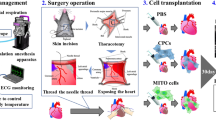
Abstract
Aims
The alternatively activated macrophages have shown a cardioprotective effect in heart failure. However, the effect of M2 adoptive transfer in non-ischemic heart failure is unknown. In this study, we evaluated the efficacy of M-CSF plus IL-4 induced M2-like macrophages transplantation in doxorubicin-induced cardiotoxicity.
Methods
Bone marrow mononuclear cells were polarized as CCR2+CD206+ M2-like macrophages by a combination of M-CSF plus IL-4 treatment. C57BL/6 mice received a single intraperitoneal injection of doxorubicin (15 mg/kg). The treatment group were treated with M2-like macrophages (1 × 10^6 cells per mouse; i.v.) once a week for 2 weeks. After 3 weeks, we examined the percentage of resident cells and cardiac function. Furthermore, we evaluated cardiac fibrosis, cardiomyocyte apoptosis and circulating inflammatory factors. Finally, we investigated the mitochondria transfer in vitro in a direct and indirect co-culture conditions.
Results
Cardiac function was significantly improved in doxorubicin-induced heart failure by adoptive transfer of M2-like macrophages. Besides, M2-like macrophages treatment attenuated cardiac fibrosis and cardiomyocyte apoptosis, as well as increased the level of circulating IL-4 and Th2 response. In vitro, M2-like macrophages could transfer mitochondria to injured cardiomyocytes in a direct and indirect way.
Conclusions
In our study, adoptive transfer of M2-like macrophages could protect against the doxorubicin-induced cardiotoxicity, which may be partly attributed to mitochondria transfer. And M2-like macrophages transplantation could become a treatment for non-ischemic heart failure in the clinical practice.
Graphical Abstract

Similar content being viewed by others
Introduction
Cardiovascular complications are becoming more and concerned in cancer therapies [1]. Doxorubicin (DOX), as a kind of anthracyclines, is limited by its cardiotoxicity in cancer chemotherapy, leading to progressive contractile dysfunction and ultimately heart failure (HF) [2]. A magnitude of investigations demonstrated that oxidative stress [3], chronic inflammation [4], and mitochondrial dysfunction [5] contributed to DOX-induced cardiac injury. However, no effective strategies were recommended for alleviating DOX-induced cardiotoxicity.
Macrophages are characterized by phenotypic diversity, including pro-inflammatory M1 (the classically activated M1-like) and anti-inflammatory M2 (the alternatively activated M2-like) populations [6]. Previous study showed that DOX administration significantly promotes production of the pro-inflammatory factors IL-1β, contributing to DOX-induced cardiac damage [7]. While M2 macrophages, characterized by production of IL-10, exerts cardioprotective effects in heart failure [8].
Previous reports showed that M2 adoptive transfer could prevent type 1 diabetes [9] and promoted locomotor recovery in spinal cord injury [10] by modulating inflammation response. Besides, M2-like macrophages could alleviate fibrosis [11] and promote cardiac repair post myocardial infarction [12]. Given that a low percentage of endogenous M2 activated macrophages during acute or chronic heart failure [13, 14], we speculated that M2 macrophage transplantation could effectively and clinical-applicably improve cardiac function in DOX-induced cardiotoxicity.
In this study, we cultured M2-like macrophages by treating bone-marrow-derived macrophages with M-CSF plus IL-4. Subsequently, we examined the effect of M2-like macrophage on doxorubicin-induced cardiomyopathic heart failure using an adoptive transfer method. Finally, we explored whether mitochondrial transfer mediated the beneficial effects of M2-like macrophages.
Materials and methods
Bone marrow derived mononuclear cells (BMDM) isolation and induction
Male C57BL/6J mice, 4 weeks old (Model Animal Research Center of Nanjing University) were euthanatized by cervical dislocation and the femur and tibia were collected. After flushed out, the red blood cells were removed using Red Blood Cell Lysis Buffer (Dakewei, China). Then the mixture was centrifuged for 5 min at 300 g and the pellet was collected. The cells were washed twice in PBS, and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA) containing 10% fetal bovine serum (FBS, Gibco, USA) and 1% penicillin/streptomycin (P/S, Invitrogen, USA). Besides, the cells were treated with M-CSF (20 ng/ml, Peprotech) for 6 days. The medium was changed every 3 days. On the 7th day, the mature macrophages were treated with IL-4 (20 ng/ml, Peprotech, USA) for 24 h to induce the formation of M2-like macrophages. We have collected M2–like macrophages after washing for 3 times to eliminate the direct effects of IL-4 and M-CSF.
Flow cytometry
After 7 days, cells were collected from the culture flasks by scraping and resuspended in PBS. After washed twice, cells were centrifuged at 300 g for 5 min and resuspended in 100 µl PBS. Then cells were incubated with antibodies or corresponding IgG controls for 30 min on ice.
For cardiac cell collection, the hearts were dissected, minced and enzymatically digested with type II collagenase (Sigma, USA), and passed through a 70-µm cell strainer. The following anti-mouse antibodies (eBioscience, USA) were used: anti-CCR2-APC, anti-CD206-PE, anti-CD3-FITC anti-CD4-APC, and anti-IL-4-PE. Data were analyzed with FlowJo software (Treestar, USA).
The H9c2 cardiomyocytes (Rat cardiomyocytes; American Type Culture Collection, Manassas, USA) were cultured in DMEM with 10% FBS and 1% P/S. The culture conditions contained 95% air and 5% CO2 at 37 ℃. An Annexin V-FITC/propidium iodide (PI) apoptosis kit (Beyotime, China) was used to analyze the apoptosis rate. The early apoptosis was gated as Annexin V+/PI− population while late apoptosis was gated as Annexin V+/PI+ population.
MitoTracker Red staining and carboxyfluorescein succinimidyl ester (CFSE)-fluorescent label
M2-like macrophages were stained with 200 nM MitoTracker Red (Beyotime, China) for 20 min at 37℃ and then washed twice with PBS. Pre-stained M2-like macrophages were collected and seeded for the following experiments.
H9c2 cardiomyocytes were seeded in 6-well plates and stained with 10 µM CFSE (Invitrogen, USA) for 20 min and washed twice with PBS. Pre-stained M2-like macrophages and H9c2 cardiomyocytes were co-cultured in six-well plates or Transwell plates. DAPI was stained for all cell nucleus after cells were fixed by paraformaldehyde.
Besides, condition medium of M2-like macrophages (M2-CM) was generated by collecting the medium of CFSE-stained M2-like macrophages after 24 h of culture. At the same time, medium of M2-like macrophages was filtered through a 0.22 μm syringe filter which could prevent mitochondria from entering the medium to generate mitochondria deleted medium of M2-like macrophages (Md-M2-CM).
Animal treatment protocol
Male C57BL/6 mice (6–8 weeks old) received a single intraperitoneal injection of doxorubicin (DOX) at a dose of 15 mg/kg. One week later, a group of mice was treated with a weekly M2-like macrophages injection (1 × 10^6 cells suspended in 0.2 ml fresh DMEM/per mouse; DOX + M2 group) for two weeks via tail vein. Another group of mice was injected with DMEM accordingly (DOX group). While the third group of mice receiving no DOX was set as Sham group. Each group has 5 mice. No mortality was associated with this dosing regimen. All procedures were approved by the Institutional Animal Care and Use Committee of Nanjing Drum Tower Hospital (2019AE01062) and were in accordance with the Guide for the Care and Use of Laboratory Animals (National Academies Press, 2011).
Transthoracic echocardiography
After 21 days, cardiac function of mice was assessed by transthoracic echocardiography using a Vevo In Vivo Micro-Imaging System (VisualSonics, Canada) under 2.0% isoflurane inhalation. Briefly, the mice were abdominally shaved, anesthetized and placed on a heat pad. The left ventricular internal systolic dimension (LVIDs) and left ventricular internal diastolic dimension (LVIDd) were measured and averaged from three consecutive cardiac cycles. And left ventricular ejection fraction (EF) and fractional shortening (FS) were calculated. EF was calculated with Simpson method [15] and FS was calculated as [(LVIDd-LVIDs)/LVIDs] x100%.
Histological analysis
Hearts from each group were washed in PBS and fixed in 4% formalin, and embedded in paraffin and sliced into 5 μm-thick tissue sections. Heart slices were stained with haematoxylin-eosin staining (HE) and observed under the light microscope (Olympus, Japan). Three HE-stained heart sections from each group were examined for the evaluation of cytoplasmic vacuolization. The cardiomyocytes with vacuoles were counted and normalized to all cardiomyocytes in the section. To observe collagen deposition, the heart sections were stained with Masson trichrome. To observe the cross-sectional area, the sections were dewaxed, rehydrated and subjected to wheat germ agglutinin-FITC for 1 h. Sections were further stained with DAPI. After washed three times, the slides were mounted with a fluorescent microscopy (Keyence, UK). The semi-quantification results were obtained using Image J (NIH, USA).
Western blotting
Heart tissues were homogenized and protein concentrations were measured using a BCA kit (Thermo, USA). Total protein lysates were separated by SDS-PAGE and transferred to PVDF membranes (Millipore, USA). The membranes were incubated with the corresponding primary antibodies overnight at 4 ℃ and then incubated with goat anti-rabbit lgG. The western blot bands were detected using ECL kit (Keygene; China). The membrane was first immunoblotted with the phosphorated protein and then washed, and immunoblotted with the total protein. The protein expression levels were normalized to GAPDH levels. Following antibodies (Abcam, USA) were used: cleaved- caspase 3, AKT, p-AKT, ERK1/2, and p-ERK1/2. The relative expression of protein was averaged using Image J.
Immunofluorescence staining
Heart cryosections from mice injected with CFSE-labelled M2-like macrophages or MitoTracker Red-labelled M2-like macrophages were stained with the DAPI to visualize the nucleus.
To show the percentage of Th2 cells within myocardium, heart sections were incubated overnight with GATA3 (CST, USA). After washing 3 times, fluorescent secondary antibodies were added.
Every section was observed using the fluorescence microscopy (Olympus, Japan) and analyzed using Image J (NIH, USA).
Cardiac apoptosis was detected by the TdT-mediated dUTP nick end labelling (TUNEL) apoptosis assay kit (Beyotime, China) according to the protocol.
Enzyme linked immunosorbent assay (ELISA)
After 21 days, the mouse serum was collected. The level of inflammatory factors were measured with the ELISA Kits (Servicebio, China) according to the manufacture’s protocol. The optical densities of the samples were detected using a microplate reader (Bioteck, USA) at a wavelength of 450 nm.
Isolation of mitochondria from M2-like macrophages and cell culture
Mitochondria were isolated from cultured M2-like macrophages using a Mitochondria Isolation Kit (Beyotime, China) according to the instructions. The freshy isolated mitochondria were used in the following study.
H9c2 cardiomyocytes were cultured at 37℃ with 5% CO2 in DMEM supplementing with 10% FBS and 1% P/S. To mimic cardiac injury in vivo, H9c2 cardiomyocytes were treated with 1 µM of doxorubicin (DOX group; Sigma, USA) for 24 h. Treatment group was added with mitochondria (from 1 × 10^6 cells) plus doxorubicin (DOX + Mito group) for 24 h.
Lactate dehydrogenase (LDH) release assay
After cultured for 24 h, the culture supernatants of H9c2 cardiomyocytes were collected to detect the level of LDH release using a LDH assay kit (Beyotime, China) according to the manufacturer’s instructions. Experiments were performed at least in triplicate. Data were expressed as percentages of control values.
Data are presented as mean ± standard deviation. Analysis was performed using Prism 7 software (GraphPad, USA). Between-group differences were assessed by Student’s t-test or one-way analysis of variance with Bonferroni post-hoc test. The statistical significance level was set at P < 0.05.
Results
M-CSF + IL-4 treatment was effective to produce M2-like macrophages
To produce M2 macrophage, mouse BM-MNCs were treated with M-CSF for 6 days, followed by IL-4 for 1 day. Under a microscope, we observed that M2-like macrophages had a typical spindle shape compared with naïve M0 macrophage with a round appearance (Fig. 1A). In addition, the results of flow cytometry (Fig. 1B) demonstrated that induced macrophages highly expressed macrophage (CCR2) and M2 macrophage (CD206) surface markers (15.8- and 79.4-fold changes respectively). In summary, after 7 days of in vitro culture with 6-day M-CSF treatment followed by 1-day of IL-4 treatment, over 70% of the BM-MNCs were polarized into M2-like macrophages. In addition, qPCR results (Fig. 1C) confirmed that M2-like macrophages had a higher expression of M2-related markers (IL-10, TGF-β和Arg-1) while a lower expression of M1-related markers (IL-1β, IL-6 和TNF-α).
Production of M2-like macrophages by M-CSF + IL-4 treatment. A The cell morphology was shown under the light microscopy. M0 macrophages (without M-CSF plus IL-4 treatment) were used as controls. Scale bar 100 μm. B The surface expression of CCR2 and CD206 was measured using flow cytometry. Below was the fluorescent intensity of CCR2 and CD206, and the quantification results. C The gene expression of M1- and M2-related markers, including IL-1β, TNF-α, iNOS and IL-10, TGF-β, Arg-1, were measured by qPCR. (Data were presented as mean ± SD. ***p < 0.001. N = 3 per group)
Adoptive transfer of M2-like macrophages prevents doxorubicin-induced left ventricular dysfunction
First, we tested the protective effects of M2-like macrophages on heart damage by DOX. A group of mice received a single injection of DOX (15 mg/kg, i.p.). While a group of mice receiving no DOX was regarded as Sham group. After 7 days, the echocardiography results suggested that the heart was already injured (Supplementary Table 1). Then we treated DOX-injured mice with DMEM (DOX group) or M2-like macrophages (1 × 10^6 cells/per mouse; DOX + M2 group) once a week for two weeks.
After 21 days, transthoracic echocardiography (Fig. 2A) in vivo showed impaired systolic function: EF was 55.1 ± 5.2% in DOX group vs. 72.9 ± 6.7% in Sham group (P < 0.001); FS was 27.8 ± 3.2% in DOX group vs. 40.7 ± 5.6% in Sham group (P < 0.001) (Fig. 2B, C). Intriguingly, adoptive transfer of M2-like macrophages resulted in a significant improvement of left ventricular dysfunction compared with DOX group: EF was 63.4 ± 2.6% (P = 0.025); FS was 33.3 ± 1.9% (p = 0.044). Even though, M2-like macrophages could not fully reverse the DOX effect compared with control group (P = 0.012 in EF; P = 0.011 in FS). However, M2 macrophage had no significant effect on increased LVID induced by DOX (Fig. 2D).
Cardiac function between groups were measured using transthoracic echocardiography between groups. A Representative echocardiography images between groups. B Ejection fraction (EF), C Fractional shortening (FS) and D left ventricular internal dimension (LVID) at systole were measure among groups. (Data were presented as mean ± SD. ***P < 0.001 vs. Sham; #P < 0.05 vs. DOX. N = 5 per group, One-way ANOVA)
M2-like macrophages transplantation prevent doxorubicin-induced cardiac remodeling and injury
Next, we explored the effect of M2-like macrophages on some hallmarks of DOX cardiotoxicity. Cytoplasmic vacuolization, an adverse architectural alteration commonly described in DOX cardiotoxicity. HE staining showed a significant increase of cardiomyocyte vacuoles in DOX hearts (Fig. 3A), which was counteracted by M2 transfer. Similar, DOX caused smaller cardiomyocyte size (Fig. 3B) and more interstitial fibrosis (Fig. 3C), which were reversed by M2 treatment. These results suggested that M2 transplantation prevented cardiac remodeling.
Histological staining was used to evaluate cardiac remodeling among groups. A HE staining for quantification of cardiomyocyte vacuoles. B Masson staining for quantification of interstitial fibrosis. C Wheat germ agglutinin staining for measurement of cell size. The arrowheads showed corresponding alterations. Three microscopic fields were analyzed per animal. (Data were presented as mean ± SD. ***P < 0.001 vs. Sham; ###P < 0.001 vs. DOX. N = 5 per group)
Adoptive transfer of M2-like macrophages increases the level of circulating IL-4
To investigate the effect of M2 macrophage infusion on the inflammatory response, we measured the level of inflammatory cytokines in mouse serum after 3 weeks. As shown in Fig. 4, DOX decreased the level of circulating IL-4, which was rescued by M2 macrophage (209.7 ± 16.3 ng/L in DOX + M2 group vs. 163.6 ± 13.0 ng/L in DOX group, P < 0.001). However, in terms of IL-1β, IL-6 and IL-10, there was no statistical difference among groups.
M2-like macrophages transplantation induced Th2 response
It was acknowledged that M2 macrophage could secrete immune cytokines to induce Th2 response. Consistently, the results of flow cytometry showed that M2 macrophage increased the percentage of CD4+IL-4+Th2 cells (Fig. 5 A). In addition, the results of immunofluorescence staining demonstrated that a higher percentage of GATA3+cells, which was a specific transcription factor of Th2 cells and used to label Th2 cells [10, 16], existed in hearts of DOX + M2 group compared with that of DOX group (Fig. 5B).
M2-like macrophages induced the Th2 response within myocardium. A The CD4+IL-4+Th2 cells were measured using flow cytometry and were quantified as the percentage of cardiac cells. B The presence of GATA3+ cells within the hearts were observed by immunofluorescence staining and were quantified as the percentage of cardiac cells. The arrowhead showed the GATA3+ cells. (Data were presented as mean ± SD. *P < 0.05 vs. Sham; #P < 0.05, ##P < 0.01 vs. DOX. N = 5 per group)
M2-like macrophages inhibit cardiomyocyte apoptosis independent of AKT and ERK pathway
Cardiomyocyte apoptosis contributed to adverse remodeling in DOX-induced cardiac injury. As shown in Fig. 6A, the results of TUNEL staining showed that DOX caused cardiomyocyte apoptosis, which was prevented by adoptive transfer of M2-like macrophages. In addition, immunoblotting results (Fig. 6B) demonstrated that the level of c-caspase 3 was decreased in DOX + M2 group compared with DOX group. Meanwhile, we observed that AKT and ERK1/2 were upregulated while p-AKT and p-ERK1/2 were not significantly changed upon M2 macrophage treatment. These results demonstrated that M2 macrophage could inhibit cardiomyocyte apoptosis independent of AKT and ERK pathway.
M2-like macrophages inhibited cardiomyocyte apoptosis. A The TUNEL staining of apoptotic cardiac cells. The arrowhead showed the TUNEL-positive cell. B The immunoblotting results among groups and the gray value of t-AKT, p-AKT, t-ERK 1/2, p-ERK 1/2, and c-caspase 3 from up-left to down-right. (Data were presented as mean ± SD. *P < 0.05, **P < 0.01 vs. Sham; #P < 0.05, ##P < 0.01 vs. DOX. N = 5 per group)
M2-like macrophages could transfer mitochondria to the cardiomyocytes
To investigated the cell engraftment within myocardium, we labelled M2-like macrophages with CFSE and treated the mice via tail injection. After 24 h, we sacrificed the mice and sliced the hearts. The green fluorescence showed that very few M2-like macrophages were resident within the myocardium (Fig. 7A). Furthermore, mice were injected with MitoTracker red pre-stained M2-like macrophages to show the distribution of M2-like macrophages derived mitochondrial. The red positive mitochondria were localized inside the myocardium, which meant that M2-like macrophages could transfer mitochondrial to the cardiomyocyte in vivo.
M2-like macrophages could transfer mitochondria to cardiomyocytes in vivo and in vitro. A The green fluorescence showed the presence of M2-like macrophages within the myocardium while the red positive mitochondria were localized inside the myocardium. The quantitative results were shown as the histogram at the left-bottom. B Red fluorescence dots (mitochondria) were incorporated into the body of CSFE-labelled cardiomyocytes. (Data were presented as mean ± SD. ***P < 0.001. N = 3 per group)
What’s more, we confirmed the transfer of mitochondrial in direct co-culture conditions in vitro. Pre-stained H9c2 cardiomyocyte (CFSE) and M2-like macrophages (MitoTracker Red) were co-cultured in six-well plate for 24 h followed by fluorescence microscopy. Figure 7B showed that MitoTracker Red labelled mitochondria were transferred from M2-like macrophages to H9c2 cardiomyocytes as evidenced by red fluorescence dots incorporated into the body of CSFE-labelled cardiomyocytes.
Transfer of Mitochondria from M2 macrophage to cardiomyocyte in an indirect co-culture condition
Meanwhile, we considered whether paracrine mechanism may also play a role in mitochondria transfer. Therefore, firstly, the pre-stained M2-like macrophages were co-cultured with H9c2 cardiomyocytes in a transwell plate (8 μm pore) for 24 h (Fig. 8 Up). Fluorescent microscopy observation showed M2-like macrophages derived mitochondrial were located inside the body of cardiomyocytes. Besides, there were more mitochondria incorporated into DOX-injured cardiomyocytes. Secondly, we cultured cardiomyocytes in the condition medium of M2-like macrophages (M2-CM) (Fig. 8 Middle). Fluorescent microscopy observation showed MitoTracker red labelled mitochondrial were internalized into the body of cardiomyocytes. And DOX-injured cardiomyocytes could take up more percentage of mitochondrial. Thirdly, we culture cardiomyocytes in mitochondria-depleted condition medium of M2-like macrophages (Md-M2-CM) (Fig. 8 Down). Fluorescent microscopy observation showed no MitoTracker red labelled mitochondria were in the body of cardiomyocytes, neither in PBS-treated cardiomyocytes or DOX-treated cardiomyocytes.
Transfer of Mitochondria from M2 macrophage to cardiomyocyte in a transwell plate (8 μm pore). A Up: M2 macrophage derived mitochondrial were located inside the body of cardiomyocytes. B Middle: M2 condition medium derived mitochondrial were internalized into the body of cardiomyocytes. C Down: No mitochondria were taken by cardiomyocytes using mitochondria-depleted condition medium of M2-like macrophages
Mitochondria internalization alleviated the cell stress induced by DOX
To explore the beneficial effects of M2-like macrophages, we isolated the intact mitochondria from M2-like macrophages and cultured them with injured cardiomyocytes. Our results showed that mitochondria (from 1 × 10^6 M2-like macrophages/ well) treatment decreased the LDH release level, which was increased upon DOX treatment (Fig. 9A). Besides, flow cytometry results showed that DOX lead to more percentage of Annexin V+PI− and Annexin V+PI+ cells, which was prevented by mitochondria treatment (Fig. 9B). These results showed that mitochondria treatment could inhibit cell apoptosis induced by DOX.
Mitochondria internalization alleviated the cell stress induced by DOX. A Mitochondria treatment decreased the LDH release level. B Mitochondria treatment decreased the percentage of Annexin V + PI- and Annexin V + PI + cells. (Data were presented as mean ± SD. *P < 0.05, ***P < 0.001 vs. Sham; #P < 0.05 vs. DOX. N = 3 per group)
Discussion
In this study, we showed that adoptive transfer of M2-like macrophages had beneficial effects on non-ischemic heart failure, and the beneficial effects could be related to the mitochondria transfer. Specially, M2-like macrophages transplantation upregulated circulating IL-4 levels and induced cardiac Th2 immune response, as well as prevented from cardiomyocytes apoptosis. In vitro, M2-like macrophages could transfer mitochondria to cardiomyocytes in a direct and indirect co-culture method [9].
Previous studies have showed a therapeutic effect of macrophage transplantation, whether originating from embryonic stem cells, induced pluripotent stem cells, or bone marrow mononuclear cells, on various organ injury, including liver [17], lung [11] and heart [18]. Using a combination of M-CSF and IL-4 treatment, we efficiently induced reparative M2-like macrophages in vitro. In ischemic heart failure, M2 macrophages transplantation enhanced cardiac repair, achieving a superior therapeutic efficacy compared to bone marrow mononuclear cells [12]. In consistent with previous studies, our results also showed that M2-like macrophages transplantation alleviated cardiac fibrosis and systolic dysfunction. Previous study found that Th1 polarization existed in heart failure, while atorvastatin exhibited beneficial effects by regulating Th1/Th2 response [19]. Immune modulation therapy improved cardiac function of patients with chronic cardiac insufficiency accompanied by increased expression of Th2 and GATA-3 mRNA [20]. AKT and ERK signaling pathways were involved in cell survival and apoptosis upon stress [21]. Previous studies have found that upregulation of AKT [22] and ERK [23] promoted the survival of cardiac myocytes. In consistent with that, we also found that M2-like macrophages induced Th2 cell activation and inhibited cardiomyocyte apoptosis, contributing to a cardioprotective role.
Regarding the possible underlying mechanism for improved cardiac function after M2-like macrophages transplantation, several paracrine factors released from transplanted cells have been suggested. M2 macrophages were reported to secrete enzymes [24, 25] cytokines [9, 12], peptides [26], exosomes [27] which could mediate its beneficial effects. What’s more, local M2-like macrophages participated in some signal pathways, including NF-kB [28], and RIG-I [29] and regulated Treg and Th17 cell responses [30]. Similarly, our results found M2-like macrophages transplantation induced Th2 response as evidenced by more filtration of IL4+ or GATA3+CD4+ T cells. Surprisingly, we found that mitochondria transfer was a novel mechanism for the beneficial effect of M2-like macrophages.
Cardiomyocytes harbor a network of mitochondria for a large energy demand. The structural and function integrity is a perquisite for mitochondria to supporting cardiac function. However, doxorubicin-induced cardiac injury was characterized by perturbed mitochondrial function, including mitochondria autophagy [31], mitochondria fission/fusion [32] and oxidative phosphorylation [33]. Mitochondrial could mediate the cell communication [34], thereby replacing the compromised mitochondria in host cells [35], and maintaining necessary function [36]. For example, mesenchymal stem cells could promote the oxidative phosphorylation of macrophages through extracellular vesicle-mediated mitochondria transfer [37]. Besides, they can transfer mitochondria to lymphoblastic leukemia cells [38] or hypoxic neurons [39] to prevent from oxidative stress. A study found that cardiac macrophages could phagocytize the mitochondria released from injury cardiomyocytes, contributing to cardiac repair [40]. In our study, we further found that mitochondria could transfer from M2-like macrophages to compromised cardiomyocytes and promote cell survival under stress.
Limitation existed in our study. Firstly, previous studies reported that extracellular mitochondrial could be packed in exosomes [41, 42], or as whole organelles [35, 43]. We speculated that they may be transferred via exosomes. The mechanism of mitochondrial transfer should be investigated in the future. Secondly, there are more than 70% of M2-macrophages and some M0 or M1 macrophages in the population. It would be better to isolate and purify M2 macrophages as a treatment method in the future.
Conclusions
In summary, we found that adoptive transfer M2-like macrophages could protect against the doxorubicin-induced cardiotoxicity, which may be partly attributed to mitochondria transfer.
Availability of data and materials
All data supporting our conclusions could be available from the corresponding author.
References
Narayan HK, Finkelman B, French B, et al. Detailed Echocardiographic Phenotyping in Breast Cancer Patients: Associations With Ejection Fraction Decline, Recovery, and Heart Failure Symptoms Over 3 Years of Follow-Up. Circulation. 2017;135:1397–412.
Li M, Sala V, De Santis MC, et al. Phosphoinositide 3-Kinase Gamma Inhibition Protects From Anthracycline Cardiotoxicity and Reduces Tumor Growth. Circulation. 2018;138:696–711.
Baxter-Holland M, Dass CR. Doxorubicin, mesenchymal stem cell toxicity and antitumour activity: implications for clinical use. J Pharm Pharmacol. 2018;70:320–7.
Riad A, Bien S, Gratz M, et al. Toll-like receptor-4 deficiency attenuates doxorubicin-induced cardiomyopathy in mice. Eur J Heart Fail. 2008;10:233–43.
Tadokoro T, Ikeda M, Ide T, et al. Mitochondria-dependent ferroptosis plays a pivotal role in doxorubicin cardiotoxicity. JCI Insight. 2020;5:e132747.
Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95.
Johnson TA, Singla DK. PTEN inhibitor VO-OHpic attenuates inflammatory M1 macrophages and cardiac remodeling in doxorubicin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2018;315:H1236–49.
Hulsmans M, Sam F, Nahrendorf M. Monocyte and macrophage contributions to cardiac remodeling. J Mol Cell Cardiol. 2016;93:149–55.
Parsa R, Andresen P, Gillett A, et al. Adoptive transfer of immunomodulatory M2 macrophages prevents type 1 diabetes in NOD mice. Diabetes. 2012;61:2881–92.
Ma SF, Chen YJ, Zhang JX, et al. Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain Behav Immun. 2015;45:157–70.
Happle C, Lachmann N, Ackermann M, et al. Pulmonary Transplantation of Human Induced Pluripotent Stem Cell-derived Macrophages Ameliorates Pulmonary Alveolar Proteinosis. Am J Respir Crit Care Med. 2018;198:350–60.
Podaru MN, Fields L, Kainuma S, et al. Reparative macrophage transplantation for myocardial repair: a refinement of bone marrow mononuclear cell-based therapy. Basic Res Cardiol. 2019;114:34.
Singla DK, Johnson TA, Tavakoli Dargani Z. Exosome Treatment Enhances Anti-Inflammatory M2 Macrophages and Reduces Inflammation-Induced Pyroptosis in Doxorubicin-Induced Cardiomyopathy. Cells. 2019;8:1224.
Peet C, Ivetic A, Bromage DI, Shah AM. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc Res. 2020;116:1101–12.
Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39 e14.
Amsen D, Antov A, Jankovic D, et al. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity. 2007;27:89–99.
Haideri SS, McKinnon AC, Taylor AH, et al. Injection of embryonic stem cell derived macrophages ameliorates fibrosis in a murine model of liver injury. NPJ Regen Med. 2017;2:14.
Ishida M, Tomita S, Nakatani T, et al. Bone marrow mononuclear cell transplantation had beneficial effects on doxorubicin-induced cardiomyopathy. J Heart Lung Transplant. 2004;23:436–45.
Cheng X, Ding Y, Xia C, et al. Atorvastatin modulates Th1/Th2 response in patients with chronic heart failure. J Card Fail. 2009;15:158–62.
Han LN, Guo SL, Li TL, Ding GL, Zhang YJ, Ma JL. Effect of immune modulation therapy on cardiac function and T-bet/GATA-3 gene expression in aging male patients with chronic cardiac insufficiency. Immunotherapy. 2013;5:143–53.
Leleu X, Jia X, Runnels J, et al. The Akt pathway regulates survival and homing in Waldenstrom macroglobulinemia. Blood. 2007;110:4417–26.
Soppert J, Kraemer S, Beckers C, et al. Soluble CD74 Reroutes MIF/CXCR4/AKT-Mediated Survival of Cardiac Myofibroblasts to Necroptosis. J Am Heart Assoc. 2018;7:e009384.
Dahl EF, Wu SC, Healy CL, Perry J, O’Connell TD. ERK mediated survival signaling is dependent on the Gq-G-protein coupled receptor type and subcellular localization in adult cardiac myocytes. J Mol Cell Cardiol. 2019;127:67–73.
Wang D, Xiong M, Chen C, et al. Legumain, an asparaginyl endopeptidase, mediates the effect of M2 macrophages on attenuating renal interstitial fibrosis in obstructive nephropathy. Kidney Int. 2018;94:91–101.
Quenum Zangbede FO, Chauhan A, Sharma J, Mishra BB. Galectin-3 in M2 Macrophages Plays a Protective Role in Resolution of Neuropathology in Brain Parasitic Infection by Regulating Neutrophil Turnover. J Neurosci. 2018;38:6737–50.
Pannell M, Labuz D, Celik MO, et al. Adoptive transfer of M2 macrophages reduces neuropathic pain via opioid peptides. J Neuroinflammation. 2016;13:262.
Dai Y, Wang S, Chang S, et al. M2 macrophage-derived exosomes carry microRNA-148a to alleviate myocardial ischemia/reperfusion injury via inhibiting TXNIP and the TLR4/NF-kappaB/NLRP3 inflammasome signaling pathway. J Mol Cell Cardiol. 2020;142:65–79.
Chu F, Shi M, Lang Y, et al. Adoptive transfer of immunomodulatory M2 macrophages suppresses experimental autoimmune encephalomyelitis in C57BL/6 mice via blockading NF-kappaB pathway. Clin Exp Immunol. 2021;204:199–211.
Chen J, Wu Y, Duan FX, et al. Effect of M2 macrophage adoptive transfer on transcriptome profile of injured spinal cords in rats. Exp Biol Med (Maywood). 2019;244:880–92.
Haribhai D, Ziegelbauer J, Jia S, et al. Alternatively Activated Macrophages Boost Induced Regulatory T and Th17 Cell Responses during Immunotherapy for Colitis. J Immunol. 2016;196:3305–17.
Pandey S, Kuo WW, Shen CY, et al. Insulin-like growth factor II receptor-alpha is a novel stress-inducible contributor to cardiac damage underpinning doxorubicin-induced oxidative stress and perturbed mitochondrial autophagy. Am J Physiol Cell Physiol. 2019;317:C235–43.
Du J, Hang P, Pan Y, et al. Inhibition of miR-23a attenuates doxorubicin-induced mitochondria-dependent cardiomyocyte apoptosis by targeting the PGC-1alpha/Drp1 pathway. Toxicol Appl Pharmacol. 2019;369:73–81.
Zhao J, Du J, Pan Y, et al. Activation of cardiac TrkB receptor by its small molecular agonist 7,8-dihydroxyflavone inhibits doxorubicin-induced cardiotoxicity via enhancing mitochondrial oxidative phosphorylation. Free Radic Biol Med. 2019;130:557–67.
Hayakawa K, Esposito E, Wang X, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535:551–5.
Yang Y, Ye G, Zhang YL, et al. Transfer of mitochondria from mesenchymal stem cells derived from induced pluripotent stem cells attenuates hypoxia-ischemia-induced mitochondrial dysfunction in PC12 cells. Neural Regen Res. 2020;15:464–72.
Wu TH, Sagullo E, Case D, et al. Mitochondrial Transfer by Photothermal Nanoblade Restores Metabolite Profile in Mammalian Cells. Cell Metab. 2016;23:921–9.
Morrison TJ, Jackson MV, Cunningham EK, et al. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am J Respir Crit Care Med. 2017;196:1275–86.
Burt R, Dey A, Aref S, et al. Activated stromal cells transfer mitochondria to rescue acute lymphoblastic leukemia cells from oxidative stress. Blood. 2019;134:1415–29.
Li H, Wang C, He T, et al. Mitochondrial Transfer from Bone Marrow Mesenchymal Stem Cells to Motor Neurons in Spinal Cord Injury Rats via Gap Junction. Theranostics. 2019;9:2017–35.
Nicolas-Avila JA, Lechuga-Vieco AV, Esteban-Martinez L, et al. A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell 2020;183:94–109 e23.
Hough KP, Trevor JL, Strenkowski JG, et al. Exosomal transfer of mitochondria from airway myeloid-derived regulatory cells to T cells. Redox Biol. 2018;18:54–64.
D’Souza A, Burch A, Dave KM, et al. Microvesicles transfer mitochondria and increase mitochondrial function in brain endothelial cells. J Control Release. 2021;338:505–26.
Boukelmoune N, Chiu GS, Kavelaars A, Heijnen CJ. Mitochondrial transfer from mesenchymal stem cells to neural stem cells protects against the neurotoxic effects of cisplatin. Acta Neuropathol Commun. 2018;6:139.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
X B and K LN designed the study; L YH and Z CX performed the experiments; W MY wrote the manuscript. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures were approved by the Institutional Animal Care and Use Committee of Nanjing Drum Tower Hospital and were in accordance with the Guide for the Care and Use of Laboratory Animals (National Academies Press, 2011).
Consent for publication
Yes.
Competing interests
There is no competing interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, Y., Wu, M., Zhong, C. et al. M2-like macrophages transplantation protects against the doxorubicin-induced heart failure via mitochondrial transfer. Biomater Res 26, 14 (2022). https://doi.org/10.1186/s40824-022-00260-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40824-022-00260-y