Abstract
Background
Obstructive sleep apnea (OSA) is one of the foremost potential severe disorders, with frequent episodes of complete or partial obstructions of the upper airway during sleep. Therefore, several attempts to find an effective pharmacotherapy by repurposing several drugs such as serotonin reuptake inhibitors (SRIs) and norepinephrine and dopamine reuptake inhibitors (NDRIs) were recently considered as alternative therapeutic strategy. So, in this review, we will present non-conventional pharmacological approaches for managing OSA via either repurposing selected natural products or traditional medicine.
Evidence acquisition
Scientific databases and literature reviewed in the last twenty years were screened using different keywords related to OSA; exclusion criteria were applied based on the accessibility and the ability of the sources to follow evidence-based approaches. The eligible resources were classified into two main categories: clinical-based studies and preclinical studies. The findings of these studies were analyzed and discussed in light of current evidence derived from recent studies.
Findings
Several natural components and traditional formulas were found to regulate several molecular targets involved in OSA pathogenesis, supported by several in-vitro and in-vivo studies. Also, natural products subjected to clinical trials give promising results. Still, there are some limitations, such as involving a small number of patients depending on subjective yet acceptable scores rather than objective scores, a lack of positive control groups, or a small number of patients.
Conclusion
Therapeutic protocols should consider non-conventional polypharmacological strategies targeting all OSA aspects. Hence, there is an opportunity to reposition some well-defined natural products, such as cinnamic acid derivatives, isoflavones, and lignans, as several evidences from in-vitro, in-vivo, and clinical trials support their potential efficacy in the management of OSA.
Similar content being viewed by others
Introduction
Obstructive sleep apnea (OSA) is a serious sleep-associated respiratory disorder that has a greater prevalence in men than women. It is characterized by complete or partial collapse of breathing during sleep, leading to reduced upper airflow. The symptoms include fragmented sleep and excessive daytime sleepiness. It has a serious impact on the patient’s health, including cognitive, intellectual, and neurological problems such as snoring, anxiety, headache, insomnia, irritability, also associated with cardiovascular diseases.
Three main factors could summarize the pathogenesis of this condition. The first one is anatomical abnormalities caused by compromised craniofacial structure, muscle injury, decreased lung volume, airway edema, and adipose soft tissue deposition, but their contribution differs among OSA patients.
The second factor includes disturbance in neuromuscular function due to an imbalance in neurotransmitters and neuromodulators affecting upper airway motoneurons such as GABA, serotonin, dopamine, norepinephrine, adenosine, and acetylcholine. Hence, several approved drugs were repurposed for treatment of OSA, such as the selective serotonin reuptake inhibitor (SSRI) Fluoxetine and Paroxetine, which were used as antidepressant [1, 2]. Also, Solriamfetol, a norepinephrine–dopamine inhibitor, was approved in 2019 for the treatment of narcolepsy and excessive daytime sleep and excessive daytime sleep [3, 4]. In the same context, Donepezil, an acetylcholinesterase inhibitor [5], and Dronabinol, which is a cannabinoid receptor (CB-1) agonist [5], were suggested as potential therapeutic to manage OSA.
The third factor involves the molecular response to inflammation and oxidative stress that might originate from other co-existing diseases, such as hypertension, and metabolic disorders, such as diabetes and obesity. This could lead to the elevation of levels of several inflammatory cytokines such as TNF-α, IL-1β, IL-6, activation of nuclear factor κB (NF-κB), and eventually hypoxia induction factor-1 (HIF-1α). The sustained upregulation of these pathways contributed to the development of several diseases [6, 7].
It is worth noting that OSA management focuses on using devices such as continuous positive airway pressure (CPAP) that prevent airway collapse. However, the low adherence of many OSA patients to CPAP has been reported extensively. Also, CPAP fails to achieve significant improvement in excessive daytime sleepiness in some cases. So, modified devices were developed to overcome these issues, such as Auto-titrating continuous positive airway pressure(ACPAP) [8, 9], Palatal implant system [10], Nasal valve dilators [11], Nasal expiratory resistor [12, 13], Oral pressure therapy [14, 15].
On the other hand, a meta-analysis of clinical trials investigating repurposed drugs such as antidepressants revealed that most of these drugs showed little to no significant clinical effect. Moreover, they demonstrated considerable side effects that accompany psychotropic drugs, with exceptions for a few examples, such as mirtazapine and trazadone [16].
Furthermore, there is a better understanding of the molecular basis of OSA, which considers other aspects of this condition rather than the breathing disorder events due to airway collapse during sleeping. Also, the comorbidity of other conditions, such as hypertension, metabolic diseases, and neuropsychological disorders in OSA patients, raised questions about the need for more effective pharmacological interventions that improve the overall therapeutic outcomes and enhance the quality of life of the patients [17].
Since nature has provided humankind with remedies for millennia, it may be worth reevaluating the pharmacological options available for natural compounds [18]. Recent studies showed the ability of several natural compounds and extracts to regulate neurological functions [19], as well as their prominent effect on reducing oxidative stress and inflammation [20].
Moreover, natural compounds are known for their ability to modulate the activity of several targets simultaneously, an advantage that could be important in a complex condition such as OSA [21]. Although several in-vitro and in-vivo studies reported the prominent effect of several natural products on neurotransmitter regulation, scarce clinical trials are investigating such effects to manage OSA, so this review will shed light on recent evidence-based studies addressing the application of plant-derived therapies for OSA. It will emphasize their benefits over conventional approved therapeutics.
Evidence acquisition
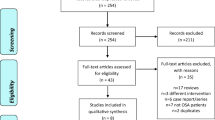
Selected studies were articles published in peer-reviewed journals between January 2000 and December 2023 and identified through searches of PubMed, Web of Science, Scopus, and SciFinder electronic databases. OSA, herbal medicine, traditional medicine, traditional medicine, Pharmacotherapy, SSRI, and NDRI were used as keywords combined with Traditional medicine, herbal medicine, or natural products, which yielded 120 results. After excluding non-English language publications, the number was reduced to thirty-eight, which was reviewed by coauthors to identify medicinal plants or natural products that were subjected to clinical trials or might be used for further clinical investigations and categorized based on three key elements, the ability to modulate neural transmitters, ventilatory stimulant activity, antioxidant, and anti-inflammatory properties. Figure 1 presents the selection criteria for the research articles to be reviewed.
Presentation of research articles (January 2000–December 2023) published in peer-reviewed journals and identified through searches of the PubMed, Web of Science, and Scopus and SciFinder electronic databases (OSA, Pharmacotherapy, SSRI, and NDRI were used as keywords combined with Traditional and herbal medicine and natural products
Literature analysis and findings
Many plants have been screened in different experimental models and a series of bioassays for their ability to modulate catecholamine contents. However, limited clinical data are available due to the lack of accurate identification of the main active ingredient responsible for such activity. However, these bioassays tend to measure phenotypic outcomes rather than elucidate the molecular targets involved in the pathogenesis of the disease, which limits their advance to clinical trials [22]. Still, few reports address the direct relationship between natural products and OSA. Hence, we will address evidence from the literature to speculate the potential mechanisms of action and molecular targets responsible for the observed experimental or clinical effect. This would be achieved by applying a reverse pharmacognosy approach where evidence supporting the efficacy of compounds or plants against molecular targets involved in other diseases yet have a prominent role in OSA could be exploited. For example, compounds targeting catecholamine release, reuptake, and metabolism could be repurposed for the management of OSA as previously mentioned natural products in the management of OSA via targeting catecholamines release, reuptake, and metabolism, which play a prominent role in sleeping apnea. Due to the scarcity of studies of animal models for OSA, few reports have addressed the direct relationship between natural products and OSA. However, several in-vitro, in-vivo, and clinical trials proved their potential use for the treatment of other related diseases such as snoring, insomnia, and depression.
Potential natural products as monotherapy for management of OSA
Cinnamon
The standardized extract of Cinnamomum cassia was found to possess a similar effect to fluoxetine in 5-hydroxytryptophan (5-HTP)-induced head twitches in mice [24], which was attributed to the presence of cinnamaldehyde (1). Interestingly, Cinnamon is a component of traditional Chinese medicine (TCM) formula that was vastly used and proven to alleviate snoring syndromes by affecting the phrenic nerve (PNA), recurrent laryngeal nerve (RLNA), and hypoglossal nerve (HNA) activity in the upper airway in animal model.
Gastrodia
Gastrodia elata (G.elata), a well-known plant that is used traditionally for its anxiolytic, antidepressant, and antiepileptic properties [23]. This could be explained by its ability to increase the concentration of both 5-hydroxytryptamine (5-HT) and dopamine (DA) while decreasing their turnover in the brain of experimental animals. [24]. Several studies showed a reproducible result that linked the CNS activity of G.elata to the presence of a phenolic glycoside known as 4-hydroxy benzyl alcohol-4-O-β-D-glucopyranoside (Gastrodin) (2) and p-hydroxy benzyl alcohol (3). Besides its ability to modulate several neurotransmitters, it was found to downregulate several proinflammatory markers and decrease oxidative stress. Also, its anti-inflammatory and antioxidant-modulating effect has been studied rigorously [25].
Ephedrine
The activity of the genioglossus (GG) muscle is vital to maintain the patency of the airway during sleeping. So, inhibition of α1-adrenergic receptors in the hypoglossal motor nucleus (HMN) will subsequently lead to airway collapse. Therefore, it was suggested that increasing adrenergic activity might be a useful approach [26]. Pseudoephedrine (4) is an α-adrenergic agonist, alone or in combination with domperidone, was found to decrease apneic episodes and improve oxygen desaturation index (ODI). However, prolonged use of pseudoephedrine needs to be closely studied before considering it as a treatment for OSA [27]. Also, Yohimbine, an α2-adrenergic antagonist that increased the release of norepinephrine (NE), was found to reverse the depressant effect of REM sleep on hypoglossal motoneurons by activating A7 and A5 neurons, which consequently alleviated OSA-induced in rats [28].
St. John’s wort
Hypericum perforatum is another example of a plant widely used for treating depression by acting as a non-selective reuptake inhibitor of a wide range of neurotransmitters, such as glutamate, DA, 5-HT, and NE. Such activity was proved to be due to Hyperforin (5), a prenylated carbocyclic acyl phloroglucinol derivative. Hyperforin inhibited neurotransmitter reuptake by inhibiting catecholamine transporters such as serotonin transporter (SERT) non-competitively. H. perforatum extract also showed weak to moderate monoamine oxidase inhibition, leading to a decrease in the metabolism of those neurotransmitters [29]. Figure 2 illustrates the molecular mechanism of Hyperforin as SSRI.
Kava Kava
Piper methysticum, also known as Kava-Kava, is a plant that several patients commonly use to reduce anxiety and insomnia. Evidence from in-vitro, in-vivo, and clinical studies supports this pharmacological activity, where the α-pyrones (6) content in the plant was found to affect several neurotransmitters by inhibiting Monoamine oxidase (MAO) and reuptake of NE and DA [30,31,32]. The clinical anxiolytic activity of kava is well studied where it was found to achieve significant clinical effects in comparison to placebo groups [33].
Moreover, Kava Kava was found to possess a benzodiazepine - action via blocking voltage-gated sodium and improving ligand binding to gamma-aminobutyric acid-A receptors [34]. However, such activity is not reversible by benzodiazepine antagonist flumazenil [35]. Since OSA patients could benefit from the administration of non-benzodiazepine hypnotic [37], the administration of standardized Kava Kava might help alleviate OSA symptoms in the patients.
Cannabis
Cannabis is a prevalent psychotropic plant utilized for recreational purposes. It has regained the interest of numerous healthcare institutes for use in various illnesses. In this context, cannabinoids can indirectly inhibit 5-HT3 receptor activity through Cannabinoid receptors (CB) receptor-dependent signaling pathways, allowing them to regulate neurotransmitter release in the nervous system. The two receptor systems are interconnected through intracellular signaling mechanisms, so activation of (CB) can inhibit serotonin-induced apnea, suggesting cannabinoids may have a function in treating OSA [36, 37].
This is supported by Preclinical and clinical studies, which demonstrated that dronabinol (7), a synthetic form of tetrahydrocannabinol (THC), decreased REM sleep and reduced apnea in experimental animals. The first activity was not affected by treatment with cannabinoid antagonists, while the second was significantly blocked [38]. Also, Dronabinol reduced OSA symptoms and decreased apnea significantly over placebo among adults. Showed good safety and tolerability profile [39]. Regardless, the use of Cannabinoids in OSA is debatable due to the lack of long-term studies and concerns about abuse [40].
Methylxanthines
Methylxanthine derivatives such as theophylline (8)and aminophylline (9)were studied to manage OSA. While the first one showed a small but significant enhancement of AHI over placebo, aminophylline did not influence the obstructive event. Still, it improved central events. Generally, both were reported to reduce sleeping efficiency and total sleep times, which might limit their use [41, 42].
Pawhuskin A
Opioid antagonists were also investigated since they acted as a respiratory stimulant and proved to decrease hypopnea and increase oxygenation in OSA. Unfortunately, several limitations face the application of synthetic drugs such as Doxapram as it has to be administrated intravenously and is contraindicated in several diseases and conditions [43]. Recently, pawhuskin A (10), a natural nonnitrogenous opioid antagonist, was isolated from Dalea purpurea, which is considered a new class of opioid antagonists. Still, there are no reports addressing its use in OSA [44].
Nicotine
While smoking is known to exacerbate OSA, nicotine (11) was suggested to be used as a respiratory stimulant due to its complex nicotinic and muscarinic pharmacological effects. When administrated using the transdermal delivery system, it showed no improvement in AHI. Moreover, it led to a decrease in total sleep time and sleep efficiency [45].
Lobeline
Lobelia, a plant commonly used in smoking cessation, was used in a preparation patented by Steven R. Frank for treating sleep apnea due to its content of lobeline (12) alkaloids. The author of the patent used other adjuvant plants to reduce adverse effects associated with nicotinic drugs, such as nausea and cramping [46]. Figure 3 shows the chemical structure of compounds (1–12), and Fig. 4 summarizes different mechanisms of natural products for the management of OSA.
Natural compounds may exert their therapeutic effect for the management of OSA by different mechanisms: A) Xanthines and B) Lobeline can act as a respiratory stimulant by affecting respiration centrally or through regulation of nicotinic and cholinergic receptors, C)Flavonoids and phenolics possess anti-inflammatory and antioxidant effect attenuating oxidative stress and restore body normal state, D) Dihydromethysticin and E)Hyperforin 2 representative compounds which is known to regulate catecholamines such as 5-hydroxytryptamine, Norepinephrine and dopamine which would eventually enhance the neuromuscular function
Utilization of natural products as polytherapy for the management of OSA
As previously demonstrated, pharmacological choices for managing OSA are limited, and their efficacy is obviously less than CPAP, which is not an unexpected situation since treating complex conditions with multiple aspects and mechanisms should comply with a polypharmacological approach instead of monotherapy. Yet, clinical trials investigating combining two or more therapeutic agents are scarce.
In this context, several traditional medicine polyherbal formulas were investigated for treating OSA. For example, Wu et al. compounded a formula from traditional Chinese medicine (TCM) based on The San-Zhong-Kui-Jian-Tang (SZ) and Nasal unblocking herbs (NUH)and studied its effect on excessive daytime sleeping and snoring, which was reduced significantly after four weeks of administration. However, the study lacks the ability to use positive control to compare the results to the known therapeutic interventions. Moreover, it depends on subjective yet acceptable scores, so other objective measures, such as AHI and ODI, should be investigated in future studies [47].
Since the authors did not give any clues about the mechanism of action of each formula component, we tried to find available studies that might explain the observed clinical effect. Radix Angelica sinensis a component of the formula was found to exert antidepressant effect in animal model through upregulation of brain derived neurotrophic factor (BDNF) [48] which is found to be important role in protection against cognitive impairment associated with OSA [49]. Moreover, the HPLC profile of A. sinensis showed the presence of ferulic acid (13) and Butylphthalide (14), which are known for their effect on serotonergic and dopaminergic systems [50] and the ability to protect against neuronal damage [51, 52].
Paeonia lactiflora, another component of (the SZ) formula, was reported extensively to possess antidepressant activity [52]; further studies revealed that the glycoside fraction isolated from this plant might be responsible for such effect by inhibiting MAO and increasing the levels of neurotransmitters. Interestingly, glycoside rich fraction containing albiflorin (15) and paeoniflorin (16) were also found to increase BDNF levels in the chronic unpredictable mild stress (CUMS) animal model, implying a possible synergistic effect with A. sinensis, also the plant was reported to possess antioxidant activity [53].
Scutellaria baicalensis is the 3rd component of the formula, which is rich in flavonoid and phenolic compounds that are known to modulate oxidative stress and protect neurons against injury induced by hypoxia Among these flavonoids, Baicalin (17) and its glycoside was reported to increase the levels of several neurotransmitters by inhibiting of MAO, exert anxiolytic activity through GABAergic ligand site rather than benzodiazepines’ site and increase BDNF levels in streptozotocin-induced depression animal model. [54, 55].
Coptis chinensis, the fourth component of the formula, is rich in several amines such as tryptophan, 5-hydroxy tryptamine, and 5-methoxytryptamine, which is a precursor for serotonin, ferulic acid, and berberine (18). Berberine is known for its effect on metabolism and has been investigated thoroughly for managing obesity and hyperglycemia [55], which could be beneficial for OSA patients. Furthermore, Berberine exerts a prominent antidepressant effect by increasing BDNF levels in the hippocampus of animal models of depression. Also, it increased the level of neurotransmitters such as NE, 5-HT, and DA [56]. Phellodendron amurense, the 5th component of the formula, is rich in alkaloids such as berberine and its analogs, so it might be safe to conclude that its pharmacological activity will be like C. chinensis [57, 58].
Anemarrhena asphodeloides is the sixth component of the formula. It is rich in hexacylic steroidal sapogenin, such as Sarsasapogenin (19). This compound was reported to increase neurotransmitter levels such as 5-HT and NE, and it also inhibited MAO A&B [59]. Mangiferin, another primary compound in A. asphodeloides, was also reported to ameliorate neurodegeneration induced by sleep deprivation by reducing inflammatory markers such as TNF-α, IL-1β, and IL-6. Remarkably, it also reverses the decrease in BDNF in plasma and hippocampal in the neurodegeneration model in mice [60]. The plant also contains the phytoestrogen lignans Nyasol(20) and its analog [61]. Since estrogen was found to enhance the function of the genioglossus muscle [62]. So, phytoestrogen may play a role in protecting the genioglossus muscle against fatigue associated with chronic intermittent [63].
Bupleurum scorzonerifolium, the seventh component known as Radix Bupleuri, has also been investigated for its antidepressant activity in CUMS model in rats, where the extract was able to increase neurotransmitters such as DA and 5-HT. Recent study showed that radix Bupleuri might exert its anti-depressant effect through modulation of three metabolic pathways such as taurine and hypotaurine metabolism, primary bile acid biosynthesis, and glyoxylate and dicarboxylate metabolism. It’s worth noting that the phytoestrogen content of this plant, such as Genistein and Daidzein (21) [64], was reported to enhance genioglossus muscle as previously discussed [62].
Radix Gentianae, the 8th component of the formula, contains Gentiopicroside (22), which is a potent anti-inflammatory compound and was proven to be effective as an antidepressant in LPS-induced depressive-like behavior by preventing tryptophan-degradation, which in turn will normalize the activation of N-methyl-D-aspartate (NMDA) receptor a target that was linked to sleep disorder occurrence [65, 66], this was in agree with a previous study showing that Gentiopicroside ameliorated reserpine induced pain/depression dyad by downregulating of GluN2B containing NMDA [67].
Radix Platycodi, the 9th component of the formula, is rich in saponin glycoside and reported extensively for its anti-hyperlipidemic effect through decreasing the expression of acetyl-CoA carboxylase, fatty acid synthase, and lipoprotein lipase and enhanced lipolysis by activating, hormone-sensitive lipase, uncoupling proteins and carnitine palmitoyl transferase 1α in liver and white adipose tissue. Furthermore, platycodon extracts downregulated the expression of important adipogenic transcriptional factors [68]. This was in agreement with a previous study indicating that platycodon extract and its major compound Platycodin D (23), could inhibit adipogenesis in the obesity model by downregulating peroxisome proliferator-activated receptor (PPARγ), CCAAT-enhancer binding protein alpha (C/EBPα), lipin-1, and adiponectin, in the other hand they activated AMPK and Sirutin-1 [69]. Figure 5 shows the chemical structure of compounds (13–23).
Forsythia suspensa, the 10th component of the formula, is known for its anti-inflammatory effect and anti-asthmatic action in ova-challenged asthma mice model due to its ability to reduce IL-4, IL-5, and IL-13and eosinophil infiltration, also the anti-inflammatory effect of this plant has been reported in several cell lines such as RAW264.7, BV2 microglia cells and PBMC. Moreover, the plant possesses remarkable antioxidant activities due to several compounds such as Forsythiaside and its analog, Phillyrin (24), Pinoresinol, and other lignans. Remarkably, forsythiaside can be considered a prodrug that, upon metabolism, will release a cinnamic acid derivative, which has a modulatory effect on neurotransmitters, as previously discussed. Furthermore, Forsythiasides decreased levels of HO-1 and nuclear transcription factor 2 (NRF2), two markers that were alleviated in the OSA model in animals [70,71,72].
Cimicifuga heracleifolia rhizomes are the 11th component of the formula. At the same time, there are no studies linking this plant to OSA, but its main active constituents, such as ferulic acid and Triterpene glycosides, may explain its use for such purpose. Ferulic acid is a cinnamic acid derivative with reported antidepressant activity through serotonergic system modulation [73]. Recently, Cimigenoside (25), a significant component of C. heracleifolia, was investigated for its ability to reduce Poly(I: C)-induced airway inflammation mice model. The study showed that Cimigenoside exerts its action by preventing neutrophil infiltration and decreasing expression of P-selectin, VCAM1, which is usually upregulated in OSA patients [74, 75].
Trichosanthes kirilowii, the 12th component of the formula, is widely used for its antioxidant and hypolipidemic activity due to the presence of unsaturated fatty acids such as linolenic acid (26) and flavonoids, as revealed in hyperlipidemia-induced by high-fat diet in rats [76].
Pueraria thomsonii or P. lobata, the 13th component of the formula, is rich in isoflavone such as Puerarin (27), which is reported to improve genioglossus muscle activity, as previously mentioned; it also has an inhibitory effect on the metabolism of Serotonin and dopamine. Moreover, several clinical trials have reported the anti-diabetic and antihyperlipidemic effects of different Pueraria species, but the outcomes of these trials are contradictory [77, 78].
Sparganium stoloniferum, the 14th component of the formula, is rich in phenylpropanoids such as ferulic acid, caffeic acid, and p-Coumaric acid. Their role in the modulation of OSA has been addressed previously. The Plant Possesses antioxidant, anti-inflammatory, and neuroprotective effects against hippocampal CA1 injury in animal models [79].
Rhizoma Curcumae, the 15th component of the formula, is rich in the famous compound Curcumin (28), which exerts a plethora of pharmacological activities related to OSA pathogenesis, such as anti-inflammatory, antioxidant, and antihyperlipidemic activities. Interestingly, Curcuma was one of the natural inhibitors of the ROS-HIF-1 endothelin axis as an approach to mitigate cardiovascular complications related to OSA [79]. Also, Curcumin was found to reduce intermittent hypoxia-induced brain injuries by regulating the AQP4 and p38 MAPK pathway. Additionally, Curcumin has been reported extensively as a neuroprotective agent through modulation of BDNF and downregulation of several inflammatory markers [80, 81].
Radix glycyrrhiza, the 16th component, is one of the most used plants in traditional medicine. Its pharmacological activity is attributed to glycyrrhizin(GN) 29, a triterpene glycoside, and its flavonoid content, such as liquidity (30). In this context, liquidation proved to exert fluoxetine-like action in depression-induced rats, which was explained by its ability to decrease oxidative stress and increase the expression of antioxidant enzymes [82].
On the other hand, GN was reported to possess antidepressant activity by increasing norepinephrine and dopamine levels but not serotonin in the case of induced depression in mice. This was in agreement with previous studies indicating the ability of GN to inhibit Monoamine oxidase [83, 84]. Besides that, glycyrrhizin was found to ameliorate metabolic disorders by improving lipid profile and reversing insulin resistance in high-fat diet-induced obesity in rats [85].
As complementary treatment for the previously mentioned formula, the same study also investigated some other herbals that are commonly used for relieving nose and airway-related symptoms, such as Thallus Laminariae also known as Laminaria japonica, Angelicae dahuricae, Flos Magnoliae, Fructus Xanthii, and Radix Asari. The first one, L. japonica, has been reported to relieve inflammation in the airway in an ovalbumin (OVA)-induced mouse asthma model. by increasing the expression of IL-12 and decreasing the gene expression of another inflammatory marker as IL-13 and TGF-β1 leading to a decrease in a number of eosinophils [86]. Moreover, its sulfated polysaccharide, Fucoidan, has been reported to protect dopaminergic neurons in experimental models by maintaining mitochondrial function through modulation of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) and (NRF2) pathway [87].
Angelica dahuricae is another plant closely related to A. sinensis and was found to possess anti-depressant activity, which is attributed to the furanocoumarin compound imperatorin. This compound increased the level of 5-HT significantly in the hippocampus and frontal cortex in prenatally stressed offspring rats [88]. In agreement with the suggested use, A. dahrucae was found to be effective against allergic inflammation by reducing the levels of TNF-α, IL-1β, and IL-4 by inhibiting NF‑κB activation [89].
Magnoliae flos, on the other hand, was reported to inhibit iNOS in human respiratory epithelial through preventing ERK Phosphorylation [92]; the most promising compounds in this plant were Epimagnolin and Fargesin which are structurally related to the Pinoresinol as mentioned above. In the same context, LEE et al. isolated seven structurally related compounds from the most active fraction against airway inflammation induced by cigarette smoke [90].
Caffeoylxanthiazonoside (31)isolated from Fructus Xanthii might explain its use in the formula as it was proved to possess anti-allergic activity comparable to ketotifen [91]. Still, other phenolic compounds, such as caffeoylquinic acids (32), have been reported to exert the same effect in passive cutaneous anaphylaxis tests and the Schultz-Dale test in comparison to second-generation antihistaminic loratadine [92].
Asarum heterotropoides, also contains lignan such as sesamin and Episesamin, which also has been shown to exert anti-inflammatory action through regulation of MAPK pathway. Unfortunately, the extract itself contains the notorious Aristolochene (33), which is reported to cause severe nephrotoxicity and is a dangerous oncogenic agent [95], so the use of an isolated compound or standardized fraction could ensure safety for use in clinical application. Figure 6 shows the chemical structure of compounds (24–33).
Since airway inflammation is evidenced in OSA patients, these plants could be a potential therapeutic agent for OSA [97]. Table 1 shows the components of the formula and their possible mechanism of action.
Chen et al. studied the effect of Qingxuan granule, a TCM formula, on patients with hypertension accompanied with or without OSA. While the formula improved the blood pressure variability, its impact was reduced in patients suffering from OSA, and among the measured OSA indices, only the hypopnea index decreased. Interestingly, Gastrodia and Radix Scutellaria were also used in this formula, which might confirm their role in the management of OSA [93].
The Chinese herbal formula, Jiawei Di Tan Tang, which contains several plants that were discussed before, was investigated for the management of OSA based on its anti-inflammatory and antioxidant actions in a randomized clinical trial. Indeed, the treated group showed increased levels of antioxidant enzymes such as superoxide dismutase (SOD) and lower levels of Malondialdehyde (MDA). Also, several cytokines serum levels decreased significantly in comparison to the control group, such as C-reactive protein, TNF-α, Interleukin-1β, and Interleukin-6 [94].
Furthermore, several OSA-related clinical parameters were measured during the treatment (12 weeks), such as AHI, Epworth sleepiness scale (ESS), Snoring, nocturnal apnea, sleepiness, fatigue, chest congestion, and lowest nocturnal blood oxygen saturation (SaO2). Interestingly, the treated group showed slight but significant improvement in these markers in comparison to their level before treatment and in the control group [95].
In another study, San’o-shashin-to-a Kampo-formula, which is composed of scutellarin Radix, Coptidis Rhizoma, and Rhei Rhizoma discussed before, was prescribed for a patient that was diagnosed with OSA. After treatment, several symptoms were improved significantly, where the apnea index decreased from 11.1 to 4.1, and abnormal movement during sleepiness disappeared completely. Still, the episodes of obstructive sleep hypopnea were not reduced significantly [96].
Remarkably, a retrospective study has shown that the use of integrative traditional Chinese medicine containing plants from the previous formulas has helped to reduce respiratory disturbance index (RDI), and body mass index (BMI) in children with obstructive sleep apnea [97].
A patent for the treatment of OSA claimed that the administration of valerian extract may enhance OSA clinical parameters based on a small clinical trial—nine patients with confirmed OSA administrated valerian for two weeks. The treatment decreased the Apnea index from 5.3 to 0.67 and the hypopnea index from 5.01 to 0.95, but there was no significant increase in sleep efficacy [98].
Mao Ting et al. used network pharmacology to identify the mechanism of action of the TCM formula for the treatment of OSA associated with atrial fibrillation. The constructed network showed that the formula exerts its action by regulating several pathways, such as the TNF signaling pathway, HIF-1 signaling pathway, PI3K-Akt signaling pathway, neuroactive ligand-receptor interaction, and calcium signaling pathway. This action was attributed to several phytochemicals such as quercetin, luteolin, kaempferol, apigenin, stigmasterol, isocorypalmine, and hyndarin [99].
A healthy diet is an important approach for enhancing the quality of life in OSA patients. Mediterranean diet (MD and prudent diet (PD), which is rich in antioxidants, were assessed for their potential to alleviate OSA clinical symptoms in a randomized clinical trial where 900 patients with apnoea-hypopnoea index > 15 were followed for six months. Besides the weight reduction of the subjects, there was a significant reduction in AHI during rapid eye movement sleep by 18.4 ± 17.6 in the MD group and by 2.6 ± 23.7 in the PD group; however, their effect was not statistically significant in other sleep parameters [100].
Moreover, Plants and compounds possessing multiple therapeutic effects such as Ginkgo biloba [101], ginseng [102], omega-3-fatty acids [103], anti-oxidant phenolics [104, 105], nutrients [106], and essential oils used in aromatherapy [107] could help in the restoration of normal state and function of the body by protecting against hypoxia-induced by OSA, which would eventually prevent the development of cardiovascular and metabolic diseases [108]. Tables 2 and 3 demonstrate representative examples of promising preclinical and clinical investigations of natural products that could be used for the management of OSA. Also, Fig. 7 summarizes the polypharmacological effect of natural products to tackle pathways of OSA pathogenesis.
Different mechanisms of naturally derived therapeutics for the management of OSA. 1) Several components of plants, such as NE, DA, 5-HT, and GABA, could modulate catecholamine activity, which helps to enhance muscle strength and maintain airway patency; also, other compounds act as ventilation activators. 2) Several components of plants are reported to suppress pathways activated by hypoxia, which would protect against Cardiovascular and central nervous system conditions induced by hypoxia. 3) natural compounds are known to counteract hypoxic states and activate oxidative stress mechanisms, leading to the release of cytokines and reactive oxygen species, which are responsible for the development of several chronic diseases 4) Nature-derived compounds work perfectly as an antioxidant and anti-inflammatory agents, which consequently protect against worsening of OSA
As previously shown, natural products could be a promising resource for the development of the next therapeutic agent for OSA. Records from traditional medicine can also be a good asset for recognizing plants with potential activity; information extracted from traditional medicine could be evaluated by an in-vivo model for OSA. Moreover, Virtual screening and system Pharmacology could be an intriguing approach since they can reveal the connections between unexplored drugs and molecular targets linked to OSA pathogenesis, which would eventually decrease the time required for preclinical testing and increase the chances for success of clinical trials [109, 110]( Fig. 8).
Concluding remarks and recommendations
The present literature mini-review intends to present the role of serious and rigorous research in the area of natural products to open a new horizon for an economical, safe, effective, reliable, and evidence-based drug /and or adjuvant medicine for OSA. Therapeutic protocols should consider a non-conventional polypharmacological strategy that targets all aspects of this disease. Hence, there is an opportunity to reposition some well-defined natural products, such as cinnamic acid derivatives, isoflavones, and lignans, as evidence from in-vitro, in-vivo, and clinical trials supports their potential efficacy in the management of OSA.
However, this will only be achieved if evidence-based confirms and justifies the presumable clinical effects. So, in conclusion, there is an opportunity for pharmacotherapy of drugs’ natural origin to play a vital role in the management of OSA. More importantly, integrating nano-based delivery systems for selected medicines and natural products could enhance the pharmacokinetic properties, significantly impact pharmacoeconomics, and limit the adverse effects associated with pharmacotherapy.
Data availability
Authors would provide the data in this manuscript upon request.
Abbreviations
- AHI:
-
Apnea-Hypopnea Index
- OSA:
-
Obstructive Sleep Apnea
- CPAP:
-
Continuous Positive Airway Pressure
- ACPAP:
-
Auto-titrating Continuous Positive Airway Pressure
- TCM:
-
Traditional Chinese Medicine
- SSRI:
-
Selective Serotonin Reuptake Inhibitor
- NDRI:
-
Norepinephrine and Dopamine Reuptake Inhibitor
- BDNF:
-
Brain-derived Neurotrophic Factor
- MAO:
-
Monoamine Oxidase
- NF-κB:
-
Nuclear Factor κB
- HIF-1α:
-
Hypoxia Induction Factor-1α
- SOD:
-
Superoxide Dismutase
- MDA:
-
Malondialdehyde
- TNF-α:
-
Tumor Necrosis Factor-α
- IL-1β:
-
Interleukin-1β
- IL-6:
-
Interleukin-6
- PPARγ:
-
Peroxisome Proliferator-Activated Receptorγ
- C/EBPα:
-
CCAAT-Enhancer Binding Proteinα
- AMPK:
-
AMP-Activated Protein Kinase
- Sirtuin-1:
-
Sirtuin-1
- OSAHS:
-
Obstructive Sleep Apnea-Hypopnea Syndrome
- MD:
-
Mediterranean Diet
- PD:
-
Prudent Diet
References
Hanzel DA, Proia NG, Hudgel DW. Response of obstructive sleep apnea to fluoxetine and protriptyline. Chest. 1991;100(2):416–21.
Berry RB, Yamaura EM, Gill K, Reist C. Acute effects of paroxetine on genioglossus activity in obstructive sleep apnea. Sleep Breath. 1999;22(8):1087–92.
Deeks ED, Heo Y-A. Solriamfetol in excessive daytime sleepiness: a profile of its use. Drugs Therapy Perspect. 2020:1–8.
Strollo PJ Jr, Hedner J, Collop N, Lorch DG Jr, Chen D, Carter LP, et al. Solriamfetol for the treatment of excessive sleepiness in OSA: a placebo-controlled randomized withdrawal study. Chest. 2019;155(2):364–74.
Prasad B, Radulovacki MG, Carley DW. Proof of concept trial of dronabinol in obstructive sleep apnea. %J Front Psychiatry. 2013;4:1.
Lavie L. Oxidative Stress—A unifying paradigm in obstructive sleep apnea and comorbidities. Prog Cardiovasc Dis. 2009;51(4):303–12.
Zhou L, Chen P, Peng Y, Ouyang R. Role of oxidative stress in the neurocognitive dysfunction of obstructive sleep apnea syndrome. oxidative med cell longev. 2016;2016:9626831.
Ip S, D’Ambrosio C, Patel K, Obadan N, Kitsios GD, Chung M, et al. Auto-titrating versus fixed continuous positive airway pressure for the treatment of obstructive sleep apnea: a systematic review with meta-analyses. Syst Reviews. 2012;1(1):20.
Berry RB, Parish JM, Hartse KM. The use of auto-titrating continuous positive airway pressure for treatment of adult obstructive sleep apnea. An American Academy of Sleep Medicine review. Sleep. 2002;25(2):148–73.
Seo J, Yun S, Shim S, Cho SW, Choi JW, Kim JW, et al. Palatal implant system can provide effective treatment for obstructive sleep apnea by recovering retropalatal patency. J Neural Eng. 2020;17(2):026017.
Kiyohara N, Badger C, Tjoa T, Wong B. A comparison of Over-the-counter Mechanical Nasal dilators: a systematic review. JAMA Facial Plast Surg. 2016;18(5):385–9.
Patel AV, Hwang D, Masdeu MJ, Chen GM, Rapoport DM, Ayappa I. Predictors of response to a nasal expiratory resistor device and its potential mechanisms of action for treatment of obstructive sleep apnea. J Clin Sleep Med. 2011;7(1):13–22.
Riaz M, Certal V, Nigam G, Abdullatif J, Zaghi S, Kushida CA, et al. Nasal expiratory positive airway pressure devices (Provent) for OSA: a systematic review and Meta-analysis. Sleep Disorders. 2015;2015:734798.
Farid-Moayer M, Siegel LC, Black J. A feasibility evaluation of oral pressure therapy for the treatment of obstructive sleep apnea. Ther Adv Respir Dis. 2013;7(1):3–12.
Colrain IM, Black J, Siegel LC, Bogan RK, Becker PM, Farid-Moayer M, et al. A multicenter evaluation of oral pressure therapy for the treatment of obstructive sleep apnea. Sleep Med. 2013;14(9):830–7.
AbdelFattah MR, Jung SW, Greenspan MA, Padilla M, Enciso R. Efficacy of antidepressants in the treatment of obstructive sleep apnea compared to Placebo. A systematic review with Meta-analyses. Sleep Breath. 2020;24(2):443–53.
Zamarron C, Vanesa García P, Riveiro A. Obstructive sleep apnea syndrome is a systemic disease. Current evidence. Eur J Intern Med. 2008;19(6):390–8.
Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discovery. 2005;4(3):206–20.
Karim N, Abdelhalim H, Gavande N, Khan I, Khan H. Natural products as an emerging Therapeutic Alternative in the treatment of neurological disorders. Evidence-Based Complement Altern Med. 2018;2018:3056847.
Koeberle A, Werz O. Multi-target approach for natural products in inflammation. Drug Discovery Today. 2014;19(12):1871–82.
Efferth T, Koch E. Complex interactions between phytochemicals. The multi-target therapeutic concept of phytotherapy. Curr Drug Targets. 2011;12(1):122–32.
Bhattacharjee M, Perumal E. Potential plant-derived catecholaminergic activity enhancers for neuropharmacological approaches: a review. Phytomedicine. 2019;55:148–64.
Chung K-T, Hsu C-H, Lin C-L, Wang S-E, Wu C-H. Traditional Chinese herbal formula relieves snoring by modulating activities of upper airway related nerves in aged rats. Drug Des Dev Therapy. 2018;12:1165.
Chen W-C, Lai Y-S, Lin S-H, Lu K-H, Lin Y-E, Panyod S, et al. Anti-depressant effects of Gastrodia Elata Blume and its compounds gastrodin and 4-hydroxybenzyl alcohol, via the monoaminergic system and neuronal cytoskeletal remodeling. J Ethnopharmacol. 2016;182:190–9.
Liu Y, Gao J, Peng M, Meng H, Ma H, Cai P, et al. A review on Central Nervous System effects of Gastrodin. Front Pharmacol. 2018;9:24.
Moran M. Reversible exacerbation of obstructive sleep apnea by α1-adrenergic blockade with tamsulosin: a case report. Respiratory Med case Rep. 2016;19:181–6.
Larrain A, Kapur VK, Gooley TA, Pope CE 2. Pharmacological treatment of obstructive sleep apnea with a combination of pseudoephedrine and domperidone. J Clin Sleep Med. 2010;6(2):117–23.
Song G, Poon C-S. α2-Adrenergic blockade rescues hypoglossal motor defense against obstructive sleep apnea. JCI Insight. 2017;2(4).
Nathan PJ. Hypericum perforatum (St John’s wort): a non-selective reuptake inhibitor? A review of the recent advances in its pharmacology. J Psychopharmacol. 2001;15(1):47–54.
Chua HC, Christensen ET, Hoestgaard-Jensen K, Hartiadi LY, Ramzan I, Jensen AA, et al. Kavain, the Major Constituent of the anxiolytic Kava Extract, Potentiates GABAA receptors: functional characteristics and molecular mechanism. PLoS ONE. 2016;11(6):e0157700.
Prinsloo D, van Dyk S, Petzer A, Petzer JP. Monoamine Oxidase Inhibition by Kavalactones from Kava (Piper Methysticum). Planta Med. 2019;85(14/15):1136–42.
Seitz U, Schüle A, Gleitz J. [3H]-monoamine uptake inhibition properties of kava pyrones. Planta Med. 1997;63(06):548–9.
Sarris J, Stough C, Bousman CA, Wahid ZT, Murray G, Teschke R, et al. Kava in the treatment of generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychopharmacol. 2013;33(5):643–8.
Boonen G, Häberlein H. Influence of genuine kavapyrone enantiomers on the GABA-A binding site. Planta Med. 1998;64(6):504–6.
White CM. The Pharmacology, Pharmacokinetics, Efficacy, and adverse events Associated with Kava. J Clin Pharmacol. 2018;58(11):1396–405.
Calik MW, Radulovacki M, Carley DW. Intranodose ganglion injections of dronabinol attenuate serotonin-induced apnea in Sprague-Dawley rat. Respir Physiol Neurobiol. 2014;190:20–4.
Carley DW, Paviovic S, Janelidze M, Radulovacki M. Functional role for cannabinoids in respiratory stability during sleep. Sleep. 2002;25(4):391–8.
Calik MW, Carley DW. Effects of Cannabinoid agonists and antagonists on Sleep and Breathing in Sprague-Dawley Rats. Sleep. 2017;40(9).
Prasad B, Radulovacki MG, Carley DW. Proof of concept trial of dronabinol in obstructive sleep apnea. Front Psychiatry. 2013;4:1.
Ramar K, Kirsch DB, Carden KA, Rosen IM, Malhotra RK. Medical Cannabis, synthetic marijuana extracts, and obstructive sleep apnea. J Clin Sleep Med. 2018;14(10):1815–6.
Mulloy E, McNicholas WT. Theophylline in obstructive sleep apnea: a double-blind evaluation. Chest. 1992;101(3):753–7.
Espinoza H, Antic R, Thornton AT, McEvoy RD. The effects of aminophylline on sleep and sleep-disordered breathing in patients with obstructive sleep apnea syndrome. Am Rev Respir Dis. 1987;136(1):80–4.
Lin CM, Huang YS, Guilleminault C. Pharmacotherapy of obstructive sleep apnea. Expert Opin Pharmacother. 2012;13(6):841–57.
Hartung AM, Beutler JA, Navarro HA, Wiemer DF, Neighbors JD. Stilbenes as κ-Selective, non-nitrogenous opioid receptor antagonists. J Nat Prod. 2014;77(2):311–9.
Davila DG, Hurt RD, Offord KP, Harris CD, Shepard JW Jr. Acute effects of transdermal nicotine on sleep architecture, snoring, and sleep-disordered breathing in nonsmokers. Am J Respir Crit Care Med. 1994;150(2):469–74.
Frank SR. Herbal preparation for sleep apnea relief. Google Patents; 2016.
Wu Y-H, Wei Y-C, Tai Y-S, Chen K-J, Li H-Y. Clinical outcomes of traditional Chinese medicine compound formula in treating sleep-disordered breathing patients. Am J Chin Med. 2012;40(01):11–24.
Shen J, Zhang J, Deng M, Liu Y, Hu Y, Zhang L. The antidepressant effect of Angelica sinensis extracts on chronic unpredictable mild stress-Induced Depression is mediated via the Upregulation of the BDNF signaling pathway in rats. Evid Based Complement Alternat Med. 2016;2016:7434692.
Flores KR, Viccaro F, Aquilini M, Scarpino S, Ronchetti F, Mancini R, et al. Protective role of brain derived neurotrophic factor (BDNF) in obstructive sleep apnea syndrome (OSAS) patients. PLoS ONE. 2020;15(1):e0227834.
Chen J, Lin D, Zhang C, Li G, Zhang N, Ruan L, et al. Antidepressant-like effects of ferulic acid: involvement of serotonergic and norepinergic systems. Metab Brain Dis. 2015;30(1):129–36.
Min J-j, Huo X-l, Qin Y-q, Chai K-q, Wu B, Jin L, et al. Protective effect of Dl-3n-butylphthalide on learning and memory impairment induced by chronic intermittent hypoxia-hypercapnia exposure. Sci Rep. 2014;4(1):1–9.
Chen Y, Wu T, Li H, Li X, Li Q, Zhu X, et al. Dl-3-n-Butylphthalide exerts dopaminergic Neuroprotection through Inhibition of Neuroinflammation. Front Aging Neurosci. 2019;11:44.
Mao Q-Q, Ip S-P, Xian Y-F, Hu Z, Che C-T. Anti-depressant-like effect of peony: a mini-review. Pharm Biol. 2012;50(1):72–7.
Sowndhararajan K, Deepa P, Kim M, Park SJ, Kim S. Neuroprotective and cognitive enhancement potentials of Baicalin: a review. Brain Sci. 2018;8(6):104.
Chang Y, Lu CW, Lin TY, Huang SK, Wang SJ. Baicalein, a constituent of Scutellaria baicalensis, reduces Glutamate Release and protects neuronal cell against Kainic Acid-Induced excitotoxicity in rats. Am J Chin Med. 2016;44(5):943–62.
Fan J, Zhang K, Jin Y, Li B, Gao S, Zhu J, et al. Pharmacological effects of berberine on mood disorders. J Cell Mol Med. 2019;23(1):21–8.
Sun Y, Lenon GB, Yang AWH, Phellodendri Cortex. A phytochemical, pharmacological, and Pharmacokinetic Review. Evid Based Complement Alternat Med. 2019;2019:7621929.
Xian X, Sun B, Ye X, Zhang G, Hou P, Gao H. Identification and analysis of alkaloids in cortex Phellodendron amurense by high-performance liquid chromatography with electrospray ionization mass spectrometry coupled with photodiode array detection. J Sep Sci. 2014;37(13):1533–45.
Ren L-X, Luo Y-F, Li X, Zuo D-Y, Wu Y-L. Antidepressant-like effects of sarsasapogenin from Anemarrhena asphodeloides BUNGE (Liliaceae). Biol Pharm Bull. 2006;29(11):2304–6.
Feng X, Xue JH, Xie KX, Liu SP, Zhong HP, Wang CC et al. Beneficial effect of Mangiferin against sleep deprivation-induced neurodegeneration and memory impairment in mice. Biomed Res. 2017.
Yang H, Baggett S, Staub R, Chow S, Bjeldanes L, Leitman D, et al. Norlignans from Anemarrhenae asphoeloides displaying selective estrogen Beta (ER) activity. Planta Med. 2008;74(03):P–78.
Manber R, Kuo TF, Cataldo N, Colrain IM. The effects of hormone replacement therapy on sleep-disordered breathing in Postmenopausal women: a pilot study. Sleep. 2003;26(2):163–8.
Lu Y, Liu Y, Li Y. Comparison of natural estrogens and synthetic derivative on genioglossus function and estrogen receptors expression in rats with chronic intermittent hypoxia. J Steroid Biochem Mol Biology. 2014;140:71–9.
Abdulmanea K, Prokudina EA, Lanková P, Vaníčková L, Koblovská R, Zelený V, et al. Immunochemical and HPLC identification of isoflavonoids in the Apiaceae family. Biochem Syst Ecol. 2012;45:237–43.
Torvaldsson S, Grote L, PEKER Y, Basun H, Hedner J. A randomized placebo-controlled trial of an NMDA receptor antagonist in sleep‐disordered breathing. J Sleep Res. 2005;14(2):149–55.
Deng Y-t, Zhao M-g, Xu T-j, Jin H, Li X-h. Gentiopicroside abrogates lipopolysaccharide-induced depressive-like behavior in mice through tryptophan-degrading pathway. Metab Brain Dis. 2018;33(5):1413–20.
Liu S-b, Zhao R, Li X-s, Guo H-j, Tian Z, Zhang N, et al. Attenuation of Reserpine-Induced Pain/Depression Dyad by Gentiopicroside through downregulation of GluN2B receptors in the Amygdala of mice. Neuromol Med. 2014;16(2):350–9.
Hwang K-A, Hwang Y-J, Im PR, Hwang H-J, Song J, Kim Y-J. Platycodon grandiflorum Extract reduces high-Fat Diet-Induced obesity through regulation of Adipogenesis and lipogenesis pathways in mice. J Med Food. 2019;22(10):993–9.
Kim H-L, Park J, Park H, Jung Y, Youn D-H, Kang J, et al. Platycodon grandiflorum A. De Candolle ethanolic extract inhibits adipogenic regulators in 3T3-L1 cells and induces mitochondrial biogenesis in primary brown preadipocytes. J Agricultural food Chem. 2015;63(35):7721–30.
Wang Z, Xia Q, Liu X, Liu W, Huang W, Mei X, et al. Phytochemistry, pharmacology, quality control and future research of Forsythia suspensa (Thunb.) Vahl: a review. J Ethnopharmacol. 2018;210:318–39.
Liu J-X, Zhang Y, Yuan H-Y, Liang J. The treatment of asthma using the Chinese Materia Medica. J Ethnopharmacol. 2021;269:113558.
Wang Y, Chai Y, He X, Ai L, Sun X, Huang Y, et al. Intermittent hypoxia simulating obstructive sleep apnea causes pulmonary inflammation and activates the Nrf2/HO-1 pathway. Exp Ther Med. 2017;14(4):3463–70.
Zeni ALB, Zomkowski ADE, Maraschin M, Rodrigues ALS, Tasca CI. Ferulic acid exerts antidepressant-like effect in the tail suspension test in mice: evidence for the involvement of the serotonergic system. Eur J Pharmacol. 2012;679(1):68–74.
Ohga E, Nagase T, Tomita T, Teramoto S, Matsuse T, Katayama H, et al. Increased levels of circulating ICAM-1, VCAM-1, and L-selectin in obstructive sleep apnea syndrome. J Appl Physiol. 1999;87(1):10–4.
Hu L, Song X, Nagai T, Yamamoto M, Dai Y, He L, et al. Chemical profile of Cimicifuga heracleifolia Kom. And immunomodulatory effect of its representative bioavailable component, cimigenoside on poly(I:C)-induced airway inflammation. J Ethnopharmacol. 2021;267:113615.
Hou Z, Zhu L, Meng R, Wang B. Hypolipidemic and antioxidant activities of Trichosanthes kirilowii maxim seed oil and flavonoids in mice fed with a high-fat diet. J Food Biochem. 2020;44(8):e13272.
Zhang Z, Lam T-N, Zuo Z. Radix Puerariae: an overview of its Chemistry, Pharmacology, Pharmacokinetics, and clinical use. J Clin Pharmacol. 2013;53(8):787–811.
Ulbricht C, Costa D, Dam C, D’Auria D, Giese N, Isaac R, et al. An evidence-based systematic review of Kudzu (Pueraria lobata) by the Natural Standard Research Collaboration. J Diet Supplements. 2015;12(1):36–104.
Jia J, Li X, Ren X, Liu X, Wang Y, Dong Y et al. Sparganii Rhizoma: a review of traditional clinical application, processing, phytochemistry, pharmacology, and toxicity. J Ethnopharmacol. 2020:113571.
Wang B, Li W, Jin H, Nie X, Shen H, Li E, et al. Curcumin attenuates chronic intermittent hypoxia-induced brain injuries by inhibiting AQP4 and p38 MAPK pathway. Respir Physiol Neurobiol. 2018;255:50–7.
Eghbaliferiz S, Farhadi F, Barreto GE, Majeed M, Sahebkar A. Effects of curcumin on neurological diseases: focus on astrocytes. Pharmacol Rep. 2020:1–14.
Zhao Z, Wang W, Guo H, Zhou D. Antidepressant-like effect of liquiritin from Glycyrrhiza uralensis in chronic variable stress induced depression model rats. Behav Brain Res. 2008;194(1):108–13.
Dhingra D, Sharma A. Antidepressant-like activity of Glycyrrhiza glabra L. in mouse models of immobility tests. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(3):449–54.
Dhingra D, Sharma A. Evaluation of antidepressant-like activity of glycyrrhizin in mice. Indian J Pharmacol. 2005;37(6):390.
Abo El-Magd NF, El-Mesery M, El-Karef A, El-Shishtawy MM. Glycyrrhizin ameliorates high fat diet-induced obesity in rats by activating NrF2 pathway. Life Sci. 2018;193:159–70.
Lin R, Liu X, Meng Y, Xu M, Guo J. Effects of Laminaria Japonica polysaccharides on airway inflammation of lungs in an asthma mouse model. Multidisciplinary Respiratory Med. 2015;10(1):20.
Zhang L, Hao J, Zheng Y, Su R, Liao Y, Gong X, et al. Fucoidan protects dopaminergic neurons by enhancing the mitochondrial function in a rotenone-induced rat model of Parkinson’s Disease. Aging Disease. 2018;9(4):590–604.
Cao Y, Liu J, Wang Q, Liu M, Cheng Y, Zhang X, et al. Antidepressive-like effect of imperatorin from Angelica Dahurica in prenatally stressed offspring rats through 5-hydroxytryptamine system. NeuroReport. 2017;28(8):426–33.
Li D, Wu L. Coumarins from the roots of Angelica Dahurica cause anti–allergic inflammation. Exp Ther Med. 2017;14(1):874–80.
Lee S-U, Ryu HW, Lee S, Shin I-S, Choi J-H, Lee J-W et al. Lignans isolated from Flower buds of Magnolia Fargesii Attenuate Airway inflammation Induced by cigarette smoke in vitro and in vivo. Front Pharmacol. 2018;9(970).
Peng W, Ming Q-L, Han P, Zhang Q-Y, Jiang Y-P, Zheng C-J, et al. Anti-allergic rhinitis effect of caffeoylxanthiazonoside isolated from fruits of Xanthium strumarium L. in rodent animals. Phytomedicine. 2014;21(6):824–9.
Peng W, Han P, Yu L, Chen Y, Ye B, Qin L, et al. Anti-allergic rhinitis effects of caffeoylquinic acids from the fruits of Xanthium strumarium in rodent animals via alleviating allergic and inflammatory reactions. Revista Brasileira De Farmacognosia. 2019;29(1):46–53.
Chen Y-y, Chen J, Zhang J-c, Chen K-j. Effect of Qingxuan Granule (清眩颗粒) on blood pressure variability of hypertensive patients with and without obstructive sleep apnea. Chin J Integr Med. 2013.
Chen Q, Lin RJ, Hong X, Ye L, Lin Q. Treatment and prevention of inflammatory responses and oxidative stress in patients with obstructive sleep apnea hypopnea syndrome using Chinese herbal medicines. Experimental Therapeutic Med. 2016;12(3):1572–8.
Chen Q, Lin RJ, Hong X, Ye L, Lin Q. Treatment and prevention of inflammatory responses and oxidative stress in patients with obstructive sleep apnea hypopnea syndrome using Chinese herbal medicines. Exp Ther Med. 2016;12(3):1572–8.
Sherman L. Herbal formula prevents inflammatory response in sleep apnoea. J Chin Med. 2016:67+.
Lai W-Y, Wei C-C, Lin C-H, Hang L-W, Shih Y-H, Huang F-W, et al. Integrative traditional Chinese medicine treatment for children with obstructive sleep apnea. J Traditional Complement Med. 2024;14(1):109–20.
Brattstroem A. Pharmaceutical composition containing valerina officinalis extract. Google Patents; 2004.
Mao T, Zhang J, Qiao Y, Liu B, Zhang S. Uncovering synergistic mechanism of Chinese herbal medicine in the treatment of atrial fibrillation with obstructive sleep apnea hypopnea syndrome by network pharmacology. Evidence-Based Complementary and Alternative Medicine. 2019;2019.
Papandreou C, Schiza SE, Bouloukaki I, Hatzis CM, Kafatos AG, Siafakas NM, et al. Effect of Mediterranean diet versus prudent diet combined with physical activity on OSAS: a randomised trial. Eur Respir J. 2012;39(6):1398–404.
LI Y. Effect of Ginkgo biloba extract on OSAS rats with secondary hypertension. Shandong Med J. 2016;2016(17):6.
Kim J-H. Pharmacological and medical applications of Panax ginseng and ginsenosides: a review for use in cardiovascular diseases. J Ginseng Res. 2018;42(3):264–9.
Papandreou C, Patil S, Dobrosielski DA. Focusing on Omega-3 fatty acids for treatment of obstructive sleep apnea and its Cardiovascular complications. J Respiratory Res. 2016;2(2):41–3.
Shahidi F, Yeo J. Bioactivities of phenolics by focusing on suppression of chronic diseases: a review. Int J Mol Sci. 2018;19(6):1573.
Zhang H, Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci. 2016;8:33–42.
Baldwin CM, Bootzin RR, Schwenke DC, Quan SF. Antioxidant nutrient intake and supplements as potential moderators of cognitive decline and cardiovascular disease in obstructive sleep apnea. Sleep Med Rev. 2005;9(6):459–76.
López V, Nielsen B, Solas M, Ramírez MJ, Jäger AK. Exploring pharmacological mechanisms of lavender (Lavandula angustifolia) essential oil on central nervous system targets. Front Pharmacol. 2017;8:280.
Belaidi E, Morand J, Gras E, Pépin J-L, Godin-Ribuot D. Targeting the ROS-HIF-1-endothelin axis as a therapeutic approach for the treatment of obstructive sleep apnea-related cardiovascular complications. Pharmacol Ther. 2016;168:1–11.
Clout AE, Della Pasqua O, Hanna MG, Orlu M, Pitceathly RD. Drug repurposing in neurological diseases: an integrated approach to reduce trial and error. J Neurol Neurosurg Psychiatry. 2019;90(11):1270–5.
Elgazar AA, Knany HR, Ali MS. Insights on the molecular mechanism of anti-inflammatory effect of formula from islamic traditional medicine: an in-silico study. J Traditional Complement Med. 2019;9(4):353–63.
Acknowledgements
The authors would like to thank Dr. Eman Mazyed, Assistant professor of Pharmaceutics, Department of Pharmaceutical Technology, Faculty of Pharmacy, Kafrelsheikh University, and Ms. Rowida Omar, Assistant lecturer of Pharmacognosy, Faculty of Pharmacy, Delta University of Science and Technology, for their great contribution and critical thinking in shaping up this manuscript in the final form.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Farid A. Badria: Concept design, data analysis. Supervision, drafting, writing, and revising the final manuscript. Abdullah A. Elgazar: Concept design, data collection, data analysis, drafting, and writing the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The manuscript has been read and approved for publication by all named authors.
Conflict of interest
The authors declare that there is no conflict of interest, and no funding was associated with this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Badria, F.A., Elgazar, A.A. Utilization of selected natural products as complementary therapeutic approach for Obstructive Sleep Apnea (OSA) management: a literature review. Clin Phytosci 10, 15 (2024). https://doi.org/10.1186/s40816-024-00375-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40816-024-00375-w