes to ecosystems: a unifying framework to study variation across biological metrics and scales. Evol Appl. 2018;11(7):1176–93.
Magurran AE. Measuring biological diversity: Wiley; Hoboken, New Jersey, 2013.
Abedi-Astaneh F, Akhavan AA, Shirzadi MR, Rassi Y, Yaghoobi-Ershadi MR, Hanafi-Bojd AA, et al. Species diversity of sand flies and ecological niche model of Phlebotomus papatasi in Central Iran. Acta Trop. 2015;149:246–53.
Jahanifard E, Yaghoobi-Ershadi MR, Akhavan AA, Akbarzadeh K, Hanafi-Bojd AA, Rassi Y, et al. Diversity of sand flies (Diptera, Psychodidae) in Southwest Iran with emphasis on synanthropy of Phlebotomus papatasi and Phlebotomus alexandri. Acta Trop. 2014;140:173–80.
Hazratian T, Vatandoost H, Oshaghi MA, Yaghoobi-Ershadi MR, Fallah E, Rafizadeh S, et al. Diversity of sand flies (Diptera: Psychodidae) in endemic focus of visceral leishmaniasis in Azar Shahr District, East Azarbaijan Province, north west of Iran. J Arthropod Borne Dis. 2016;10(3):328.
Schowalter TD. Insect ecology: an ecosystem approach: Academic Press; Cambridge, Massachusetts, 2016.
Jalali H, Enayati AA, Fakhar M, Motevalli-Haghi F, Charati JY, Dehghan O, et al. Reemergence of zoonotic cutaneous leishmaniasis in an endemic focus, northeastern Iran. Parasite Epidemiol. Control. 13(2021):e00206.
Shirzadi M, Esfahania S, Mohebalia M, Ershadia M, Gharachorlo F, Razavia M, et al. Epidemiological status of leishmaniasis in the Islamic Republic of Iran, 1983–2012. East Mediterr Health J. 2015;21(10):736–42.
Seyedi-Rashti MA, Nadim A. The genus of Phlebotomus (Diptera: Psychodidae: Phlebotominae) of the countries of the eastern Mediterranean region. Iran J Public Health. 1992;21(1-4):11–50.
Nadim A, Javadian E. Key for species identification of sandflies (Phlebotominae; Diptera) of Iran; 1976.
Galati EA, Galvis-Ovallos F, Lawyer P, Léger N, Depaquit J. An illustrated guide for characters and terminology used in descriptions of Phlebotominae (Diptera, Psychodidae). Parasite. 2017;24(26):1–35.
Barrios SPG, Pereira LE, Nazário Monaco NZ, Graciolli G, Casaril AE, Infran JOM, et al. Synanthropy and diversity of Phlebotominae in an area of intense transmission of visceral leishmaniasis in the south Pantanal floodplain, Midwest Brazil. PLoS One. 2019;14(5):e0215741.
Marí RB, Jiménez-Peydró R. Differences in mosquito (Diptera: Culicidae) biodiversity across varying climates and land-use categories in eastern Spain. Entomol Fenn. 2011;22(3):190–8.
Nikookar SH, Fazeli-Dinan M, Azari-Hamidian S, Nasab SNM, Aarabi M, Ziapour SP, et al. Fauna, ecological characteristics, and checklist of the mosquitoes in mazandaran province, northern Iran. J Med Entomol. 2018;55(3):634–45.
Ott AP, Carvalho GS. Comunidade de cigarrinhas (Hemiptera: Auchenorrhyncha) de uma área de campo do município de Viamão, Rio Grande do Sul, Brasil. Neotrop Entomol. 2001;30(2):233–43.
Thukral AK. A review on measurement of alpha diversity in biology. Agric Res J. 2017;54(1):1–10.
Chao A, Gotelli NJ, Hsieh T, Sander EL, Ma K, Colwell RK, et al. Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr. 2014;84(1):45–67.
Mascari TM, Hanafi HA, Jackson RE, Ouahabi S, Ameur B, Faraj C, et al. Ecological and control techniques for sand flies (Diptera: Psychodidae) associated with rodent reservoirs of leishmaniasis. PLoS Negl Trop Dis. 2013;7(9):e2434.
Nadim A, Seyedi-Rashti M, Mesghali A. On the nature of leptomonads found in Sergentomyia sintoni in Khorassan, Iran and their relation to lizard leishmaniasis. J Trop Med Hyg. 1968;71(9):240.
Akhoundi M, Mirzaei A, Baghaei A, Alten B, Depaquit J. Sand fly (Diptera: Psychodidae) distribution in the endemic and non-endemic foci of visceral leishmaniasis in northwestern Iran. J Vector Ecol. 2013;38(1):97–104.
Jafari R, Najafzadeh N, Sedaghat M, Parvizi P. Molecular characterization of sandflies and Leishmania detection in main vector of zoonotic cutaneous leishmaniasis in Abarkouh district of Yazd Province, Iran. Asian Pac J Trop Med. 2013;6(10):792–7.
Salimi M, Jesri N, Javanbakht M, Farahani LZ, Shirzadi MR, Saghafipour A. Spatio-temporal distribution analysis of zoonotic cutaneous leishmaniasis in Qom Province, Iran. J Parasit Dis. 2018;42(4):570–6.
Ghatee MA, Taylor WR, Karamian M. The geographical distribution of cutaneous leishmaniasis causative agents in Iran and its neighboring countries, a review. Front Public Health. 2020;8:11.
Norouzi B, Hanafi-Bojd A-A, Moin-Vaziri V, Noorallahi A, Azari-Hamidian S. Ecology of sand flies (Diptera: Psychodidae, Phlebotominae) in a new focus of leishmaniasis in northern Iran. Acta Trop. 2020;212:105649.
Perfil'ev PP. Phlebotomidae (sandflies): Israel Program for Scientific Translations; 1968.
Aghaie Afshar A, Rasi Y, Ebaie M, Aghaie AM. Determination of fauna and monthly activity of sandflies in the south of Baft district, Kerman province in 2004. J Kerman Univ Med Sci. 2005;12(2):136–41.
Mawloudi C, Salehzadeh A, Zahirnia AH, Davari B, Jamaat SR, Mawludi S. Species composition and seasonal activity of phlebotomine sand flies (Diptera: Psychodidae) in Paveh County, west of Iran. Iran J Health Saf Environ. 2017;5(1):911–7.
Forouzani A, Khajehiean A, Darabi H, Fouladvand MA, Nabipour I, Bahramian F. Fauna and Monthly activity of sand flies in the focus of cutaneous Leishmaniasis in Bushehr District (2007-2008). ISMJ. 2011;14(1):31–40.
Kavarizadeh F, Vazirianzadeh B, Rassi Y, Jalali Glusang A, Abbas MS. A Faunistic study of sand flies of Musian District, southwestern of Iran. Pak J Zool. 2013;45(2):549–54.
Sayyadi M, Vahabi A, Sayyad S, Gharib A, Vahabi B. An entomological survey of phlebotomine sand flies (Diptera: Psychodidae) in Ravansar County, Kermanshah Province, west of Iran. Life Sci J. 2013;10(12s):873–7.
Rafatbakhsh-Iran S, Salehzadeh A, Nazari M, Zahirnia AH, Davari B, Latifi M, et al. Ecological aspects of the predominant species of Phlebotominae sand flies (Diptera: Psychodidae) in Hamadan, Iran. Zahedan J Res Med Sci. 2016;18(2):e5994.
Kassiri H, Javadian E. Some ecological characteristics of Phlebotomine sandflies in a focus of cutaneous Leishmaniasis, Chabahar, Iran. Zahedan J Res Med Sci. 2012;14(8):21–4.
Barata R, Ursine R, Nunes F, Morais D, Araújo H. Synanthropy of mosquitoes and sand flies near the Aimorés hydroelectric power plant, Brazil. J Vector Ecol. 2012;37(2):397–401.
Uribe-M N, Wolff M, de Carvalho CJ. Synanthropy and ecological aspects of Muscidae (Diptera) in a tropical dry forest ecosystem in Colombia. Rev Bras Entomol. 2010;54(3):462–70.
Zouirech M, Faraj C, Belghyti D. Entomological and epidemiological investigations of an emerging focus of cutaneous leishmaniasis in Bzou, Morocco. Entomol faun; 2015.
Fazeli-Dinan M, Asgarian F, Nikookar S, Ziapour S, Enayati A. Defining and comparison of biodiversity components of hard ticks on domestic hosts at Highland, woodland and plain in northern Iran. Trop Biomed. 2019;36(1):114–30.
Nikookar S, Moosa-Kazemi S, Oshaghi M, Vatandoost H, Yaghoobi-Ershadi M, Enayati A, et al. Biodiversity of culicid mosquitoes in rural Neka township of Mazandaran province, northern Iran. J Vector Borne Dis. 2015;52(1):63.
Strand TM, Lundkvist Å. Rat-borne diseases at the horizon. A systematic review on infectious agents carried by rats in Europe 1995–2016. Infect Ecol Epidemiol. 2019;9(1):1553461.
Byers KA, Lee MJ, Patrick DM, Himsworth CG. Rats about town: a systematic review of rat movement in urban ecosystems. Front Ecol Evol. 2019;7:13.
Acknowledgments
The authors would like to sincerely thank the staff of the health centers and the experts of the entomology department of the School of Health for their help in the project.
Funding
This research was supported by Research Deputy of Mazandaran University of Medical Sciences, Project No. 2728 (ethical code: IR.MAZUMS.REC.1398.936).
Author information
Authors and Affiliations
Contributions
NHV, AAE and FMH designed the study, HJ was student in MSc and collected all data. SHN, EJ and JYC analyzed the data. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jalali, H., Nikookar, S.H., Hosseini-Vasoukolaei, N. et al. Ecology of sand flies (Diptera: Psychodidae, Phlebotominae) in Jajarm County, an area with high risk of cutaneous leishmaniasis, in North Khorasan, Iran. BMC Zool 7, 14 (2022). https://doi.org/10.1186/s40850-022-00113-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40850-022-00113-0