Abstract
Background
Reproductive strategies and evolutionary pressures differ between males and females. This often results in size differences between the sexes, and also in sex-specific seasonal variation in body mass. Seasonal variation in body mass is also affected by other factors, such as weather. Studies on sex-specific body mass patterns may contribute to better understand the mating system of a species. Here we quantify patterns underlying sex-specific body mass variation using a long-term dataset on body mass in the Siberian flying squirrel, Pteromys volans.
Results
We show that female flying squirrels were larger than males based on body mass and other body measures. Males had lowest body mass after the breeding season, whereas female body mass was more constant between seasons, when the pregnancy period was excluded. Male body mass did not increase before the mating season, despite the general pattern that males with higher body mass are usually dominant in squirrel species. Seasonal body mass variation was linked to weather factors, but this relationship was not straightforward to interpret, and did not clearly affect the trend in body mass observed over the 22 years of study.
Conclusions
Our study supports the view that arboreal squirrels often deviate from the general pattern found in mammals for larger males than females. The mating system seems to be the main driver of sex-specific seasonal body mass variation in flying squirrels, and conflicting selective pressure may occur for males to have low body mass to facilitate gliding versus high body mass to facilitate dominance.
Similar content being viewed by others
Background
Reproductive strategies and evolutionary pressures differ between sexes and often lead to sex differences in body size, in body mass as well as in bone measurements [1–3]. The same factors may also lead to sex-specific seasonal variation in body mass, depending on energy expenditure and condition of individuals [4, 5]. Studies on sex-specific seasonal patterns of body mass are relatively scarce [6–9], but may contribute to understand the mating system of a species.
In mammals, lactation is one of the main factors contributing to the seasonal difference in energy expenditure between sexes. In addition, the levels of intra-sexual competition during the breeding season typically differ between sexes, depending on the mating system of a species [3, 10–13]. An interesting mating system to study intra-sexual patterns in body mass is the so called scramble competition mating system [14]. This mating system has been frequently reported for insects [15], but is poorly studied in mammals, although it may occur, for example, in arboreal squirrels (subfamily Sciurinae; [16, 17]). In a scramble competition mating system, females are solitary and males move to visit different females which may be in oestrus during different days. Within this context, selection may favour males that are effective in locating female territories scattered across the landscape [16, 18].
Mating system and sexual selection are, however, not the only factors shaping differences in body mass between sexes [19]. For example, the seasonality of resource availability (such as food) and weather conditions (e.g. harsh winter periods) may also affect body mass difference between sexes beyond the more commonly recognised effect of reproductive strategies [20, 21]. Consequently, climate change can have sex-specific effects on body mass [22], potentially creating temporal changes also in sexual body mass dimorphism of the species.
In this study, we broadly aim to quantify patterns underlying sex-specific body mass variation between and within years in the Siberian flying squirrel, Pteromys volans (Linnaeus, 1758) in Central-Western Finland. We used long-term datasets on body mass of individuals spanning 22 years and also a short-term dataset on body measurements. In flying squirrels, the locomotor system of gliding likely places a unique set of selective forces related to body mass in this group [23, 24]. Female flying squirrels live in isolation and males need to rapidly move between females during the mating season [17]. In addition, Siberian flying squirrels, like tree squirrels [25], perform mating chases, whereby a few males glide and run one after another from tree to tree following a female [26]. These behaviours may promote high movement abilities of males in competition with each other, and may favour fast gliders with low body mass. However, within the mating system of tree and flying squirrels, the defence of females by large dominant males may promote high body mass necessary for dominating mating opportunities and prevent smaller subdominant males from reproducing [17, 25, 27]. Earlier studies indicate that flying squirrels may have female-biased sexual dimorphism, with females being larger than males based on body mass [17] and body measurements of few museum specimens [28]. However, these studies are based on a limited number of individuals. In addition, the links between the mating system and the seasonal variation in body mass are still poorly understood in squirrels. For example, conflicting pressures between a defence mating system and a scramble competition mating system [16, 18] may affect seasonal variation in body mass in male flying squirrels. In addition, seasonal sexual dimorphism in body mass may be related to different seasonal patterns in energy expenditure between the sexes.
We predict (i) that seasonal patterns in body mass underlie the specific mating system: if males have highest body mass before the breeding season and lowest body mass after the breeding season, then the female defense mating systems is dominant in this species [2, 9]. Instead, if the scramble competition mating system is operative, then we predict that males should not have the highest body mass before the mating season in order to be fast in mating chases and in locating females. We also predict (ii), against the general pattern in mammal species, that in flying squirrels the females will have a larger body mass (measured outside of the breeding season) and other body measurements than males. Finally, we predict (iii) that weather conditions affect seasonal variation in body mass. Spring weather, which corresponds to the start of the breeding season of flying squirrels and, thus, may have sex-specific effects on body mass, has significantly warmed in Finland during our study period [29, 30]. Thus, climate change has the potential to affect the 22 year trend in body mass of the two sexes in flying squirrels.
Methods
Study species
The Siberian flying squirrel is a nocturnal arboreal rodent which nests in tree cavities, nest-boxes and dreys (twig nests) in spruce-dominated boreal forests. Flying squirrels feed on deciduous trees within spruce-dominated forests. The mating season starts in mid-March and the first litter is born in late April [26]. Females can sometimes have a second litter which is born in late June. Females are territorial and live in non-overlapping home ranges (on average 4 ha in size), whereas males have much larger home ranges (average size of 60 ha) that can overlap with several other male and female home ranges [17]. The movement activity of males increases during the mating season when males actively move between territories of different females. Females come into oestrus, albeit not synchronously, within a short period starting from mid-March [17, 26].
Study areas and data collection
The study was carried out in two areas: Luoto (63°49’N, 22°49’E) and Vaasa (63°3’N, 22°41’E). In Luoto, flying squirrels were studied between 1993 and 2014 within an area of 44 km2. The main land-uses in Luoto are shoreline spruce-dominated mixed forests, clear-cuts, and cultivated Scots pine plantations. The Vaasa study area is located about 90 km southwest of Luoto. The marking of flying squirrels started in 1992 in Vaasa within an area of 4 km2, after the year 2000, the area was expanded to cover 25 km2. Vaasa is covered by spruce-dominated forest patches, clear-cuts, and agricultural fields (for more information see [31, 32]).
The studied populations bred in nest-boxes. Nest-boxes were placed in forest patches of various sizes in groups of 2 to 4 nest-boxes per site, on average 2 nest-boxes per mature spruce forest hectare. The nest-boxes were made from a piece of aspen or spruce trunk, so that they resembled natural cavities. No known differences are apparent in behaviour or reproductive output, e.g. in number of offspring produced or communal nesting patterns, between individuals living in nest-boxes and those living in dreys or natural cavities (unpublished data; [32]), nor are we aware of significant differences in predator communities between sites. The nest-boxes have an entrance-hole diameter of 4.5 cm. This diameter is the same of that of the entrance of cavities made by the Great spotted woodpecker, Dendrocopus major, which represent the most common natural nesting site for flying squirrels in our study area. This size of the entrance-hole prevents main predators (e.g. the pine marten, Martes martes, and also large owls) from entering the nest-box. The same size of the entrance-hole, as well as the cavity size, between nest boxes and natural cavities makes the former readily accepted by flying squirrels for breeding and resting.
In total 489 male and 562 female flying squirrels were captured by hand from nest-boxes, sexed, weighed, and marked with ear-tags (Hauptner 73850, Hauptner, Germany). The main nest-box checking session was in June, and sites found occupied were checked again in August. In addition, in the years 1992–2003 part of the nest boxes were checked also between September and March, but for the following years there were only sporadic observations during these months. In total there were 1812 observations in June and August and 284 observations for October-March (for the 1051 studied individuals). The same observer (R. Wistbacka) was responsible for measurements taken in Luoto study area and also in Vaasa after 2001. Before 2001, weighing in Vaasa was made by R. Wistbacka and A. Mäkelä. The same weighing scale type was used (Pesola) and the scale was calibrated with a similar scale used in another flying squirrel population [33]. Thus, biases due to observer error between study areas were reduced.
We knew the age of 182 out of 489 males and 239 out of 562 females, because those individuals had been previously captured and marked as juveniles. Recapturing probability of individuals was high, above 0.8 for females and 0.7 for males [31], and we can conclude that new unmarked adult individuals located within our study area were very likely new recruits to our study system. Recruits arrive during the natal dispersal period, typically concentrated in September, whereas breeding dispersal is rare in flying squirrels [34, 35]. In other words, within our study areas the likelihood of an unmarked adult individual of being 1 year old is very high ([31], unpublished data). We used age based on this assumption in our models, because it was better aiming to control for the possible effect of age than leaving age out of the analyses (the same approach was used by [16]). The results for age were similar when only individuals captured as juveniles were used (Additional file 1: Figure S1).
Female body mass is affected by pregnancy between the start of mating season in mid-March and the birth of summer litters. Based on our data, second litters are born by mid-July at the latest. Thus, for the analysis of the effect of age and year on body mass, we excluded female observations recorded between 15 March and end of July. Nevertheless, the energy expenditure during the breeding season may have carry over impacts on body mass of females still in August.
For analysing sexual dimorphism in size, in addition to body mass, we measured skull length of 72 individuals, femur length of 60 individuals, and tail length of 56 individuals, all adults, during June 2014 and 2015 (the same individuals were used for measurements of skull, femur and tail, but femur and tail length were missing for some individuals). All these measurements were taken by the same observer (A. Santangeli) to avoid observer-induced measurement bias.
Weather variables
We used weather information from the weather station (maintained by the Finnish Meteorological Institute) nearest to each study area. For Vaasa, the closest weather station was located within our study area, and for Luoto it was 10 km southeast of the study area. Weather recording stations were at the same altitude as the study areas. We used monthly average temperature and precipitation indices between December and June. Early winter weather was averaged for December and January, late winter was February and March. Spring season was represented by April and May, and June described the summer weather. For the spring weather, we also used the starting date of the tree growing season. Temperatures consistently above +5 °C indicate the beginning of tree growing season (Finnish Meteorological Institute; http://en.ilmatieteenlaitos.fi/seasons-in-finland), and have also been shown to correlate with birch bud burst in Finland [36].
Statistical analyses
Models on sexual differences in body measurements
We first built three generalized linear mixed models (GLMM) using as a response the skull, femur and tail length (n = 56–72). Because the main rationale for these models was to quantify sex differences in the abovementioned three measurements, we used sex as a categorical predictor, and territory identity nested within year in the random part to account for multiple observations from different individuals in the same territory (e.g. measurements of male and female from the same territory). We then used all observations where body mass was measured (n = 695) during the months from January to March, and from August to November to explore sex differences in body mass. Thus we excluded the months when females were pregnant in order to make the comparison in body mass between the two sexes. Additionally, we fit a GLMM with similar structure as those explained above, but now also with age and month controlled for in the model.
Models on seasonal and weather effects on body mass
We used GLMMs to investigate the relationship between body mass of flying squirrels (separately for sex and for two seasons, see below) in relation to environmental, life-history and temporal predictors. Specifically, we ran four GLMMs using in turn the body mass of adult females or males separately within two different seasons (winter and summer) as the response variable. Here we considered as winter all measurements of body weight collected between January and March from males and females (n = 120 and 99, respectively), and as summer the measurements collected between June and November for males (n = 804), and between August and November for females (n = 284). We excluded female measurements collected in June from all analyses as these are affected by the breeding state (pregnancy). We run separate sex-specific models for the winter and summer season because data for the winter season were only collected up to the year 2003, whereas those from the summer period spanned until the year 2014 (see above).
In each of the four models, we included the individual identity nested within the study area (Luoto or Vaasa) in the random part of the model to account for pseudo-replication (i.e. multiple measurements collected on the same individual in the same study area over the years). We then included, in each of the four models, the month when body mass was measured (as a categorical variable), the age of the individual and the year (both as continuous variables). Finally, for the winter models we also included as predictors the average temperature and average precipitation during December and January, and for the summer models, the average temperature and precipitation in December and January combined, February and March combined, as well as average temperature and precipitation in May, April and June separately. In the summer models we also included the starting date of the tree growth season, with the rationale that an early start of the growth season would result in higher body mass later in the summer. We also tested the effect of age squared (to fit non-linear trends) by including this variable in each full model, and removing it if non-significant. We assessed the significance of each level combination within the categorical variables (e.g. between body mass in January and February within the month variable) by means of post-hoc comparisons adjusted for multiple testing using the Tukey method.
Before fitting the models we checked for collinearity using variance inflation factor (VIF) analyses. All variables had a VIF value lower than 2.5, indicating low collinearity levels and no need for excluding any of them from the models. We then built the four full models (i.e. the ones with all candidate predictor variables), one for each sex-class and season combination (see above). Next we applied model selection based on the Akaike’s information criterion (AIC), followed by multi-model inference and averaging [37] using the MuMin package in R [38]. We derived averaged model coefficients and p-values for each variable from across the set of best ranked models (i.e. with ∆AIC < 4; listed in Additional file 1: Table S1).
Finally, we tested whether there was any temporal trend in body mass during winter (from early January to 15th of March), i.e. before the start of the mating season. This model was similar to the above models for body mass of males and females, but month was replaced with the day of the year so that 1st January got a value of 0 and 15th of March a value of 105. For this latter analysis we did not have repeated measurements from the same individual, therefore there was no need to include the individual identity as a random effect, whereas study area was included as a class variable.
All analyses were performed in R software v. 3.0.3 [39].
Results
Sexual differences in body measurements
Females body mass was on average 12 g higher than that of males (using data from the 22 years study period: t = -14.61, p < 0.001). Moreover, based on data from the years 2014 and 2015 only, females appear to have longer femur than males (t = -3.10, p = 0.01), whereas skull (t = 0.30, p = 0.77) and tail length (t = -1.32, p = 0.22) were similar between the two sexes (Fig. 1).
Seasonal and weather effects on body mass
We found considerable model uncertainty when running all possible combinations of body mass sub-models for the four separate analyses (see Additional file 1 for the list of 10 best supported models for males and females). This underscores the need for multi-model averaging, from which results are shown below.
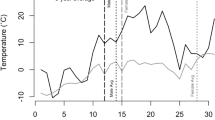
Body mass of adult flying squirrels did not vary significantly between the different winter months (Fig. 2) and there was no change in body mass over the period preceding the start of the mating season for male or female flying squirrels. This was tested with the correlation between date, from January to mid-March, and body mass: males: n = 104, F 1, 95.6 = 0.35, p = 0.55; females: n = 93, F 1, 21.5 = 0.81, p = 0.38. However, we show that male body mass declined after the breeding season, amounting to about 10 % loss of weight from the winter body mass (Fig. 2). This decline was observable right after the start of the mating season as the pattern seems clear already in April (i.e. the decline from March to April was on average 5 g from the raw data, n = 32 measurements in April). Conversely, the body mass of females did not vary between seasons and summer months (Fig. 2).
Male and female body mass (least square means and standard errors) for each month from the winter model (1 January to 3 March) and for the summer model (6 June to 11 November). For females, June was omitted because they may still be pregnant in June. Lines above the bars join months for which body mass was significantly different after post-hoc testing (adjusted for multiple testing using the Tukey method). For females, there were no significant differences in body mass between the months of the winter season and between the months of the summer season
For both sexes, low temperatures in late winter, as well as the early start of the tree growth season, resulted in increased body mass during the following summer season (Fig. 3, Table 1). Moreover, for male flying squirrels higher temperature in spring was associated to lower body mass in summer, whereas increased rain in June was related to lower body mass of females. Winter body mass was not related to any weather variable (Tables 1 and 2). The only significant temporal trend observed over the years of study was an increasing trend in female weight measured in winter. Moreover, body mass increased with age in both sexes (Tables 1 and 2).
Decrease in summer body mass of a) male and b) female flying squirrels with the increase in average temperature during the preceding winter (February and March). c Change in summer body mass of female flying squirrels in relation to the onset of tree growth season. d Trend in winter body mass of female flying squirrels over the years of study. Dots depict predicted values from the full model, with fitted lines and confidence intervals
Discussion
We did not observe changes in male body mass before the breeding season. This was against our prediction for the female defence mating system. The only observable seasonal pattern we found was a decrease in male body mass after the breeding season. As we predicted, female flying squirrels were larger than males, but there were no detectable seasonal variation in female body mass outside of the pregnancy period. Unexpectedly, cold winter and cold spring conditions were linked to an increase in body mass of males and females in the following summer. The only temporal trend observed over the 22 year study period was a slight increase in winter body mass of females. Thus, no measurable temporal trends that might be related to sexual-size dimorphism were detected.
The observed female-biased sexual size dimorphism in the Siberian flying squirrels of this study is consistent with patterns observed for the southern flying squirrel Glaucomys volans [24]. In our study, the female-biased body mass was largest after the breeding season, because female body mass did not vary much within the year (outside of the pregnancy period). Similar observations of a stable female body mass between seasons have been noted for North American red squirrels [40]. We observed no difference in skull size between sexes. Conversely, femur length was longer in females than in males. The explanation for this may be the need for females to secure gliding potential when pregnant, and, thus, increase the aerofoil area compared to males [23]. For example, it is known that for flying mammals (i.e. bats) pregnancy restricts the ability to move [41]. Larger female body mass, as compared to that of males in flying squirrels may be related to reproductive benefits from large size due to the territoriality of females ([17, 24], see also [42]). Alternatively, it may also be due to the benefits for males to be smaller and thus more vagile within a scramble competition mating system.
We predicted changes in male body mass before the start of the mating season, but did not find any indication for these changes, within winter or between autumn and winter. Thus, there was no clear pattern in male body mass that would support the female defence mating system. For example in North American red squirrels [40] and grey-headed flying-foxes, Pteropus poliocephalus [9], body mass of males increased before the start of the mating season and declined rapidly then after. However, it is known that in the Siberian flying squirrel the male body mass is positively related to reproductive success [17]. Thus, some aspects of the female defence mating system may be operative in flying squirrels, as is the case for tree squirrels [25, 27]. However, the stable body mass of males during winter seems to fit the hypothesis that extra weight just before breeding may not enhance fast movement to locate females. This fits the scramble competition mating system. Perhaps in the case of the Siberian flying squirrel, the selective forces for increased gliding potential versus dominance (i.e. low versus high body mass, respectively) at some level cancel each other out.
Body mass before the mating season is likely affected by environmental factors during the preceding winter. However, food availability for flying squirrels during the winter months is relatively stable, since the main winter food, catkins of deciduous trees, develop already during the autumn and persist on the trees during winter. In other words, food availability does not vary within the winter season [30]. Only after bud burst in spring do flying squirrels start consuming leaf material, but this is after the start of the mating season (from mid-May onwards in our study area). In addition, we did not find any weather variable to have an effect on body mass in winter, which is in line with a previous study where no effect of winter temperature on communal nesting behaviour of flying squirrels was found [32].
Surprisingly, male body mass did not increase during summer, from June to August, although the breeding season for male flying squirrels ends already in the spring after they have fertilised the females. Males had even lower body mass in August than in June. Instead, for some other species male mass is observed to decline rapidly during the mating season, but also increased rapidly during the summer [8, 43, 44]. In August, male flying squirrels frequently move between forest patches occupied by different females [45], perhaps to increase social links with new females which might affect future reproductive success. This may increase the energy expenditure of male flying squirrels and explain the decline in body mass observed during the late summer months. Moreover, another possible explanation for the observed monthly variation in body mass might also relate to the food available to flying squirrels in the different periods, because in early summer flying squirrels eat mainly leaves [46]. However, food would affect body mass in both sexes. In addition, whether the nutritional value of leaves differs from that of catkins for flying squirrels is still unknown. In any case, food obviously is related to observed increase in male body mass from August to October, as squirrels likely prepare for winter by increasing body mass in late autumn.
Body mass increased with the age of Siberian flying squirrels in both sexes, which is in line with earlier studies reporting body mass increases with age in adult squirrels [25, 27, 47]. It should be noted that our analysis did not separate mortality and individual growth curves and is not suitable for determining growth patterns related to senescence [48, 49]. Nevertheless, age, body mass, dominance hierarchies and reproductive success are linked in squirrels, as older individuals are usually heavier, higher in dominance hierarchy, and consequently have increased reproductive success [17, 25, 47].
Our results do not suggest any impact of climatic trends on body mass dimorphism in flying squirrels. The only temporal pattern observed during our study period was a slight increase in female body mass during winter. Our temporal data for winter months was limited (winter body mass data ended in year 2003) and it remains unclear what process is behind the observed temporal trend, because winter body mass was not linked to winter temperatures. In addition, the observed trend was against the expectation that climate warming would result in smaller body size ([50, 51]; but see [52]). The increasing trend in early spring temperatures over our study period ([29], for more detailed analysis on weather changes in our study area, see [30]) indicates potential for temporal changes in sexual dimorphism in body mass. However this was not the case, as we did not observe any trends in summer body mass of males or females.
Weather in winter had unexpected effects on summer body mass of flying squirrels. The summer body mass of both sexes increased when the preceding winter and spring seasons were colder. The reasons for this pattern remain unknown, but a possible explanation could be that warm weather at the beginning and during the breeding season may increase the intensity of mating season and result in high energy expenditure and low body mass after a successful reproduction. Lower body mass in summer was also associated with delayed tree growth in spring. This correlation might indicate a poor food situation in the late spring, because after the flowering of catkins in early spring leaves represent the main food for flying squirrels. Late start of the tree growing season indicates delay in leave growth. We also found a negative correlation between increased rain in June and female body mass in August, which may indicate that rainy conditions in summer have negative impacts on female body mass. In earlier studies, weather has been observed to affect timing of reproduction and reproductive success in Siberian flying squirrels [30] and red squirrels [53, 54]. In addition, for example in ungulates, body mass interacts strongly with weather and food availability in spring and summer [55, 56].
Conclusions
Our study supports the general view that tree and flying squirrels often deviate from the general pattern in mammals for males being larger than females [24, 57, 58]. Seasonal body mass patterns of male flying squirrels support the hypothesis that competition during the breeding season is the main driver of seasonal body mass variation in males. Instead, female body mass was more constant between seasons, when the effects of pregnancy are not considered. However, we also suggest that, in the case of Siberian flying squirrels, competing forces may play a role in selecting for male size that would represent an optimal balance between fast gliding (i.e. low body mass) and dominant (i.e. high body mass), with these competing pressures potentially masking some of the seasonal variation (before breeding season) in male body mass. However, verifying this hypothesis would require further studies. Our results also support the view that the effects of temperature on body mass may be complex and in correlative studies not necessarily straightforward to interpret [52]. Nevertheless, no indication that climate change had affected sexual body mass dimorphism was detected.
References
Ralls K. Mammals in which females are larger than males. Quart Rev Biol. 1976;51:245–76.
Andersson M. Sexual Selection. Princeton: Princeton University Press; 1994.
Clutton-Brock TH. Sexual selection in males and females. Science. 2007;318:1882–5.
Lindstedt SL, Boyce MS. Seasonality, fasting endurance, and body size in mammals. Am Nat. 1985;125:873–8.
Gittleman JL, Thompson SD. Energy allocation in mammalian reproduction. Am Zool. 1988;28:863–75.
Soderquist TR. Ontogeny of sexual dimorphism in size among polytocous mammals: tests of two carnivorous marsupials. J Mammal. 1995;76:376–90.
Boratynski Z, Koteja P. Sexual and natural selection on body mass and metabolic rates in free-living bank voles. Funct Ecol. 2010;24:1252–61.
Rughetti M, Festa-Bianchet M. Seasonal changes in sexual size dimorphism in Northern chamois (Rupicapra rupicapra). J Zool. 2011;284:257–64.
Welbergen JA. Fat males and fit females: sex differences in the seasonal patterns of body condition in grey-headed flying-foxes (Pteropus poliocephalus). Oecologia. 2011;165:629–37.
Mitchell B, McCowan D, Nicholson IA. Annual cycles of body weight and condition in Scottish red deer, Cervus elaphus. J Zool. 1976;180:107–27.
Michener GR, Locklear L. Differential costs of reproductive effort for male and female Richardson’s ground squirrels. Ecology. 1990;71:855–68.
Wolff JO. Breeding strategies, mate choice, and reproductive success in American bison. Oikos. 1998;83:529–44.
Korine C, Speakman J, Arad Z. Reproductive energetics of captive and free-ranging Egyptian fruit bats (Rousettus aegyptiacus). Ecology. 2004;85:220–30.
Ims RA. Spatial clumping of sexually receptive females induces space sharing among male voles. Nature. 1988;335:541–3.
Thornhill R, Alcock J. The evolution of insects mating systems. Cambridge: Harvard University Press; 1983.
Lane JE, Boutin S, Gunn MR, Coltman DW. Sexually-selected behaviour: red squirrel males search for reproductive success. J Anim Ecol. 2009;78:296–304.
Selonen V, Painter JN, Rantala S, Hanski IK. Mating system and reproductive success in the Siberian flying squirrel. J Mammal. 2013;94:1266–73.
Schulte-Hostedde AI, Millar JS. “Little chipmunk” syndrome? Male body size and dominance in captive yellow-pine chipmunks (Tamias amoenus). Ethology. 2002;108:127–37.
Isaac JL. Potential causes and life-history consequences of sexual size dimorphism in mammals. Mammal Rev. 2005;35:101–15.
Chan-McLeod AC, White RG, Russell DE. Comparative body composition strategies of breeding and nonbreeding female caribou. Can J Zool. 1999;77:1901–7.
Beck CA, Bowen WD, Iverson SJ. Sex differences in the seasonal patterns of energy storage and expenditure in a phocid seal. J Anim Ecol. 2003;72:280–91.
Sheriff MJ, Richter M, Buck CL, Barnes BM. Changing seasonality and phenology of free-living arctic ground squirrels; the importance of sex. Phil Trans R Soc B. 2013;368:20120480.
Fokidis HB, Risch TS. Does gliding when pregnant select for larger females? J Zool. 2008;275:237–44.
Fokidis HB, Risch TS, Glenn TC. Reproductive and resource benefits to large female body size in a mammal exhibiting female-biased sexual size dimorphism. Anim Behav. 2007;73:479–88.
Koprowski JL. Alternative reproductive tactics and strategies of tree squirrels. In: Wolff JO, Sherman PW, editors. Rodent Societies: an Ecological and Evolutionary Perspective. Chicago: University of Chicago Press; 2007. p. 86–95.
Hanski IK, Mönkkönen M, Reunanen P, Stevens PC. Ecology of the Eurasian flying squirrel (Pteromys volans) in Finland. In: Goldingay R, Scheibe J, editors. Biology of gliding mammals. Furth: Filander; 2000. p. 67–86.
Wauters L, De Vos R, Dhondt AA. Factors affecting male mating success in red squirrels (Sciurus vulgaris). Ethol Ecol Evol. 1990;2:195–204.
Nandini R-R. The evolution of sexual size dimorphism in squirrels. PhD dissertation. Alabama: Auburn University; 2011.
Mikkonen S, Laine M, Mäkelä HM, Gregow H, Tuomenvirta H, Lahtinen M, Laaksonen A. Trends in the average temperature in Finland, 1847–2013. Stoch Env Res Risk Assessm. 2015;29:1521–9.
Selonen V, Wistbacka R, Korpimäki E. Food abundance and weather modify reproduction of two arboreal squirrel species. J Mammal. 2016; doi:10.1093/jmammal/gyw096.
Lampila S, Wistbacka A, Mäkelä A, Orell M. Survival and population growth rate of the threatened Siberian flying squirrel (Pteromys volans) in a fragmented forest landscape. Ecosci. 2009;16:66–74.
Selonen V, Hanski IK, Wistbacka R. Communal nesting is explained by subsequent mating rather than kinship or thermoregulation in the Siberian flying squirrel. Behav Ecol Socio. 2014;68:971–80.
Koskimäki J, Huitu O, Kotiaho J, Lampila S, Mäkelä A, Sulkava R, Mönkkönen M. Are habitat loss, predation risk and climate related to the drastic decline in a Siberian flying squirrel population? A 15 year study. Popul Ecol. 2014;56:341–8.
Hanski IK, Selonen V. Female-biased natal dispersal in the Siberian flying squirrel. Behav Ecol. 2009;20:60–7.
Selonen V, Hanski IK. Condition-dependent, phenotype-dependent and genetic-dependent factors in the natal dispersal of a solitary rodent. J Anim Ecol. 2010;79:1093–100.
Rousi M, Heinonen J. Temperature sum accumulation effects on within-population variation and long-term trends in date of bud burst of European white birch (Betula pendula). Tree Physiol. 2007;27:1019–25.
Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. 2nd ed. New York: Springer; 2002.
Bartoń K. Package “MuMIn” - Multi-Model Inference. 2014.
R Core Development Team. R: A language and environment for statistical computing. Version 3.0.3. 2013.
Koprowski JL. Annual cycles in body mass and reproduction of endangered Mt. Graham Red Squirrels. J Mammal. 2005;86:309–13.
Hayssen V, Kunz TH. Allometry of litter mass in bats: maternal size, wing morphology, and phylogeny. J Mammal. 1996;77:476–90.
Bondrup-Nielsen S, Ims RA. Reversed sexual size dimorphism in microtines: are females larger than males or are males smaller than females? Evol Ecol. 1990;4:261–72.
Falls JB, Falls EA, Fryxell JM. Fluctuations of deer mice in Ontario in relation to seed crops. Ecol Monogr. 2007;77:19–32.
Hoogland JL. Sexual dimorphism of prairie dogs. J Mamm. 2003;84:1254–66.
Hanski IK, Stevens P, Ihalempiä P, Selonen V. Home-range size, movements, and nest-site use in the Siberian flying squirrel, Pteromys volans. J Mammal. 2000;81:798–809.
Mäkelä A. Liito-oravan (Pteromys volans L.) ravintokohteet eri vuodenaikoina ulosteanalyysin perusteella (diet of flying squirrel, in Finnish). WWF Finland Rep. 1996;8:54–8.
Wauters L, Dhondt AA. Lifetime reproductive success and its correlates in female Eurasian red squirrels. Oikos. 1995;72:402–10.
Nussey DH, Coulson TN, Delorme D, Clutton-Brock TH, Pemberton JM, Festa-Bianchet M, Gaillard JM. Patterns of body mass senescence and selective disappearance differ among three species of free-living ungulates. Ecology. 2011;92:1936–47.
Mumby HS, Chapman SN, Crawley JAH, Mar KU, Htut W, Thura Soe A, Aung HH, Lummaa V. Distinguishing between determinate and indeterminate growth in a long-lived mammal. BMC Evol Biol. 2015;15:214.
Millien V, Lyons SK, Olson L, Smith FA, Wilson AB, Yom-Tov Y. Ecotypic variation in the context of global climate change: revisiting the rules. Ecol Lett. 2006;9:853–69.
Blois JL, Feranec RS, Hadly EA. Environmental influences on spatial and temporal patterns of body-size variation in California ground squirrels (Spermophilus beecheyi). J Biogeogr. 2008;35:602–13.
Teplitsky C, Millien V. Climate warming and Bergmann’s rule through time: is there any evidence? Evol Appl. 2014;7:156–68.
Williams CT, Lane JE, Humphries MM, McAdam AG, Boutin S. Reproductive phenology of a food-hoarding mast-seed consumer: resource- and density-dependent benefits of early breeding in red squirrels. Oecologia. 2014;174:777–88.
Studd EK, Boutin S, McAdam AG, Krebs CJ, Humphries MM. Predators, energetics and fitness drive neonatal reproductive failure in red squirrels. J Anim Ecol. 2015;84:249–59.
Mysterud A, Yoccoz NG, Langvatn R, Pettorelli N, Stenseth NC. Hierarchical path analysis of deer responses to direct and indirect effects of climate in northern forest. Proc R Soc B. 2008;363:2357–66.
Herfindal I, Saether B-E, Solberg EJ, Andersen R, Hdga KA. Population characteristics predict responses in moose body mass to temporal variation in the environment. J Anim Ecol. 2006;75:1110–8.
Don BAC. Home range characteristics and correlates in tree squirrels. Mammal Rev. 1983;13:123–32.
Lurz PWW, Gurnell J, Magris L. Sciurus vulgaris. Mamm Species. 2005;769:1–10.
Acknowledgements
We thank all of the field workers Timo Hyrsky, Rune Jakobsson, Antero Mäkelä and Markus Sundell, who have assisted during data gathering. Three referees in peerage of science are thanked for their useful comments that helped to improve the manuscript.
Funding
The study was financially supported by Academy of Finland (grant number 259562 to VS), Kone Foundation (to AS), Oskar Öflunds stiftelse (to RW), Societas Pro Fauna et Flora Fennica (to RW), Svensk-Österbottniska samfundet (to RW), and Vuokon luonnonsuojelusäätiö (to RW).
Availability of data and material
The data set supporting the results of this article is available in the European Boreal Forest Biodiversity (EBFB) database repository, https://www.earthcape.com/.
Authors’ contributions
RW collected data, VS and AS analysed data and wrote the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. No ethical approval was required from an institutional or national ethics review board.
Author information
Authors and Affiliations
Corresponding author
Additional file
Additional file 1: Table S1.
The best ranked models explaining body mass of male Siberian flying squirrels measured in summer and winter (upper and lower panel respectively). Columns show the variables included in each model (see main text for an explanation of the variable names), the degrees of freedom of each model, the Akaike value corrected for small sample size (AICc) and the difference in AIC between best ranked and the target model (∆AICc) as well as the AIC weight of each model. List is restricted to 10 best ranked models. Table S2. The best ranked models explaining body mass of female Siberian flying squirrels measured in summer and winter (upper and lower panel respectively). Columns show the variables included in each model (see main text for an explanation of the variable names), the degrees of freedom of each model, the Akaike value corrected for small sample size (AICc) and the difference in AIC between best ranked and the target model (∆AICc) as well as the AIC weight of each model. List is restricted to 10 best ranked models. Figure S1. Male and female body mass (least square means and standard errors) for each month from the winter model (1 January to 3 March) and for the summer model (6 June to 11 November). Only observations where the exact age was known have been used for this supporting analysis (see methods). For females, June was omitted because they may still be pregnant in June. Lines above the bars join months for which body mass was significantly different after post-hoc testing (adjusted for multiple testing using the Tukey method). For females, there were no significant differences in body mass between the months of the winter season and between the months of the summer season. (DOCX 127 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Selonen, V., Wistbacka, R. & Santangeli, A. Sex-specific patterns in body mass and mating system in the Siberian flying squirrel. BMC Zool 1, 9 (2016). https://doi.org/10.1186/s40850-016-0009-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40850-016-0009-3