Abstract
Background
The plant Kingiodendron pinnatum (DC.) Harms, belonging to the family Fabaceae is endemic to the Western Ghats of India and is commonly used for various ailments, especially by the tribes. K. pinnatum is occasionally used as a substitute for Saraca asoca in Asokarishta, a well-known uterine tonic in Ayurveda. Recent studies revealed a pharmacological similarity between the plants. S. asoca is reported to have anti-cancer properties, but there are no reports on K. pinnatum except for antioxidant and antimicrobial activities. Therefore, the study is aimed to investigate the anticancer potential of the plant.
Methods
Cytotoxicity of methanolic bark extract of the plant was analysed on different cancer cell lines by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Dalton's lymphoma ascites (DLA) cell-induced solid and Ehrlich ascites carcinoma (EAC) cell-induced ascites tumour models in mice were used to study the antitumor potential. Phytochemical screening of the extract was also performed.
Results
The extract was found cytotoxic to DLA, EAC, HCT15, MDA-MB-231, T47D and PC3 with inhibitory concentration (IC50) values of 50.09, 74.74, 67.02, 119.22, 149.04 and 194.5 μg/mL, respectively. In the solid tumour model, a significant (P < 0.001) reduction in tumour weight of 0.7 ± 0.15 g was observed in 500 mg/kg b.wt. extract treated group compared to the control group (3.6 ± 0.24 g) by oral administration for 30 days. In the ascites tumour model, a high survival rate of 28.2 ± 8.72 days (P < 0.01) was found by the extract treatment compared to the control animals. Phytochemicals like alkaloids, flavonoids, phenols, phytosterols, saponins, tannins, steroids and terpenoids were detected in the extract.
Conclusion
Results obtained by the cytotoxic and anti-tumour studies revealed the anticancer potential of K. pinnatum. The plant exhibits more cytotoxicity towards cancer cell lines of the reproductive system such as the breast and prostate.
Similar content being viewed by others
Introduction
Cancer is one of the leading causes of death globally and the number of cases is mounting gradually. According to the International Agency for Research on Cancer (IARC), 18.1 million new cancer cases and 9.6 million cancer deaths were accounted for in 2018. The incidence rate of commonly diagnosed cancer types such as lung (2.09 million), breast (2.08 million), colorectal (1.8 million), prostrate (1.3 million) and stomach cancer (1 million) are increased [1]. Currently, there are many drugs available on market for different cancers but they are not completely effective and safe. Present treatment modalities like chemotherapy and radiotherapy have serious side effects affecting some vital organs. Hence, the studies are more focused on plant-derived products as they have shown to be effective and have fewer side effects. Many natural products and their analogues including camptothecin, resveratrol, taxol, vinblastine and sulforaphane have been recognised as anti-cancer drugs and various plants with anticancer potential are being identified day by day [2, 3]. Biologically active compounds are often characterized by unique structures, making natural product research an effective method for discovering new compounds with distinct mechanisms of action. Despite the advances that have enabled the use of natural products in the discovery of new therapeutic agents, there are still challenges to be addressed. In most cases, bioactive natural products must be produced at a large scale to meet the manufacturing requirements, which constitutes a major hurdle before they are eventually available for clinical use. For these issues to be resolved, it will be necessary to develop innovative therapeutic concepts and new technologies, thereby advancing the transformation of the field [4].
Kingiodendron pinnatum, the plant belonging to the family Fabaceae is endemic to the Western Ghats of India and is mainly distributed in the evergreen hill and deciduous forests of Karnataka, Kerala and Tamil Nadu states. Traditionally, an oleo-gum-resin extracted from the tree is being used by tribes for gonorrhoea, catarrhal conditions of genitourinary and respiratory tracts and curing sores in elephants [5]. The resin obtained by piercing the trunk has been used for joint pains and to get relief for the fissured foot by Kanikkar, a predominant tribal community of Kalakad-Mundanthurai of Western Ghats, Tirunelveli, Tamil Nadu, India [6]. Phytochemicals such as phenols, flavonoids, tannins, glycosides and terpenes were reported from the plant [7]. The plant is reported to have antioxidant, antifungal and antibacterial activities [5]. K. pinnatum is occasionally used as a substitute for Saraca asoca (Asoka), which is the prime raw material in the preparation of Asokarishta, a fermented formulation, commonly used to treat gynaecological ailments especially abnormal uterine bleeding (menorrhagia). The population of the tree is less in wild but is generally used as a substitute due to its massive size and the chance of getting a good amount of bark compared to the Asoka tree [8]. A previous study revealed the pharmacological efficacy of K. pinnatum as an alternative for S. asoca in Asokarishta by demonstrating the inhibitory effect of estrogen-induced uterus endometrial thickening in immature female rats, giving scientific validation for its use in polyherbal formulations [8]. S. asoca is reported to have anticancer properties [9], but there are no reports on K. pinnatum. Therefore, the present study is intended to analyse the cytotoxic effect of the plant on reproductive cancers such as breast and prostate and its antitumour potential using mouse solid and ascites tumour models. The study is expected to provide insights into the anticancer potential of K. pinnatum.
Materials and methods
Chemical and reagents
Dulbecco's Modified Eagle Medium (DMEM), Fetal Bovine Serum (FBS) was procured from Thermo Fisher Scientific Inc., USA. Streptomycin, penicillin was purchased from Sigma Aldrich, USA. MTT, trypan blue dye, phosphate buffer saline were procured from Sisco Research Laboratory Pvt. Ltd., India and methanol, isopropanol, HCl, Triton X 100 from Merck, India.
Collection of the plant sample
The stem bark of K. pinnatum was collected from the Wayanad region of Western Ghats, Kerala, India. The plant was authenticated by Dr. N. Sasidharan, Taxonomist, Kerala Forest Research Institute (KFRI), Thrissur, Kerala (India). The voucher specimen of K. pinnatum (No. KFRI 4725) was deposited in the Herbarium of KFRI. The collected stem bark was shade dried, powdered and stored in air-tight containers until use.
Preparation of the extract
About 20 g of the powder was extracted with 250 mL methanol by stirring overnight. The extract was filtered using Whatman no. 1 filter paper and evaporated to dryness. It was weighed to determine the percentage yield of the soluble constituents using the formula.
The residue thus obtained was stored at 4 °C until use.
Phytochemical analysis
The extract obtained was dissolved in methanol and subjected to various analysis to find out the presence of different phytochemicals. The total phenolic and flavonoid contents of K. pinnatum extract was determined by Folin–Ciocalteau colorimetric reagent (FCR) [10] and aluminium chloride colorimetric methods [11], respectively. Dragendorff’s, Hagers and Mayer's tests were used to detect the presence of alkaloids. Shinoda's, ferric chloride, Froth formation, Salkowski and Liebermann-Burchard tests, lead acetate and Salkowski tests were used to detect the presence of flavonoids, phenols, saponins, sterols, tannins and terpenoids [12], respectively.
Animals
Female Swiss albino mice (25–30 g) were purchased from the Small Animal Breeding Station (SABS), College of Veterinary, KVASU, Thrissur, Kerala. The animals were kept in the animal house facility of Amala Cancer Research Centre following standard conditions of 24–28 °C, 60–70% humidity, 12 h dark/light cycle and fed with standard rat feed bought from Sai Durga Feeds, Bangalore, India and water ad libitum. All the animal experiments were carried out with the prior permission of the Institutional Animal Ethics Committee (IAEC) and were conducted strictly according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) constituted by the Ministry of Environment and Forest, Government of India.
Cell lines
Breast cancer cell lines (MDA-MB-231, T47D), colorectal cancer cell line (HCT-15), prostate cancer cell line (PC3) and normal African green monkey kidney epithelial cells (Vero) obtained from National Centre for Cell Science (NCCS), Pune (India) were cultured in DMEM medium supplemented with FBS (10% v/v), streptomycin (100 μg/mL) and penicillin (100 U/mL). All cells were maintained at 37 °C, 5% CO2, 95% air and 100% relative humidity. Daltons Lymphoma Ascites (DLA) and Ehrlich's Ascites Carcinoma (EAC) cell lines were obtained from Amala Cancer Research Centre’s animal house facility. The cells were maintained in the intra-peritoneal cavity of mice.
In vitro cytotoxicity by trypan blue dye exclusion method
The short-term cytotoxic activity of the extract was evaluated by determining the percentage viability of murine tumour cells like DLA and EAC using the trypan blue exclusion method [13]. Crude methanolic extract of the plant was used for the study. The cells were grown in the peritoneal cavity of female mice (8 weeks old, 25–30 g) by injecting 1 × 106 cells/mL intra-peritoneally. Cells were aspirated aseptically from the cavity of mice after 15 days of inoculation, washed with PBS and centrifuged at 1000 rpm for 5 min. Pellets were resuspended in PBS and the cell count was adjusted to 1 × 106 cells/mL. The cells were pipetted out and added into each tube having PBS with different concentrations of the drug. It was then incubated for 3 h at 37ºC. After incubation, trypan blue dye was added and left for 3 min before observation. It was then observed under a light microscope using a haemocytometer. The experiments were performed in triplicate and the percentage of cytotoxicity was determined by counting the number of dead cells to that of live cells and substituting in the equation:
The graph was plotted and the half-maximal inhibitory concentration (IC50) was calculated.
In vitro cytotoxic analysis by MTT assay
MDA-MB-231, T47D, HCT-15, PC3 and Vero cell lines were used to study the cytotoxic activity of crude methanolic extract of K. pinnatum using MTT assay [14]. Approximately, 1 × 105 cells were seeded in a 12-well plate containing medium and incubated at 37 °C for 24 h. The cells were incubated with different concentrations of the extract at 37 °C for 24 h. After incubation, 100 μL of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) was added to each well and incubated for 4 h. The dark blue formazan crystals were dissolved in a 1 mL solubilization solution containing isopropanol, HCl and Triton X 100 by continuous aspiration and re-suspension. The absorbance of the coloured product was measured at 570 nm. Three experiments with comparable outcomes were carried out in duplicate and the cytotoxicity was determined by comparing the percentage of death of the treated cell population with the untreated control indicated by their respective absorbance assessed with the MTT assay.
Acute toxicity studies
The extract of K. pinnatum, at the concentration 2500 mg/kg b.wt. was administrated to 3 male and 3 female Swiss albino mice (25–30 g) orally according to the Organization of Economic Co-operation and Development (OECD) guideline for testing chemicals [15]. The animals were monitored for 14 successive days for any visible changes in behaviour, body weight, water and food intake, hair loss etc. At the end of the experimental period, the animals were sacrificed and the internal organs were examined for any sign of change by conducting a necropsy. The experiment was repeated with another set of animals.
Anti-tumour analysis in mouse models
For the study, Swiss albino mice were grouped into 5 groups comprising 6 animals. Group I: control-untreated; group II: vehicle control (propylene glycol); group III: KPLD—K. pinnatum (KP) low dose (250 mg/kg b.wt.); group IV: KPHD—KP high dose (500 mg/kg b.wt.); group V: standard—cyclophosphamide (10 mg/kg b.wt.). DLA and EAC cells were aspirated from the peritoneal cavity of the tumour-bearing mice and washed with PBS. DLA cell suspension (100 µL) containing approximately 1 × 106 cells was injected intramuscularly into the right hind limb for the development of solid tumour and EAC cells into the peritoneal cavity of the animals for the ascites tumour development. The extracts were administered 24 h after the induction of the tumour and continued for 10 consecutive days. Solid tumour development was determined by measuring the diameter of the tumour growth in two perpendicular planes using a Vernier calliper. The readings were taken at a 3 days interval basis up to the 30th day [16]. The tumour volume was calculated according to the following formula,
where, r1 is the minor radius and r2 is the major radius.
The percentage inhibition of tumour growth was calculated according to the formula,
Where C is the tumour volume of control animals on the 30th day and T is the tumour volume of treated animals on the 30th day. The tumours excised from the animal were weighed. In EAC model, the number of survival days of animals was recorded.
Statistical analysis
Data were presented as mean ± standard deviation. Data analysis was performed by one-way ANOVA method followed by Dunnett's multiple comparison test and Kaplan–Meier survival curve by Mantel-Cox test in Graphpad Prism 7. The level of significance was considered as p < 0.05*, p < 0.01** and p < 0.001***.
Results
Phytochemicals present in the extract
The total yield of the extract from 20 g of powdered bark using 250 mL methanol was 5.6022 g. Preliminary phytochemical screening revealed the presence of alkaloids, flavonoids, phenols, phytosterols, saponins, tannins and terpenoids in the methanolic extract (Tab. 1). The total phenol and flavonoid contents were found to be 2.297 mg GAE/g extract and 0.246 mg QE/g extract, respectively.
Cytotoxic effect of the extract on cancer cells
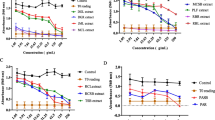
The extract was shown to have cytotoxicity against murine tumour cells like DLA and EAC cells in a dose-dependent manner with IC50 values of 50.09 and 74.74 μg/mL, respectively (Fig. 1). In the MTT assay, the extract was found to be cytotoxic on breast cancer cell lines MDA-MB-231 and T47D with IC50 of 70.22 and 149.04 μg/mL, respectively. It also showed cytotoxicity towards a colorectal cancer cell line, HCT-15 in a dose-dependent manner with an IC50 value of 67.02 μg/mL and prostate cancer cell line, PC3 with an IC50 of 198 μg/mL. The extract shows less cytotoxicity in the normal African green monkey kidney epithelial cell line, Vero even at the concentration of 280 μg/mL (Figs. 2 and 3).
Acute toxicity
The animals treated with the extract appeared healthy and no mortality was observed. There was no significant change in the behaviour of animals including, breathing, skin effects, water and food consumption, body weight etc. No changes in colour, texture and relative organ weights of the liver, heart, spleen, kidney, uterus and ovary were observed. Therefore, the extract seems to be safe up to a dose level of 2500 mg/kg. The parameters observed after the administration of the extract are represented in (Table 2).
Anti-tumour potential of the extract
The methanolic extract of K. pinnatum was found to inhibit the DLA-induced solid tumour in mice. The tumour weight in the control and vehicle group of animals was found to be 3.6 ± 0.24 and 3.1 ± 0.3 g, respectively on day 30th of tumour inoculation. High dose of K. pinnatum treated animals shows a significant reduction in tumour weight (0.7 ± 0.15 g, P < 0.001) compared to the control group animals (3.6 ± 0.24 g). The high dose of the extract reduced the tumour volume to 65.69 ± 18.1 cm3 from 426.25 ± 36.61 cm3 in the control group animals (Fig. 4). The tumour size and weight of control and treatment group animals are represented in Fig. 5.
Effect of K. pinnatum extract on DLA induced mouse solid tumor. Results are presented as mean ± SD, n = 5. One-way ANOVA was used to determine the statistical comparison followed by Dunnett's multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001, statistically significant as compared to the control group
A Comparison of tumour weight (in grams) (B) tumour size. Results are presented as mean ± SD, n = 5. One-way ANOVA was used to determine the statistical comparison followed by Dunnett's multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001, statistically significant as compared to the control group
K. pinnatum also shows a significant anti-tumour effect in the ascites tumour model. Animals in the control and vehicle control groups show almost the same mean survival rate with values of 20.4 ± 4.33 and 20.4 ± 4.77 days, respectively. The animals administrated with 500 mg/kg. b.wt. dose K. pinnatum extract showed a high survival rate of 28.2 ± 8.72 days (P < 0.01) compared to the control group animals (20.4 ± 4.33 days). Animals treated with the standard drug cyclophosphamide survived up to 30.8 ± 7.52 days, which was close to animals administrated with a high dose of K. pinnatum extract (Fig. 6).
Effect of K. pinnatum extract on the survival of ascites bearing animals. Results are presented as mean ± SD, n = 5. Kaplan–Meier survival curve was used to analyse the statistical comparison followed by Mantel-Cox test. *P < 0.05, **P < 0.01, ***P < 0.001, statistically significant as compared to the control group
Discussion
It has been reported that Kingiodendron pinnatum has a pharmacological similarity to that of S. asoca and can be used as a substitute in Ayurvedic preparations [8]. There are reports of cytotoxic and anticancer activities of S. asoca against different cancer cell lines [9, 17] and mouse tumour models [18], but no reports on K. pinnatum even though the plant is reported to have antioxidant, antifungal and antibacterial activities [5]. In our study, the plant K. pinnatum shows a cytotoxic and anti-proliferative effect on different types of cancer cell lines such as breast, colorectal, prostate and murine tumour cells and inhibits the development of mouse solid and ascites tumours suggesting its anticancer potential. K. pinnatum exhibited considerable in vitro cytotoxicity towards reproductive cancers such as breast and prostate cancer cell lines. In DLA induced solid tumour model, the extract treated group showed a decrease of the tumour growth. The mean tumour weight of the control was comparable to other groups except for the standard group. In the EAC ascites model, the survival curves were statistically significant for the high extract treated group and standard, compared to other groups.
S. asoca is reported to have phytoestrogens such as quercetin, kaempferol, β-sitosterol and luteolin [18] with anticancer potential. The mechanisms of action of these are suggested to be the modulation of estrogen receptors. Many studies have stated a connection between phytoestrogens and their possible role in cancer therapy or prevention [17, 19]. Phytoestrogens are reported to induce apoptosis in breast cancer cells and inhibit prostate and ovarian cancer growth [20,21,22]. They can interact and modulate different growth factors and activate/inhibit cytokine signalling pathways. genistein, a phytoestrogen, induced apoptosis in MCF-7 breast cancer cells through the down-regulation of the Akt signalling pathway [23]. Also, it inhibits triple-negative breast cancer cell, MDA-MB-231 growth by inhibiting NF-kB activity via the Notch-1 pathway [24]. In prostate cancer cells, it inhibits the activation of NF kB via the Akt signalling pathway [25].
The phytochemical screening of K. pinnatum has shown the presence of important phytoestrogens and revealed a similar phytochemical profile as that of S. asoca. In a pharmacological study on estradiol-induced keratinization, K. pinnatum was found to reduce cornification in immature rat uterus. The elevated level of estrogen in estradiol-administered animals has reduced and inhibited acute as well as chronic inflammations in mice [8]. This study gives a scientific validation for the use of K. pinnatum in polyherbal uterine tonic, Asokarishta as a substitute for S. asoca. In the present study, K. pinnatum exhibits more inhibitory effects toward reproductive cancers such as breast and prostate cancers. Thus, the activity of K. pinnatum may be due to the presence of similar phytoestrogens seen in S. asoca as they are pharmacologically related.
Conclusion
The results obtained by the cytotoxic and antitumour studies indicate the anticancer potential of the plant K. pinnatum, especially on reproductive cancers. The study also suggests the presence of phytoestrogens which are reported to have a variety of biological activities. The phytoestrogens can bind to reproductive cancer cells with estrogen receptors and can be a possible target of some phytoestrogens. This modulation of estrogen receptors may be the reason for the cytotoxic properties of some phytoestrogens against breast cancer cells. Thus, to comprehend the subsequent mechanism, it is necessary to analyse how phytoestrogens of K. pinnatum interact with estrogen receptors.
Availability of data and materials
Data supporting the findings of this study are available on request from the corresponding author
Abbreviations
- BWT:
-
Body weight
- DLA:
-
Dalton's lymphoma ascites
- EAC:
-
Ehlich ascites carcinoma
- IC50 :
-
Half maximal inhibitory concentration
- KP :
-
Kingiodendron pinnatum
- KPLD:
-
Kingiodendron pinnatum Low dose
- KPHD:
-
Kingiodendron pinnatum High dose
- MTT:
-
(3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Prakash O, Kumar A, Kumar P. Anticancer potential of plants and natural products. J Am J Pharmacol Sci. 2013;1(6):104–15.
Cragg GM, Pezzuto JM. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med Princ Pract. 2016;25(Suppl. 2):41–59.
Huang M, Lu J-J, Ding J. Natural products in cancer therapy: past, present and future. Nat prod bioprospect. 2021;11(1):5–13.
Kumar KJ, Prasad AGD, Richard SA. Biochemical activity of endangered medicinal plant Kingiodendron pinnatum. Asian J Plant Sci. 2011;1(4):70–5.
Sutha S, Mohan VR, Kumaresan SS, Murugan C, Athiperumalsami T. Ethnomedicinal plants used by the tribals of Kalakad-Mundanthurai Tiger Reserve (KMTR), Western Ghats, Tamil Nadu for the treatment of rheumatism. Indian J Tradit Knowl. 2010;9:502–9.
Sheik S, Chandrashekar KR. Antimicrobial and antioxidant activities of Kingiodendron pinnatum (DC.) Harms and Humboldtia brunonis Wallich: endemic plants of the Western Ghats of India. J Natl Sci Found. 2014;42(4):307.
Shahid AP, Sasidharan N, Salini S, Padikkala J, Meera N, Raghavamenon AC, et al. Kingiodendron pinnatum, a pharmacologically effective alternative for Saraca asoca in an Ayurvedic preparation, Asokarishta J Tradit Complement Med. 2017;8(1):244–50.
Yadav NK, Saini KS, Hossain Z, Omer A, Sharma C, Gayen JR, et al. Saraca indica bark extract shows in vitro antioxidant, antibreast cancer activity and does not exhibit toxicological effects. Oxid Med Cell Longev. 2015;205360(10):16.
Kaur C, Kapoor HC. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Technol. 2002;37(2):153–61.
Chang C-C, Yang M-H, Wen H-M, Chern J-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:3.
Dey PM. Oligosaccharides. Methods in plant biochemistry (ed), Vol. 2. London: Academic Press; 1990. p. 189–218
Moldeus P, Hogberg J, Orrenius S, Fleischer S, Packer L. Methods in enzymology. Academic Press, New York. 1978;52:60–71.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63.
OECD. OECD guideline for testing of chemicals. 2001. https://www.oecd.org/chemicalsafety/risk-assessment/1948378.pdf. Accessed 17 Dec 2001
Ma Y, Mizino T, Ito H. Antitumor activity of some polysaccharides isolated from a Chinese mushroom, Huangmo, the fruiting body of Hohenbuehelia serotina. Agric Biol Chem. 1991;55(11):2701–10.
Virk-Baker MK, Nagy TR, Barnes S. Role of phytoestrogens in cancer therapy. Planta Med. 2010;76(11):1132–42.
Swar G, Shailajan S, Menon S. Activity based evaluation of a traditional Ayurvedic medicinal plant: Saraca asoca (Roxb.) de Wilde flowers as estrogenic agents using ovariectomized rat model. J Ethnopharmacol. 2017;195:324–33. https://doi.org/10.1016/j.jep.2016.11.038.
Basu P, Maier C. Phytoestrogens and breast cancer: In vitro anticancer activities of isoflavones, lignans, coumestans, stilbenes and their analogs and derivatives. Biomed Pharmacother. 2018;107:1648–66.
Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey A, Harper P. Dietary phytoestrogen intake is associated with reduced colorectal cancer risk. J Nutr. 2006;136(12):3046–53.
Raffoul JJ, Banerjee S, Che M, Knoll ZE, Doerge DR, Abrams J, et al. Soy isoflavones enhance radiotherapy in a metastatic prostate cancer model. Int J Cancer. 2007;120(11):2491–8.
Obiorah IE, Fan P, Jordan VC. Breast cancer cell apoptosis with phytoestrogens is dependent on an estrogen-deprived state. Cancer Prev Res. 2014;7(9):939–49. https://doi.org/10.1158/1940-6207.capr-14-0061.
Anastasius N, Boston S, Lacey M, Storing N, Whitehead SA. Evidence that low-dose, long-term genistein treatment inhibits oestradiol-stimulated growth in MCF-7 cells by down-regulation of the PI3-kinase/Akt signalling pathway. J Steroid Biochem Mol Biol. 2009;116(1–2):50–5. https://doi.org/10.1016/j.jsbmb.2009.04.009.
Pan H, Zhou W, He W, Liu X, Ding Q, Ling L, et al. Genistein inhibits MDA-MB-231 triple-negative breast cancer cell growth by inhibiting NF-κB activity via the Notch-1 pathway. Int J Mol Med. 2012;30(2):337–43.
Li Y, Sarkar FH. Inhibition of nuclear factor kappa B activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res. 2002;8(7):2369–77.
Acknowledgements
The authors are thankful to the Indian Council of Medical Research (ICMR), New Delhi for the financial
support (No. 3/1 /3/JRF -2015(2)/HRD).
Funding
Indian Council of Medical Research (ICMR),No. 3/1 /3/JRF -2015(2)/HRD, Chennattu M Pareeth
Author information
Authors and Affiliations
Contributions
All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics of approval and consent to participate
All the animal experiments were carried out with the prior permission of the Institutional Animal Ethics Committee (IAEC) (Approval No: ACRC/IAEC/17(l)/P-05 dt: 22–07-2017) and were conducted strictly according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) constituted by Ministry of Environment and Forest, Government India.
Consent for publication
All authors agreed to the publication of the research.
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pareeth, C.M., Meera, N., Silpa, P. et al. Analysis of anticancer potential of Kingiodendron pinnatum (DC.) Harms. Clin Phytosci 9, 4 (2023). https://doi.org/10.1186/s40816-023-00356-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40816-023-00356-5