Abstract
Background
Type 1 Diabetes (T1D) is associated with increased risk of fractures, worsened by presence of microvascular complications. This study’s objective is to determine the impact of progressive decline in estimated glomerular filtration rate (eGFR) on bone biomarkers and bone microarchitecture in youth with T1D.
Methods
Slopes of eGFR were calculated using measures obtained at four timepoints from adolescence to young adulthood. Participants were identified as eGFR decliners if eGFR decreased ≥ 3ml/min/1.73m2/year. Bone health was assessed in young adulthood by high resolution peripheral quantitative computed tomography (HRpQCT Xtreme CTII) and bone biomarkers; osteocalcin, procollagen 1 intact n-terminal pro-peptide (P1NP), c-terminal telopeptide (CTX), and bone specific alkaline phosphatase. The relationship between diabetes duration, glycated hemoglobin, body mass index (BMI) and vitamin D level on bone biomarkers and microarchitecture was evaluated. Linear regression analysis was used for the statistical analysis in this study.
Results
Ninety-nine study participants were studied with longitudinal evaluation of eGFR over 7.4 ± 1.0 years with mean age of 14.7 ± 1.7 years at baseline. Cross sectional evaluation of bone was performed at 21.3 ± 2.1 years. 44% participants had eGFR decline and showed 5% higher cortical porosity diameter than non-decliners (p = 0.035). Greater diabetes duration was associated with higher trabecular separation (p = 0.004) and lower trabecular number (p = 0.01). Higher level of 25 hydroxy-vitamin D was associated with lower trabecular separation (p = 0.01). Elevated glycated hemoglobin (p = 0.0008) and BMI (p = 0.009), were associated with lower markers of bone formation.
Conclusion
Mild increase in cortical porosity diameter was found in youth with T1D and eGFR decline, however, overall measures of bone microarchitecture on HR-pQCT were similar between both groups and there were no statistically significant changes in bone biomarkers. Hence, skeletal impairments were limited in youth with different eGFR trajectories near peak bone mass. Longitudinal HR-pQCT studies are needed to further understand the impact of eGFR decline on bone microarchitecture. Optimal glycemic control, normal BMI and vitamin D status were supported by this study as important markers for good bone health.
Similar content being viewed by others
Background
Type 1 diabetes (T1D) is associated with increased risk of fractures that is 2 to fourfold higher relative to the general population [1]. Fracture risk is increased in the presence of diabetic kidney disease (DKD) [2]. Newer imaging modalities, such as high resolution peripheral computed tomography (HRpQCT) have improved sensitivity compared to commonly used imaging modalities to evaluate and detect changes in bone microarchitecture [3]. HRpQCT is a non-invasive imaging modality that provides information on bone mineral density with additional information on trabecular and cortical bone compartments [3]. HRpQCT studies in adolescents with T1D have shown significant differences compared to healthy controls including lower tibia trabecular thickness and higher tibia cortical porosity consistent with reduced bone quality [4]. Furthermore, HRpQCT studies in adults report markers of worsened bone microarchitecture in adults with T1D compared to healthy controls [2]. Additional impairments, including lower total volumetric bone mineral density, trabecular volumetric bone mineral density, trabecular thickness and failure load and increased trabecular separation, have been shown in T1D patients with microvascular complications compared to those without microvascular complications [2].
Albuminuria is a well-recognized and clinically evaluated marker to assess diabetes-related kidney changes. However, there is evidence of changes in kidney function of patients with T1D prior to development of microalbuminuria or eGFR < 60ml/min/1.73m2 [5,6,7]. Progressive decline in eGFR is reported to be a predictor of progression to DKD that can be used as a surrogate marker in younger T1D patients, to predict long term changes in kidney function [5,6,7]. Prior to the development of chronic kidney disease in patients with T1D, it is hypothesized that the effects of type 1 diabetes with hyperglycemia, increased cytokines, advanced glycated end products not only affects the kidneys but also has an effect on bone [8, 9]. Presence of microvascular complications in T1D are associated with increased levels of vascular endothelial growth factor (VEGF) production and action, impairing bone healing [8, 9]. There is also decreased blood vessel formation and reduced bone regeneration [8, 9].
T1D is associated with increased risk of fractures worsened by presence of kidney disease, therefore a major challenge in preventing bone related complication is identifying youth at higher risk. As such, the aim of this study was to evaluate bone health in young adulthood in a cohort of youth with T1D followed longitudinally from adolescence to young adulthood to assess the association of progressive eGFR decline on bone microarchitecture and biomarkers, utilizing second generation HRpQCT imaging and bone biomarkers. Secondary aims were to assess the relationships between HbA1c, diabetes duration, BMI, and 25 hydroxy-vitamin D on bone microarchitecture in T1D. The overarching hypothesis is that progressive eGFR decline, in addition to diabetes disease variables, are associated with impairments in bone microarchitecture and biomarkers.
Methods
Study population and design
This was a secondary use analysis of longitudinal data of adolescents who participated in the Adolescent Type I Diabetes Cardio-Renal Intervention Trial (AdDIT, EudraCT Number: 2007-001039-72, Trial Registration Number: ISRCTN91419926) in Canada from 2009-2015, and were recruited into a longitudinal, observational study evaluating cardio-renal-bone health in young adults with T1D from 2016 - 2022 at the Hospital for Sick Children in Toronto, Canada [10, 11]. There were pre-specified timelines for data collection in both studies, i.e. AdDIT study baseline, AdDIT midpoint, AdDIT final and cardio-renal-bone study baseline.
This study was approved by the Sick Kids Research Ethics Board. All patients provided informed consent to participate in this study. The study was conducted in accordance with the Declaration of Helsinki.
Demographic and clinical data
Demographic characteristics including age, sex and ethnicity, along with age of T1D diagnosis, duration of T1D, fracture history (i.e., number and type of fracture), insulin regimen, total daily insulin dose (units/kg/day) and route of administration (pump or injection), history of celiac disease, hypothyroidism, and smoking status were collected from the cardio-renal-bone health study baseline visit. Height, measured using a wall-mounted stadiometer and weight measured by an electronic scale were collected from all study visits.
Biochemical investigations
HbA1c and creatinine were measured in the Department of Pediatric Laboratory Medicine at SickKids using standard laboratory methods (including an isotope dilution mass spectrometry-traceable assay) and were collected at all four time points. HbA1c was averaged to determine cumulative glycemic exposure.
Serum calcium, phosphate, magnesium, 25 hydroxyvitamin D, bone-specific alkaline phosphatase (Bs-ALP), procollagen-type 1 N-terminal-propeptide (P1NP) and serum cross-linked C-telopeptide (CTX) were measured in the Department of Laboratory Medicine & Pathobiology, Toronto General Hospital, Toronto, Canada using standard laboratory measures. Osteocalcin was measured by Eve Technologies, Calgary, Canada. Osteocalcin was run on multiplex immunoassay and CTX was measured using immunoassay test on Diagnostics e411 analyzer (Roche, Elecsys beta CrossLaps serum assay). Total P1NP and Bs-ALP were run on manual Elisa assay, immunoenzymetric assay with SPECTRA MAX 384 PLUS. Cut-off for Vitamin D sufficiency, insufficiency and deficiency was based on best practice guidelines [12].
Estimated Glomerular Filtration Rate (eGFR)
eGFR for each study participant was calculated using CKiD U25 creatinine, sex adjusted formula; males = 41.8 x (height /serum creatinine), females = 37.6 x (height /serum creatinine); height in meters, creatinine in mg/dL. This eGFR formula was developed for youth ages 1 – 25, facilitating evaluation of eGFR from adolescence to young adulthood [13]. It has been shown to be the most precise and accurate eGFR equation with the least bias when compared to measured GFR in our cohort [14].
Linear mixed effect regression modeling was used to determine participant-specific eGFR slopes. Participants with eGFR decline \(\ge\) 3ml/min/1.73m2/year, were classified as progressive eGFR decliners while participants with eGFR \(<\) 3 ml/min/1.73m2/year were classified as eGFR non-decliners. This eGFR slope cut off was based on prior research studies in patients with diabetes [5,6,7].
High Resolution Peripheral Quantitative Computer Tomography (HRpQCT) imaging
In young adulthood, at the final data collection point, images of tibia from the non-dominant ankle and wrist using HRpQCT second generation scanner, (Xtreme CTII, Scanco Medical AG, Brutisellan, Switzerland) was performed on all study participants. The opposite ankle or wrist was scanned if an individual had a history of fracture at the non-dominant side. A trained nuclear medicine technician in performing HRpQCT scans, under supervision of a radiologist performed all scans. Daily and weekly scans were performed as part of quality control. The international guidelines for HRpQCT scanners by Whittier et al., was used to perform the scans [15]. Fixed scanning approach was performed for ankle and wrist measurements and threshold-based approach was used to assess cortical porosity.
Statistical analysis
Data analysis was conducted using R studio version 1.4.1717. Normally distributed continuous variables were described as mean ± SD or as median (Q1, Q3), depending on distribution; frequency and proportions were used to describe categorical variables. Linear regression analysis was performed to assess the relationship between eGFR decline and HRpQCT bone parameters, adjusted for age, sex, and body mass index (BMI). To evaluate the relationship between eGFR decline and bone biomarkers, linear regression analysis was performed, adjusting for age, sex and 25 hydroxyvitamin D level. The adjusted covariates were based on prior literature. P value less than 0.05 was considered statistically significant for the primary relationship of eGFR decline on HRpQCT bone parameters. P value less than or equal to 0.01 was chosen to evaluate secondary outcomes of relationships between demographic and diabetes variables on bone microarchitecture as well as relationship between eGFR decline on bone biomarkers.
Results
One hundred and three study participants completed HRpQCT scans, four of which had missing data. Total of 99 study participants were evaluated in this study over an average of 7.4 (± 1.0) years. Average age of study participants at time zero was 14.7 (± 1.7) years and 21.3 (± 2.1) years at the final assessment. Median diabetes duration was 13.7 (11.8, 16.0) years with cumulative HbA1c of 8.25% (± 0.95).
Forty four percent (73% male) of study participants were eGFR decliners, compared to 56% (24% male) eGFR non-decliners. The median eGFR slope for the whole cohort was -2.8 (-4.7, -0.7) ml/min/1.73m2/year, with the median eGFR slope for eGFR decliner group, -4.9 (-6.2, -4.0) ml/min/1.73m2/year and eGFR non-decliner group -0.7 (-2.3, - 0.08) ml/min/1.73m2/year.
Among all study participants, 30% reported fracture history with mean number of 0.4 (± 0.8) lifetime fractures. Reported fractures were traumatic fractures, predominantly sport related injuries. There were no significant differences in fracture history or 25 hydroxy-vitamin D level between eGFR decliners and non-decliners (Table 1). Calcium, PTH, phosphate and magnesium were within normal range based on the normative reference ranges of lab assay. Significant differences were found between groups, with eGFR decliner group having a male predominance, younger age at diagnosis and younger age at end of study (Table 1).
Primary outcomes: HRpQCT
In the adjusted linear regression analysis, being in the eGFR decliner group was associated with 5% higher tibia cortical porosity (p = 0.035). There were no other statistically significant associations between eGFR decline and other HRpQCT bone parameters (Table 2).
Secondary outcomes
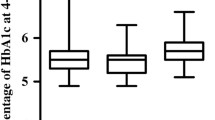
In the adjusted model, there were no statistically significant associations between progressive eGFR decline and bone biomarkers (Table 3). Higher HbA1c was associated with lower P1NP (p = 0.0008) (Fig. 1a). Increasing BMI was associated with lower osteocalcin (p = 0.009), (Fig. 1b). Increasing diabetes duration was associated with increasing radius trabecular separation (p = 0.004) and decreasing radius trabecular number (p = 0.01), (Fig. 1 c, d). Lower 25 hydroxyvitamin D concentration was associated with higher tibia trabecular separation (p = 0.01), (Fig. 1e). The relationship between HbA1c, diabetes duration, BMI and vitamin D level and bone microarchitecture at the tibia and radius are outlined in Tables 4 and 5.
Discussion
The primary objective of this study was to assess the effects of progressive eGFR decline, an early marker of microvascular kidney injury in T1D on bone microarchitecture [5,6,7]. A statistically significant change was found with 5% higher cortical porosity diameter in young adults with T1D and progressive eGFR decline compared to participants with a lack of eGFR decline. This is a novel finding, not previously reported. This is likely due to the study’s use of second generation HRpQCT, which provides better resolution in contrast to previous reported studies using first generation HRpQCT. Previous studies have reported changes in cortical porosity; a recent study of adolescents with T1D showed a 21.5% increase in cortical porosity in participants with T1D compared to healthy controls [4]. It is proposed that a change in cortical porosity diameter would occur prior to cortical porosity.
Although a statistically significant difference was found in cortical porosity diameter, there were no other significant differences among the other HRpQCT parameters assessing bone microarchitecture or with bone biomarkers. The limited findings of microarchitectural changes in this study may be due to the young adult age of the subjects which is when peak bone mass is achieved. Additionally, shorter diabetes duration and limited glycemic exposure could limit the extent of the effects of advanced glycated end products, glycemia and oxidative stress on microvasculature and bone re-modeling [8].
T1D has been shown to be associated with a low bone turnover state and reduced BMD [16]. Presence of kidney disease in T1D has features of exocrine insufficiency, and a degree of malabsorption that can lead to lowered BMD and mineralization abnormalities [16]. Hyperglycemia may be associated with increased loss of calcium in urine, however due to other regulatory systems, most notably parathyroid hormone, serum calcium is usually maintained [17]. Lower levels of 25 hydroxyvitamin D and higher levels of trabecular separation was found in this study. A systematic review and meta-analysis showed a 2.6% increase in total body bone mineral content and 1.7% increase in lumbar spine bone mineral density from baseline in children with low vitamin D who were supplemented [18].
In this study, higher HbA1c was associated with lower levels of P1NP, a marker of bone formation. A recent study found lower P1NP with higher HbA1c in adolescents with T1D [4]. Secondary analysis of the Diabetes Control and Complications Trial (DCCT)/ Epidemiology of Diabetes Interventions and Complications (EDIC) study found worse glycemic control to be associated with lowered bone formation [19]. This finding is likely due to the negative effects of high glucose on osteoblast response to mechanical loading and differentiation of mesenchymal stem cells [20, 21].
In a recent adolescent study with sub-analysis of patients with T1D duration of 2 years or longer, an association was found with lower trabecular number and increased trabecular separation [22]. Similar microarchitectural changes were observed in this study with longer diabetes duration having an association with higher trabecular separation and lower trabecular number.
T1D was previously associated with a lean phenotype, however there are recent reports of increasing rates of overweight and obesity in T1D [23]. The average BMI of this cohort was 25.7kg/m2, which is in the overweight category in BMI classification [24]. Increasing BMI was associated with lower levels of osteocalcin, a marker of bone formation. There is conflicting evidence regarding BMI and its effect on bone health [25]. There is evidence of potential role of high BMI as a protective factor to bone loss with it increasing bone mineral density due to higher levels of 17 ß-estradiol, higher mechanical load and decreasing bone turnover [25]. However, there is concern of potential microarchitectural changes that might be resulting in increased fracture rates observed with obesity [26].
While this study showed a small but statistically significant difference between cortical porosity diameter and progressive eGFR decline; it is noted that there were minimal differences observed in the other HRpQCT parameters and bone biomarkers in the adjusted linear regression analysis. Further longitudinal HRpQCT studies are needed to better understand the long-term impact of progressive eGFR decline on bone microarchitecture and biomarkers and fracture risk.
There are a few study limitations. This study was a secondary analysis of previously collected data, and the initial goal of the original research study was not specifically to address this study’s hypothesis. This cohort had overall good metabolic control and high technology, pump use which may correspond to lower elevations in glycemic exposure and reduced glucotoxicity effect on bone microarchitecture and biomarkers. HRpQCT provides extensive information with multiple parameters assessed at the tibia and radius, therefore a well-developed statistical plan was established a priori to the analysis, in order to limit potential type 1 error. There was a lack of study participants with substantial reduced kidney function. eGFR decline was chosen as a surrogate marker of early kidney disease in T1D for this study based on previous longitudinal studies, however another surrogate marker or addition of multiple markers could have provided better evaluation of early signs of microvascular changes in the kidney [5,6,7, 27]. The study population was primarily Caucasian, 62%, therefore lack of ability to evaluate ethnicity related factors.
Strengths of this study include addressing gaps in literature and specifically, the evaluation of the impact of early signs of kidney microvascular disease in adolescents with T1D on bone microarchitecture in young adulthood. A second generation HRpQCT scanner was used which has better resolution, providing improved evaluation of bone micro-architecture. Additionally, this study utilized the updated CKiD U25 formulae which allows evaluation of eGFR from childhood to young adulthood. This study was a secondary analysis of a well phenotype longitudinally evaluated cohort, providing a wealth of information.
Conclusion
In summary, while a single outcome with 5% increased cortical porosity diameter was observed to be statistically significant in the eGFR decliner group compared to those with stable eGFR, overall measures of HR-pQCT and bone biomarkers were similar between both groups and were not statistically significant. These data suggest skeletal impairments are limited with youth with T1D near peak bone mass with different eGFR trajectories. Significant relationships were found with markers of increased skeletal fragility; increased diabetes duration, higher HbA1c, lower 25 hydroxyvitamin D and higher BMI. While further studies with longitudinal HR-pQCT evaluation are needed to determine the long-term effects of progressive eGFR decline on bone microarchitecture and fracture risk, these results emphasize the importance of optimal glycemic, blood pressure control, healthy lifestyle with weight bearing physical activity, along with appropriate dietary calcium and vitamin D intake in patients with T1D.
Availability of data and materials
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
Abbreviations
- Bs-ALP:
-
Bone specific alkaline phosphatase
- Ct:
-
Cortical
- Ct.Pm:
-
Cortical perimeter
- Ct.Po:
-
Cortical porosity
- Ct.Po.Dm:
-
Cortical porosity diameter
- CTX:
-
C-terminal telopeptidee
- GFR:
-
Estimated glomerular filtration rate
- HbA1c :
-
Glycated hemoglobin
- HRpQCT:
-
High resolution peripheral quantitative computed tomography
- N:
-
Number
- P1NP:
-
Procollagen 1 intact n-terminal pro-peptide
- Sp:
-
Separation
- Tb:
-
Trabecular
- T1D:
-
Type 1 diabetes
- Th:
-
Thickness
- vBMD:
-
Volumetric bone mineral density
References
Shah VN, Shah CS, Snell-Bergeon JK. Type 1 diabetes and risk of fracture: meta-analysis and review of the literature. Diabet Med. 2015;32(9):1134–42.
Shanbhogue VV, et al. Bone geometry, volumetric density, microarchitecture, and estimated bone strength assessed by HR-pQCT in adult patients with type 1 diabetes mellitus. J Bone Miner Res. 2015;30(12):2188–99.
Di Iorgi N, et al. Update on bone density measurements and their interpretation in children and adolescents. Best Pract Res Clin Endocrinol Metab. 2018;32(4):477–98.
Mitchell DM, et al. Elevated HbA1c is associated with altered cortical and trabecular microarchitecture in girls with type 1 diabetes. J Clin Endocrinol Metab. 2020;105(4):e1648–56.
Westreich KD, et al. Trajectories in estimated glomerular filtration rate in youth-onset type 1 and type 2 diabetes: the SEARCH for diabetes in youth study. J Diabetes Complications. 2021;35(2):107768.
Colombo M, et al. Predicting renal disease progression in a large contemporary cohort with type 1 diabetes mellitus. Diabetologia. 2020;63(3):636–47.
Skupien J, et al. The early decline in renal function in patients with type 1 diabetes and proteinuria predicts the risk of end-stage renal disease. Kidney Int. 2012;82(5):589–97.
Shah VN, et al. Bone health in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2018;25(4):231–6.
Cipriani C, et al. The interplay between bone and glucose metabolism. Front Endocrinol (Lausanne). 2020;11:122.
Levin A, Adams E, Barrett BJ, et al. Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease (Can-SOLVE CKD): Form and Function. Can J Kidney Health Dis. 2018;5:1–12.
Marcovecchio ML, et al. Adolescent Type 1 Diabetes Cardio-Renal Intervention Trial (AdDIT): urinary screening and baseline biochemical and cardiovascular assessments. Diabetes Care. 2014;37(3):805–13.
Bouillon R, Carmeliet G. Vitamin D insufficiency: definition, diagnosis and management. Best Pract Res Clin Endocrinol Metab. 2018;32(5):669–84.
Pottel H, Björk J, Delanaye P, Nyman U. Evaluation of the creatinine-based chronic kidney disease in children (under 25 years) equation in healthy children and adolescents. Pediatr Nephrol. 2022;37(9):2213-16.
Gaebe K, et al. Evaluation of novel glomerular filtration rate estimation equations in adolescents and young adults with type 1 diabetes. J Diabetes Complications. 2022;36(1):108081.
Whittier DE, et al. Guidelines for the assessment of bone density and microarchitecture in vivo using high-resolution peripheral quantitative computed tomography. Osteoporos Int. 2020;31(9):1607–27.
Santos Monteiro S, et al. Bone mineral density progression following long-term simultaneous pancreas-kidney transplantation in type-1 diabetes. Ann Endocrinol (Paris). 2023;84(4):454–9.
Starup-Linde J, Vestergaard P. Biochemical bone turnover markers in diabetes mellitus - A systematic review. Bone. 2016;82:69–78.
Winzenberg T, et al. Effects of vitamin D supplementation on bone density in healthy children: systematic review and meta-analysis. BMJ. 2011;342:c7254.
Rubin MR, et al. Biochemical markers of bone turnover in older adults with type 1 diabetes. J Clin Endocrinol Metab. 2022;107(6):e2405–16.
Gopalakrishnan V, et al. Effects of glucose and its modulation by insulin and estradiol on BMSC differentiation into osteoblastic lineages. Biochem Cell Biol. 2006;84(1):93–101.
Seref-Ferlengez Z, et al. P2X7R-Panx1 complex impairs bone mechanosignaling under high glucose levels associated with type-1 diabetes. PLoS One. 2016;11(5):e0155107.
Devaraja J, et al. Impact of type 1 diabetes mellitus on skeletal integrity and strength in adolescents as assessed by HRpQCT. JBMR Plus. 2020;4(11):e10422.
Corbin KD, et al. Obesity in type 1 diabetes: pathophysiology, clinical impact, and mechanisms. Endocr Rev. 2018;39(5):629–63.
Nuttall FQ. Body mass index: obesity, bmi, and health: a critical review. Nutr Today. 2015;50(3):117–28.
Hou J, et al. Obesity and bone health: a complex link. Front Cell Dev Biol. 2020;8:600181.
Taes YE, et al. Fat mass is negatively associated with cortical bone size in young healthy male siblings. J Clin Endocrinol Metab. 2009;94(7):2325–31.
Hoj Thomsen L, et al. Smad2 phosphorylation in diabetic kidney tubule epithelial cells is associated with modulation of several transforming growth factor-beta family members. Nephron. 2017;135(4):291–306.
Acknowledgements
The authors are grateful for the support of the SPOR Can-SOLVE CKD and the Canadian Pediatric Endocrine Group (CPEG) for the CPEG fellowship award funding for Dr. Babalola’s research fellowship.
Funding
The authors have no funding to declare.
Author information
Authors and Affiliations
Contributions
FB designed the study, collected, analyzed, interpreted the data and wrote the manuscript. FM was the supervisor for the study overseeing the study process, the analysis and the interpretation and reviewed the manuscript. JH and MZ were involved in the reviewing the study design, analysis, interpretation and reviewing the manuscript. RM was involved in assisting in data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Sick Kids Research Ethics Board. All patients provided informed consent to participate in this study. The study was conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Babalola, F., Hamilton, J., Zappitelli, M. et al. Bone health in young adults with type 1 diabetes and progressive eGFR decline. Clin Diabetes Endocrinol 10, 12 (2024). https://doi.org/10.1186/s40842-024-00169-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40842-024-00169-6