Abstract
Background
The medicinal plant Citrullus colocynthis (L.) Schrad. (C. colocynthis) may benefit patients at different phases of diabetes by attuning to contrasting situations. Our primary objective was to find the mechanism(s) behind the antidiabetic/anti-hyperlipidemic effects of C. colocynthis seed aqueous extract (CCAE) in two different stages of type 2 diabetes (T2D) in rats.
Methods
Fasting blood sugar (FBS) levels, body weights, and the degree of impaired glucose tolerance (IGT) were measured in healthy nondiabetic control rats (Con), as well as rats with early and late stages of T2D, denoted as ET2D and LT2D, respectively. CCAE was intraperitoneally (IP) injected for 28 days. In the end, the hepatic mRNA expression levels of the following genes were determined by RT-PCR: glucose-6-phosphatase (G6Pase), phosphoenolpyruvate carboxykinase (PEPCK), insulin-dependent sterol regulatory element-binding protein-1c (SREBP-1c), acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), peroxisome proliferator-activated receptor alpha (PPARα), and carnitine palmitoyltransferase I (CPT1). The liver was examined by hematoxylin and eosin (H&E) and Oil-Red O staining. CCAE was partially analyzed by HPLC-DAD.
Results
ET2D and LT2D were characterized by differentially elevated FBS, deteriorated bodyweight, and significant IGT compared to Con. Hepatosteatoses of varying morphologies and higher hepatic expression of G6Pase than PRPCK in ET2D versus the opposite in LT2D further confirmed the divergent nature of metabolic aberrations. At the end of 28 days, the high levels of FBS, alkaline phosphatase (ALP), triglyceride (TG), urea, hepatic protein carbonyl content (PCC), and alanine and aspartate aminotransferases (AST and ALT, respectively) persisted in untreated LT2D. CCAE ameliorated oxidative stress and upregulated PPARα expression in diabetic groups and Con; it downregulated CPT1 expression in the LT2D group. CCAE’s ability to lower FBS and serum and hepatic TG in both ET2D and LT2D indicated its ability to act via different mechanisms. Ferulic acid (Fer A) and rutin hydrate (RH) were detected in CCAE.
Conclusion
CCAE lowered the FBS in ET2D via inhibiting the hepatic G6Pase expression (glycogenolysis). In LT2D, CCAE abated sugar levels by diverting PEPCK activity, preferably towards glyceroneogenesis than gluconeogenesis. The preserved triglyceride/fatty acid (TG/FA) cycle, the upregulated PPARα, and the downregulated CPT1 gene expressions reduced serum and hepatic TG.
Similar content being viewed by others
Introduction
Type 2 diabetes (T2D), the most prevalent diabetes mellitus, is globally on the rise. The causes and risk factors of T2D are not entirely understood. The upsurge in blood glucose levels after a meal or when hungry are the early signs of diabetes. Two subtypes of T2D can be called early-stage (or mild) (ET2D) and late-stage (or severe) (analogous to uncontrolled T2D or LT2D). These diabetes subtypes can be created in laboratory animals using streptozotocin (STZ) and niacinamide (NIA) [1, 2] to compare the metabolic aberrations and to test the efficacies of purified drugs and traditional remedies.
Citrullus colocynthis (L.) Schrad (C. colocynthis) is a member of the Cucurbitaceae family and grows in arid regions of Asia, North Africa, and the Middle East, including Iran. C. colocynthis is known as Abujahl melon in Iran [3], but bitter apple, bitter watermelon, fox melon, and colocynth are also used [4]. Throughout history, humans have used the fruits, seeds, leaves, and roots of C. colocynthis to prevent and treat various illnesses, including dermatological conditions as leprosy, digestive disorders as constipation and jaundice, respiratory diseases as asthma and bronchitis, and many other ailments such as rheumatism, hypertension, mastitis, and pain [4,5,6,7]. Other biomedical applications of C. colocynthis include anti-cancer [8,9,10,11,12,13,14,15], anti-diabetic [16,17,18], anti-lipidemic [19,20,21,22], antimicrobial [23, 24] and insecticidal [25, 26]. Most recent investigations have further hinted at its preventive and healing effects on various complications of diabetes, including diabetic nephropathy [27,28,29], glycated hemoglobin [30], and deteriorated cognitive performance [31]. The seed is known to be less toxic [32] and more influential in lowering blood sugar levels [33, 34], and the aqueous extracts have better hypoglycemic effects than organic solvent extracts [35, 36]. Also, the aqueous extract would preserve the constituent phytochemicals in their most natural shapes since plants are 80% water by weight.
Many modern-day diseases are rooted in chronic oxidative stress [37, 38], which occurs when the sum of oxidants and prooxidants concentration exceeds endogenous and exogenous antioxidant concentrations [39]. The persistent overabundance of oxidants and prooxidants in the body due to a sedentary lifestyle, consumption of processed foods, overnutrition, and environmental pollution leads to their inefficient neutralization by the body’s free radical scavenging mechanisms. Under such stressful conditions, plant antioxidants may protect the body from long-term harm [40]. Like most plants, C. colocynthis is rich in secondary metabolites and antioxidants [41, 42]. However, C. colocynthis may be more critical from a pharmacological viewpoint for treating diabetes because it possesses both acute [36] and long-term sugar-lowering effects [43], and its hypoglycemic potency is comparable to metformin [44].
In the present study, phytochemical analysis and HPLC-DAD were conducted to obtain a basic idea about the quality of extract ingredients and marker compounds. The levels of fasting blood sugar (FBS), glycosylated hemoglobin (HbA1C), c-peptide, body weights, impaired glucose tolerance (IGT), and other clinical chemistry parameters were measured to define the hallmark characteristics of each disease stage as much as possible. In addition, since T2D means concomitant carbohydrate and fatty acid (FA) metabolic disorders [45], the mRNA expression levels of the rate-limiting enzymes in hepatic carbohydrate and lipid metabolic pathways were determined. Glucose 6-phosphatase (G6Pase, involved in glycogenolysis and gluconeogenesis pathways) and phosphoenolpyruvate carboxykinase (PEPCK, regulator of gluconeogenesis) were selected to study carbohydrate metabolism. Insulin-dependent sterol regulatory element-binding protein-1c (SREBP-1c, regulator of hepatic de novo lipogenesis or DNL), Acetyl-CoA carboxylase (ACC, regulator of FA synthesis and degradation), fatty acid synthase (FAS, regulator of FA synthesis), peroxisome proliferator-activated receptor alpha (PPARα, regulator of β-oxidation), and carnitine palmitoyltransferase I (CPT1, regulator of β-oxidation) genes were measured to examine the lipid metabolic pathway. The histological investigation provided insight into the morphology and structure of liver tissue, and protein carbonylation content (PCC) served as a measure of hepatic oxidative stress.
The present study aimed to find the phytochemical characteristics and marker compounds in CCAE, to see how diverse the metabolic aberrations in ET2D and LT2D can be concerning each other and concerning the nondiabetic state, and to see if CCAE has similar mechanistic actions in ET2D and LT2D, or would it have differentiating impacts and benefit a particular stage of diabetes better than the other.
Materials and methods
Chemicals and reagents
Standards and chemicals used in this study, including gallic acid (GA), cinnamic acid (Cin A), ferulic acid (Fer A), gentistic acid, p-coumaric acid, chicoric acid (Chi A), caffeic acid, chlorogenic acid (Chl A), quercetin, rutin hydrate (RH), catechin, Folin-Ciocalteu reagent (FGR), 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•), aluminum chloride hexahydrate, sodium nitrite, ferric chloride, ketamine, xylazine, STZ, and NIA were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO, USA). The ascorbic acid and vanillic acid (Van A) were bought from Merck (Hohenbrunn, Germany), and analytical grade hydrochloric acid, sulfuric acid, and glacial acetic acid from Merck (Darmstadt, Germany). Gradient grade acetonitrile was purchased from Merck (Darmstadt, Germany) and Dr. Mojallali Industrial Chemical Complex Co. (Tehran, Iran). Ultragradient methanol was obtained from Carlo Erba Reagents (Val de Reuil Cedex, Italy). All solvents were filtered through 0.45 filters before use.
Preparation of C. colocynthis seed extract (CCAE)
Bitter apple fruits were collected from the Salehabad District, in Mehran County, Ilam Province, Iran (latitude: 33°28′11″N, longitude: 46°11′17″E, Altitude: 136 m above sea level) and authenticated by Dr. Amin, a botanist of the Faculty of Pharmacy, Tehran University of Medical Sciences (TUMS). A voucher sample of the seeds (PMP-648) was deposited in the herbarium of the Faculty of Pharmacy, TUMS. The seeds were washed and air-dried under shade before pulverization using an electric mill. CCAE was prepared by soaking 100 g of the seed powder in 1 L of distilled water (100 g/L) and refluxing for 20 min in a water bath (80 °C) [46]. The vacuum filtered extract was concentrated by a rotary evaporator at 80 °C to facilitate its freeze-drying process (EYELA Freeze dryer FD-1, Japan). Based on our definition of the average extract yield as the amount of freeze-dried extract (grams) obtained per 100 g of powdered seeds, the average yield of lyophilized powder from three separate batches was 2.06 ± 0.11 g/L, with a percent yield of about 2%.
Phytochemical analysis
Determination of total phenolic (TP) content
The TP content in CCAE was measured using FCR [47]. Briefly, 900 μL of FCR (diluted 1:10 with distilled water) was added to 100 μL from various concentrations of CCAE and shacked vigorously. To the mixture, 750 μL of 7% NaHCO3 solution was added, followed by mixing. After incubation at room temperature for 90 min, 200 μL aliquots were transferred to 96-well plates, and the absorbance was measured at 725 nm using a microplate reader (BioTek Instruments, VT, USA). GA standard curve (0–0.125 mg/mL) was used to quantify TP content which we defined as the amount of total phenolic compounds (milligram) in 1 g of freeze-dried extract and expressed using the following equation: GAE (mg/g Freeze-dried extract = GA (mg/mL)/sample (g/mL).
Total flavonoid (TF) content
We determined the total flavonoid content of CCAE according to [48]. Briefly, 5 min after mixing 250 μL of the extract solution with 75 μL of 5% NaNO2, 150 μL of 10% AlCL3 solution, and then after another 5 min, 500 μL of 1 M NaOH were added, and the final volume was adjusted to 2.5 mL with deionized water. Absorbance was measured at 510 nm (BioTek Instruments, VT, USA). Total flavonoid contents were calculated using a calibration curve for (+)catechin (0–1.25 mg/mL). The amount of TF was reported as (+)catechin equivalents (CE, mg (+)catechin/1 g freeze-dried extract).
DPPH free radical scavenging activity
DPPH, which is a free radical, forms a purple solution whose color gradually disappears when exposed to free radical scavenging compounds such as plant extracts. This feature of a 0.4 mM DPPH solution in methanol was used to measure the free-radical scavenging activity of CCAE [49]. Different dilutions of GA (0–0.125 mg/mL) were prepared; 50 μL of CCAE and each standard was added to separate wells of a 96-well plate in quadruplicate, followed by the addition of 150 μL of DPPH solution. After one hour of incubation at room temperature in the dark, the absorbance was recorded at 515 nm. GA standard curve was used as a positive control to calculate the half-maximal (50%) inhibitory concentration (IC50) for GA using % inhibition values graphically. The percent scavenging effect of CCAE was calculated using the following equation: % scavenging effect = (“A Blank” - “A CCAE”)/"A Blank” × 100. “A Blank” implied the absorbance of the control (containing all reagents except the extract). IC50 for CCAE was compared to that of GA.
Frap assay (ferric ion reducing antioxidant power)
Briefly, from the sample (5 mg/mL) and GA standard (0–2.5 mg/mL) solutions, 10 μL was added to a 96-well plate with subsequent addition of 190 μL of freshly prepared FRAP reagent (mixture of 10 mM TPTZ solution in 40 mM HCl, 300 mM acetate buffer, pH 3.6, and 20 mM FeCl3 solution, in 1:10:1 ratio). After 30-min incubation in the dark, the absorbance was recorded at 593 nm [47]. The Frap capacity of CCAE was determined according to the GA standard curve.
HPLC analysis of CCAE
Sample hydrolysis
A sample of CCAE (0.2 g) was dissolved in 10 mL of 50% aqueous methanol solution containing 1.2 M HCl and 0.04% (w/v) ascorbic acid. The hydrolysis was performed at 80 °C under reflux for two h. The supernatant obtained from the hydrolysis was cooled and stored at − 20 °C [50].
HPLC-DAD
A sample (4 mg) of CCAE and each standard dissolved in DMSO and the supernatant obtained from the hydrolysis step above were filtered (0.45 μm, 13 mm syringe filter, Waters, USA). Five microliter samples were injected into a Knauer analytical HPLC (PLATIN blue, Knauer, Berlin, Germany) equipped with a Rheodyne injector system and a variable wavelength spectrometric detector (Knauer PDA-1). The separation was accomplished on a Nucleosil column (RP-C18, 25 × 0.46 cm; 5 μm) at 0.8 mL/min flow rate and 280 nm at room temperature (30 °C). The mobile phase comprising deionized water (with 0.5% glacial acetic acid, A) and HPLC grade acetonitrile/methanol (70:30, B) was applied as follows: 10–15% (0 to 5 min); 15–20% (5 to 18 min); 20–70% (18 to 40 min), kept at 70% (40 to 45 min); 70–10% (45 to 50 min), and kept at 10% (50 to 55 min) [50]. The PDA was operated to give the spectra from 200 to 500 nm. The standard compounds were used to build a homemade library. Data acquisition, processing, and control of chromatographs were performed using EZChrom Elite 3.3.2 software (Knauer, Berlin, Germany). GA, RH, Fer A, and Chl A linear range (LR) calibration curves were used to calculate their limit of detection (LOD), their limit of quantitation (LOQ), and their recoveries using quality control (QC) samples.
Animals
Seven-week-old male Wistar-Albino rats (average mass of 100 to 160 g) were purchased from the School of Public Health, TUMS. The animals were housed in standard and clean cages (2 per cage) under controlled environmental conditions at room temperature 22 ± 2 °C and 12-h light-dark cycle with access to standard rat chow and water ad libitum. Animal handling and treatment were performed at the Clinical Biochemistry Department of the School of Medicine of Tehran University of Medical Sciences (TUMS) according to the guidelines for laboratory animals. The animal experiments were approved by the ethical committee of Tehran University of Medical Sciences under ethical code number IR.TUMS.MEDICINE.REC.1396.2639.3.
Induction of early- and late-stage diabetes in rats
Following several acclimatization days, we placed the rats with the closest body weights in each group to obtain low standard deviations for the average weights. As explained elsewhere, the rats with the lowest body weight were assigned to nondiabetic control rats (Con) [51]. After an overnight (8 h) fasting period, diabetes was induced following the procedure reported previously [46, 51]. Briefly, rats destined for LT2D were IP injected with STZ alone (55 mg/kg of body weight in experiment I and 65 mg/kg in experiment II, as explained below), and those destined for ET2D groups were injected with STZ (the same concentrations as above) and NIA (200 mg/kg, injected 15 min after STZ administration). STZ and NIA were freshly prepared in 0.3 mL cold normal saline as a vehicle; IP injections were delivered using insulin syringes. Diabetes was confirmed by measuring glucose levels in peripheral blood obtained from the tail vein on days 4 and 10 after diabetes induction (GlucoSure STAR, ApexBio, Taiwan). The rats in Con were injected with the vehicle. STZ-injected rats with fasting blood sugar (FBS) above 300 mg/dl on both occasions (day-4 and day-10) were classified as LT2D rats, and NIA/STZ-injected rats with stable FBS ranging between 140 and 220 mg/dl were regarded as ET2D, as explained elsewhere [51]. Rats were induced with diabetes for two purposes, as mentioned in the following.
Experiment I. To select CCAE dosage by intraperitoneal glucose tolerance test (IPGTT)
When LT2D and ET2D were induced in two groups of rats (n = 10; STZ, 55 mg/kg; NIA, same as above), 3 of the STZ-injected rats and 7 of the NIA/STZ-injected ones developed the corresponding types of diabetes, respectively. After measuring FBS in the fasting rats, IPGTT was performed in two representative diabetic rats in each group as follows: glucose (2 g/kg dissolved in ~ 0.3 mL of normal saline) mixed with one of the two nontoxic doses of CCAE (90 mg/kg or 110 mg/kg) was IP injected [18, 34, 52] and blood sugar was recorded at 15 min intervals in the tail blood. This experiment aimed to select the CCAE dosage, but it also showed the acute or short-term hypoglycemic effect of CCAE. By using metformin instead of CCAE, we tried to compare the potency of CCAE versus one dose of metformin (100 mg/kg [53]) (Supplement 1, Fig. S1A).
Experiment II. To study subtle metabolic differences between ET2D and LT2D and to investigate the effects of daily usage of CCAE on sugar and fat metabolism during 28 days
A total of 68 rats were allocated into three groups of Con (n = 10), LT2D (n = 40), and ET2D (n = 20). After the induction of ET2D and LT2D (STZ, 65 mg/kg; NIA, as above), 14 of 40 (35%) STZ-injected rats and 16 of 20 (80%) NIA/STZ-injected rats developed LT2D and ET2D, respectively. Rats that did not become diabetic (n = 28) were injected with a second dose of STZ (55 mg/kg), of which six died, eight did not develop diabetes, and the remaining fourteen that developed LT2D (FBS above 300 mg/dl on days 4 and 10) were distributed in the STZ (6) and CCAE-STZ (8) groups (Supplement 1, Fig. S1B, shows the study groups). The methods explained in the following paragraphs concern the groups of animals in this section (Experiment II).
Evaluation of IGT by IPGTT
IPGTT was performed similarly as above, using only glucose (without added drugs) to evaluate glucose tolerance status in LT2D and ET2D groups. As a significant degree of IGT is predictable in severe diabetes and to prevent the loss of rats from this group, IPGTT was conducted on three representative rats from the LT2D groups. However, all the rats from ET2D groups were subjected to IPGTT to see if modest FBS increases could lead to significant IGT. Four out of 20 NIA/STZ-injected (ET2D) rats that had normal blood sugar at the end of IPGTT were excluded from the study (Supplement 1, Fig. S1C). It took 5 days to perform IPGTT on all the ET2D rats since the procedure required overnight fasting. These 5 days plus the previous 10 days spent for the induction of diabetes and its approval added up to a total of 15 days. The area under the curve (AUC) was calculated using the GraphPad Prism software.
Treatment with CCAE for 28 days (the ultimate study groups)
Fifteen days after induction of diabetes, we started the CCAE treatment with the selected dose of CCAE for half of the rats in each of the main groups. The treatment proceeded for 28 days. At the end of the study, the six ultimate groups of rats were: Con (n = 4), CCAE-Con (n = 4), NIA/STZ (n = 8), CCAE-NIA/STZ (n = 8), STZ (n = 12), CCAE-STZ (n = 14, alive on day 28) (Supplement 1, Fig. S1B).
Measuring weekly FBS and bodyweights
At the end of each week, we measured FBS and body weights. The amount (mg) of CCAE for the rats’ daily injections was determined according to their weekly body weights. Rats in Con were injected with an equal volume of the vehicle.
Blood and liver tissue sample collection
After 28 days of treatment, blood (4–5 mL) was collected from the heart under anesthesia by ketamine (87 mg ketamine/kg) and xylazine (13 mg xylazine/kg). A sample (2.5 mL) of the collected blood from each animal was transferred into tubes containing ethylenediaminetetraacetic acid (EDTA, 3 mg/mL) for HbA1c determination, and sera were prepared from the remaining blood samples. After dissection, liver tissues were frozen in liquid nitrogen. All samples were stored at − 80 °C until analysis.
Fasting blood chemistry
C-peptide concentration and percent HbA1c were determined using Human/Rat/Mice c-peptide ELISA (BioVision, America) and hemoglobin A1C (Biosystem, Spain) kits. The concentrations of AST, ALT, ALP, urea, creatinine, TG, cholesterol, LDL, and HDL were measured at the Diabetes Center of Shariati Hospital, Tehran, Iran, using the respective kits (Human diagnostics).
Real-time PCR
Total RNA was extracted from frozen liver tissue and purified using Hybrid-R Blood RNA (GeneAll, South Korea) and RNA Riboclear (GeneAll, South Korea) kits. The quantity and quality of extracted RNA were inspected by spectroscopic absorption at 260 nm and electrophoresis. Reverse transcription (RT) was performed with the Revert Aid First Strand cDNA Synthesis kit (Takara, Japan). Real-time PCR was performed with Rotor-Gene Q (Qiagen, Germany) using SYBR Premix Ex Taq (Takara, Japan). Primer sequences used in this study are listed in Table 1. The relative changes in gene expression were determined using the ΔCt method and normalized against the housekeeping β-actin expression.
Protein and protein carbonyl content (PCC) determination
Frozen liver samples (300 mg) were homogenized in ice-cold PBS buffer (3 mL, pH 7.4) containing phenylmethylsulfonyl fluoride (PMSF) (5 mM) and an anti-protease cocktail. After centrifugation at 1000×g at 4 °C to remove cell debris, the supernatants were collected, and aliquots were stored at − 80 °C until use to measure protein concentration by BCA Protein Quantification kit (Pars-Tous, Iran) [54] and PCC by 2,4-dinitrophenylhydrazine (DNPH) reagent [55].
Hepatic TG measurement
A small piece of each frozen liver (~ 150 mg) was homogenized in 3 mL of chloroform/methanol (2:1). The hepatic lipids were extracted using the method suggested by Folch et al. [56]. TG content was measured using a colorimetric-enzymatic kit (Parsazmun, Iran) and expressed as mg/g tissue.
Liver histology
For hematoxylin and eosin (H&E) staining, two representative samples from frozen liver tissues were fixed in 10% formalin and embedded in paraffin. After cutting into 5 mm thickness, the samples were stained with H&E. Two other samples from each group were cryostat sectioned, and after immersing in 60% isopropanol for 5 min, stained with freshly filtered Oil Red O solution for 45 min at room temperature. After washing the sections with 60% isopropanol and then distilled water, the cryosections were stained with hematoxylin and examined under an optical microscope.
Statistical analysis
Statistical analyses were performed using ordinary one-way ANOVA or non-parametric test followed by the Kruskal-Wallis test in the GraphPad Prism software, version 8.0. Results are presented as mean ± SEM unless otherwise stated. A statistical significance was defined as p < 0.05.
Results
Phytochemical analysis of the extract (CCAE)
Representative HPLC chromatograms before and after the HCl-hydrolysis of CCAE, the PDA-UV spectra of the prominent peaks, the name(s) of the component(s) with the most analogous UV spectrum to every peak, and the degree of similarity (in brackets) are presented in Fig. 1A and B. According to a library search against UVTOX library (Knauer, Germany) and a homemade library, RH, Fer A, Chl A, GA, Chi A, Van A, and quercetin appeared to be the significant possible marker compounds in CCAE. Upon mild HCl-hydrolysis, as described in the Methods section, three new peaks (peaks 4, 5, and 6) emerged, displaying UV spectra similar to canrenoic acid (an aldosterone antagonist), formestane (a synthetic aromatase inhibitor), and Cin A. From among the compounds suggested by library search, the presence of only Fer A (peak 7) and RH (peak 10) could be confirmed via spiking, and the other suggested standard compounds were not present in CCAE (Supplement 2, Fig. S2–1 - Fig. S2–3).
Typical HPLC chromatogram recorded at 280 nm of CCAE with UV spectra of CCAE before (A) and after (B) mild hydrolysis. The CCAE contained 7 corresponding to ferulic acid (0.81 ± 0.48 mg/g), and 10 corresponding to rutin hydrate (17.67 ± 1.66 mg/g). GA, gallic acid; RH, rutin hydrate; Fer A, ferulic acid; Chl A, chlorogenic acid; SA, sorbic acid; Van A, vanillic acid; Canrenoic A, canrenoic acid; Cin A, Cinnamic acid; Chi A, chicoric acid; Oxa C, oxabolone cypionate
The estimated concentrations of FerA and RH and the results of the phytochemical analysis are summarized in Table 2. The scavenging effect of CCAE on the DPPH radical was 50% at a concentration of 4.36 mg/mL compared to the scavenging effects of GA at 0.016 mg/mL. Due to being at levels lower than its LOQs, the concentration of peak 7 assigned to Fer A was determined using the peak area of a single standard concentration at around the CCAE peak area for that compound using the formula: Marker compound content in mg = Mass in mg of the standard × Peak area of the sample (CCAE)/Peak area of the standard.
Experiment 1: selection of CCAE dosage using IPGTT
According to IPGTT results, CCAE had a prompt (short-term) hypoglycemic effect in ET2D but not in LT2D; and the hypoglycemic potency of 90 mg/kg of CCAE was comparable to 100 mg/kg of metformin consistent with Mojaz Dalfardi et al. [44] (Supplement 1, Fig. S1A). We chose this dosage (90 mg/kg of CCAE) for the daily treatment of rats during the 28-day experiment.
Experiment II. To show differences in metabolic disarray between ET2D and LT2D and to investigate the differential effects of daily usage of CCAE on sugar and fat metabolism after 28 days
Evaluation of IGT by IPGTT
Both groups of rats, ET2D and LT2D, suffered significant IGT as indicated by their higher AUCs than Con; IGT was significantly higher in LT2D than ET2D as expected (Supplement 1, Fig. S1C, Table S1).
Weekly FBS and body weights
The average FBS levels were increased in both diabetic models (NIA/STZ group, 133.8 ± 7.9 mg/dl, p = 0.004; STZ group, 453.0 ± 41.7 mg/dl, p = 0.001; compared to Con, 95 mg/dl ± 5.5). FBS levels were lowered by CCAE to near-control levels in both diabetic groups (p > 0.05 vs. Con) (Fig. 2), showing that CCAE exerted chronic (long-term) sugar-lowering effects in both ET2D and LT2D. CCAE did not change the FBS in the nondiabetic control group (CCAE-Con), consistent with Lahfa et al. [36] but against Abdel-Hassan et al. [57].
Weekly FBS and body weights. After diabetes induction (i.e., on day zero), FBS increased ~ 0.44 (44%) and ~ 3.5 (352%) times in NIA/STZ and STZ groups, respectively. By day 28, CCAE had lowered the FBS in both early (CCAE-NIA/STZ group, 133.8 ± 7.9 mg/dl, p = 0.004 vs. NIA/STZ) and late (CCAE-STZ group, 453.0 ± 41.7 mg/dl, p = 0.001 vs. STZ) diabetes groups to a value very close to Con (p = 0.23 for NIA/STZ group and p = 0.10 for STZ group, vs. Con), a demonstration of the chronic or long-term hypoglycemic effect of CCAE in both early- and late-stage diabetes. On day 28, the body weights of the treated groups were similar to those of corresponding controls (Con vs. CCAE-Con, p = 1.0; NIA/STZ vs. CCAE-NIA/STZ, p = 0.72; STZ vs. NIA/STZ, p = 0.69). Day “-15” indicates the starting day of diabetes induction by STZ or NIA/STZ injection, and day 0 marks the start of daily treatment with CCAE that lasted for 28 days. Con: nondiabetic control, CCAE-Con: nondiabetic control treated with CCAE, NIA/STZ: early-stage diabetic group, CCAE-NIA/STZ: the early-stage diabetic group treated with CCAE, STZ: late-stage diabetic group, CCAE-STZ: the late-stage diabetic group treated with CCAE
Body weights of rats in the Con and CCAE-Con, as well as ET2D groups (NIA/STZ and CCAE-NIA/STZ), increased significantly during the study time (day 28 vs. day 0: Con, p = 0.027; CCAE-Con, p = 0.062; NIA/STZ, p = 0.005; and CCAE-NIA/STZ, p = 0.003) due to normal growing up. The weight loss in LT2D rats after diabetes induction (p < 0.001, day 0 vs. day − 15) continued further in the CCAE-treated groups (day 28 vs. day 0; CCAE-STZ, p < 0.001 and STZ, p = 0.538). Nevertheless, on day 28, the body weights of the treated groups (CCAE-ET2D vs. ET2D and CCAE-LT2D vs. LT2D) were not significantly different from their corresponding controls (Fig. 2).
Serum c-peptide levels on day 28
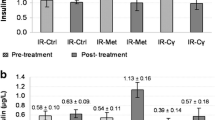
The lower-than-control levels of c-peptide in ET2D (NIA/STZ) and its complete depletion in LT2D (STZ) groups indicated limited and complete destruction, respectively, of pancreatic beta cells in these rats (Con vs. STZ, p = 0.047). Treatment with 90 mg/kg CCAE for 28 days did not change the status of insulin concentration in the treated groups (Fig. 3).
C-peptide and HbA1c levels on day 28. C-peptide levels indicate total or partial destruction of pancreatic β-cells in STZ- and NIA/STZ- rat groups, respectively, and that treatment with CCAE for 28 days did not increase insulin secretion remaining β-cells. HbA1C levels increased in diabetic groups; 28 days of treatment with CCAE did not produce any change in HbA1C levels of treated diabetic groups relative to untreated counterparts. Con: nondiabetic control, CCAE-Con: nondiabetic control treated with CCAE, NIA/STZ: early-stage diabetic group, CCAE-NIA/STZ: the early-stage diabetic group treated with CCAE, STZ: late-stage diabetic group, CCAE-STZ: the late-stage diabetic group treated with CCAE. *, p < 0.05; †, p = 0.001. #, p < 0.05 and ##, p < 0.01 show significant differences between the diabetic group (STZ or NIA/STZ, respectively) versus Con
Blood HbA1c levels on day 28
The HbA1C levels in the STZ group were higher than those in the NIA/STZ group at the end of the experiment (Fig. 3), as rats in the STZ group were exposed to significantly higher glucose levels than those in the NIA/STZ group during the same period (28 days + 5 days after confirmation of diabetes = 33 days). However, the reduction of HbA1c percentage in the CCAE-treated groups was insignificant relative to untreated counterparts (Fig. 3).
Fasting blood analysis on day 28
Lowering the serum TG levels was the most significant biochemical effect of CCAE in both diabetic models. TG levels rose in the sera of diabetic rats (NIA/STZ group vs. Con: p = 0.04) in agreement with some reports [58, 59]; then significantly decreased towards normal in the CCAE-treated groups (p = 1.0, treated groups vs. Con) consistent with others [60]. Concerning cholesterol, our results showed no significant difference between all groups (p = 0.9) in contrast to [61]. Other biochemical markers changed predominantly in the LT2D rats (STZ vs. Con: urea, p = 0.005; uric acid, p < 0.01; AST, p < 0.05; ALP, p < 0.01, ALT, p < 0.01) and CCAE treatment improved some of the biomarkers (Table 3).
Hepatic PEPCK and G6Pase gene expression on day 28
ET2D induction led to a ~ 5-fold increase in the mRNA levels of G6Pase and a slight increase in mRNA levels of PEPCK in the NIA/STZ-group (Con vs. NIA/STZ, p = 0.005 for G6Pase and p = 0.987 for PRPCK). On the contrary, we noticed a ~ 12-fold increase in the mRNA levels of PEPCK in the STZ-group with only a slight rise in G6Pase mRNA levels (Con vs. STZ, p = 0.981 for G6Pase and p = 0.023 for PEPCK) (Fig. 4). Treatment with CCAE caused suppression of G6Pase expression in ET2D. However, the suppression of PEPCK expression by CCAE in LT2D was not statistically significant. A previous study has reported similar results on the suppression of hepatic G6Pase, and fructose 1, 6-bisphosphatase enzymatic activities (not mRNA expressions) in alloxan-induced rats by a leaf suspension of C. colocynthis [62], but interestingly the inhibition of PEPCK activity or its mRNA expression by C. colocynthis has not been reported.
Hepatic PEPCK and G6Pase gene expression on day 28. After diabetes induction, there was a significant (~ 5 fold) rise in the mRNA levels of G6Pase (p = 0.005) and a slight insignificant rise of PEPCK in ET2D (NIA/STZ group, p = 0.987); treatment with CCAE (for 28 days) resulted in a significant decrease in the expression of G6Pase (p = 0.003). In LT2D, the expression of PEPCK increased significantly (~ 12 folds) after induction of diabetes (p = 0.023) and did not decrease significantly after CCAE treatment (p = 0.072). Con: nondiabetic control, CCAE-Con: nondiabetic control treated with CCAE, NIA/STZ: early-stage diabetic group, CCAE-NIA/STZ: the early-stage diabetic group treated with CCAE, STZ: late-stage diabetic group, CCAE-STZ: the late-stage diabetic group treated with CCAE. We used non-parametric tests to compare the variables between independent samples, followed by Mann-Whitney Test. We presented the data as the mean ± SEM. **, p < 0.01; ***, p < 0.001. Every bar is the average of 2 duplicate analyses for all rats of all groups
Hepatic mRNA expression of SREBP-1c, ACC, FAS, PPARa, and CPT1 on day 28
On day 28, the mRNA expression levels of SREBP-1c, ACC, FAS, and PPARα were significantly low in diabetic rats (STZ and NIA/STZ groups vs. Con, p < 0.05) (Fig. 5), consistent with previous reports [63, 64]. Treatment with CCAE normalized the mRNA levels of PPARα in both CCAE-STZ and CCAE-NIA/STZ groups (Fig. 5).
Hepatic mRNA expression of SREBP-1c, ACC, FAS, PPARα, and CPT1 on day 28. Con: nondiabetic control, CCAE-Con: nondiabetic control treated with CCAE, NIA/STZ: early-stage diabetic group, CCAE-NIA/STZ: the early-stage diabetic group treated with CCAE, STZ: late-stage diabetic group, CCAE-STZ: the late-stage diabetic group treated with CCAE. *p < 0.05; ** p < 0.01; *** p < 0.001. We have presented the data as the mean ± SEM. #, p < 0.05 and ##, p < 0.01 show significant differences between the diabetic group (STZ or NIA/STZ, respectively) versus Con
In line with our results, low ACC activity (not mRNA expression) was detected by Jeyanthi et al. [20] in the sera of alloxan-induced diabetes, which was found to rise, in contrast to our results, after treatment with C. colocynthis. The mRNA levels of CPT1 increased in the diabetic groups (1.5 and 2.0 folds in ET2D and LT2D, respectively). Treatment with CCAE lowered CPT1 mRNA levels back to near control values in LT2D rats (STZ group vs. CCAE-STZ, p < 0.001) (Fig. 5).
Hepatic PCC on day 28
PCC levels decreased significantly upon treatment with CCAE, although, contrary to Cumaoglu et al. [65], its increase due to diabetes was insignificant in ET2D and LT2D groups compared to Con (Fig. 6).
Effect of CCAE on hepatic protein carbonylation and triglyceride levels. We observed increased levels of PCC (LT2D and ET2D vs. Con, p = 0.73 and p = 0.19, respectively) and TG in the liver tissue of both diabetic groups compared to Con. Treatment with CCAE led to a significant reduction of both variables in all treated groups compared to their untreated controls. Con: nondiabetic control, CCAE-Con: nondiabetic control treated with CCAE, NIA/STZ: early-stage diabetic group, CCAE-NIA/STZ: the early-stage diabetic group treated with CCAE, STZ: late-stage diabetic group, CCAE-STZ: the late-stage diabetic group treated with CCAE. we have expressed the values as mean ± SEM. *, p = 0.016; **, p = 0.008, †, p = 0.029. #, p < 0.05 and ##, p < 0.01 show significant differences between the diabetic group (STZ or NIA/STZ, respectively) versus Con
Hepatic TG concentration on day 28
The measurement of hepatic TG concentration (Fig. 6) as well as H&E and Oil-Red stained liver tissues (Fig. 7) showed high levels of fat (fatty liver) in the untreated diabetic groups compared to Con parallel to TG levels in serum (Fig. 6). Unlike our results, some researchers noted a reduction of TG in the liver of STZ-induced rats, which they attributed to increased hepatic β-oxidation [66] or declined rates of lipogenesis [63, 67]; yet others reported no change in the hepatic lipid content [68]. There was a significant decrease in liver TG after treatment with CCAE (Fig. 6).
Effect of CCAE on liver histology. Liver histology was studied using H&E (200 ×) and Oil Red O staining. According to H&E, large macrovesicular LDs accumulated in the liver of early-stage T2D; CCAE treatment eliminated the large LDs. Lipid in the LT2D liver was not noticeable, perhaps due to its different (microvesicular) morphology. Fibrosis of the portal area and fibroblast accumulation in the liver were other remarkable manifestations in the late-stage diabetic liver tissues, which did not seem to improve by CCAE treatment. Oil Red O staining approved the greater extent of fat accumulation in diabetic livers and the capacity of CCAE to decrease hepatic fat
Liver histology on day 28
H&E staining showed large lipid droplets (LDs) in the liver tissues of NIA/STZ but not those of the STZ group (Fig. 7). Congested central vein, dilatation of hepatic sinusoids (Fig. 7), fibrosis (Supplement 3), and excessive glycogen accumulation (Supplement 4) were complications detected in livers of LT2D rats. Treatment with CCAE reduced hepatic lipid load in CCAE-NIA/STZ and CCAE-STZ groups (Fig. 7) in agreement with Khalil et al. [69]; but, in contrast to Oryan et al., the structural damages to the liver tissue did not improve by C. colocynthis in the present study in CCAE-STZ [70].
Discussion
Due to the growing popularity of plant-based medicines and herbal treatments, various medicinal plants are under intense scientific investigation to confirm their efficacy in the treatment of diabetes and prevention of its complications, for elucidation of their constituent compounds and mechanism of action, and assessment of drug quality and standardization. To our knowledge, this is the first study that compares the hypoglycemic and hypolipidemic effects of C. colocynthis in the early- and late- stages of diabetes. A concise list of experiments and their results are summarized in Table 4 to facilitate the perception of the pattern of the significant changes in one glance.
T2D is a baffling, gradually aggravating disease that is diagnosed many years after initiation. Our results in Fig. 2 and Supplement 1, Fig. S1C verify the existence of an undeniable load of IGT during the prediabetes period when patients and their physicians are unaware of the condition, which can explain the actual presence of complications at the time of diagnosis.
CCAE’s short-term hypoglycemic effect in ET2D (Supplement 1, Fig. S1A) was consistent with Lahfa et al. [36]. The long-term hypoglycemic effect of CCAE in LT2D was in line with Benariba et al. [71] with the FBS levels in CCAE-treated groups at the end of 28 days, almost fitting the time frame mentioned by Benmehdi et al. regarding the action of saponosides isolated from C. colocynthis seeds in STZ-diabetic rats [43].
CCAE did not hinder an increase of body weight during growth in CCAE-Con and CCAE-ET2D. Nevertheless, the CCAE-STZ group experienced a more significant weight loss than the untreated STZ group in disagreement with Benariba et al., who observed prevention of weight loss by C. colocynthis extract [72] and with Ahangarpour and Oroojan, who reported a weight increase in fructose-induced insulin-resistant rat models [32]. At the end of the study, the body weights of the treated groups were not significantly different from their corresponding controls (Fig. 2), perhaps because the dosage of 90 mg/kg of CCAE was a sub-toxic dose. Various studies have suggested that high doses of C. colocynthis can lead to significant loss of weight despite or as a result of its toxic side effects [73].
T2D is commonly associated with elevated HbA1c, increased serum and hepatic TG levels, altered liver enzymes, and a progressive failure of pancreatic beta cells to produce insulin. Due to a longer half-life in blood than insulin, c-peptide may be a more reliable indicator of beta-cell status [74]. Although the correlation between c-peptide and insulin concentrations is not linear [75], the pattern of c-peptide levels in the untreated ET2D and LT2D groups closely matched that of insulin when measured directly [51]. The results suggested that treatment with 90 mg/kg of CCAE for 28 days was insufficient to approve the plant’s previously reported insulinotropic effect [76,77,78,79] (Fig. 3); instead, an insulin-enhancing activity leading to a promotion of peripheral (adipose and muscle) tissue glucose uptake has been proposed recently [80].
In contrast to the reported potential of C. colocynthis extracts to diminish the formation of glycated hemoglobin [30, 81, 82], the decrease of HbA1c percentage in the CCAE-treated groups compared to their untreated counterparts was insignificant in the present study (Fig. 3).
Treatment with CCAE improved the biomarkers of liver function [83, 84] and significantly reduced serum and liver TG levels in both diabetic models, in agreement with several reports [20,21,22, 28, 60, 61, 85, 86] (Table 3).
Two of the three objectives of the present study were to investigate, in the liver tissue of ET2D and LT2D rats, 1) the stereotype sugar and fat metabolic disarrangements and 2) the mechanisms of hypoglycemic-hypolipidemic activities of CCAE. Concerning the first, we noted the differential expressions of gluconeogenic enzymes, G6Pase and PEPCK (Fig. 4), and the presence of architecturally distinctive fatty liver in ET2D and LT2D (Fig. 7).
Architectural differences in hepatic fat implied that ET2D rats had macrovesicular steatosis (large LDs that displace the nucleus), whereas LT2D rats suffered from microvesicular steatosis (small LDs with preserved cellular architecture) [87].
The buildup of large LDs in the ET2D liver is because the conditions required for some remarkable amount of DNL are available; i.e., insulin is present - even if its concentration is only 40% of Con, and the hepatic expression of SREBP-1c and FAS are less inhibited (ET2D compared to LT2D) (blue boxes in Fig. 8). It appears that macrovesicular LDs grow during enhanced local TG synthesis [89] because the increased glucose uptake into hepatocytes due to hyperglycemia-induced GLUT2 expression and the activation of SREBP-1c by insulin would give rise to DNL [90]. The newly synthesized hepatic fat and fat absorbed from food, called “new fat,” is expected to act as a PPARα agonist and promote FA β-oxidation leading to the prevention of fat accumulation [91]. Meanwhile, though, the ongoing production of malonyl CoA by the remaining ACC activity (Fig. 5) and its inhibition of CPT1 (Fig. 5) and, therefore, its prevention of FA entrance into the mitochondria for β-oxidation caused an accumulation of macrovesicular type LD in the liver of ET2D rats [92, 93]. Further activation of PPARα by CCAE without affecting CPT1 expression can explain the anti-fatty liver activity of CCAE in ET2D (Fig. 7).
The effect of CCAE on the course of events in ET2D (blue boxes) and LT2D (black boxes). Low FA β-oxidation/disposal may give rise to the fatty liver when accompanied by increased DNL, as it may occur in ET2D. Enhanced β-oxidation of FAs is expected to prevent fatty liver unless the rate of FA entry into the liver overwhelms the capacity of the mitochondria, as it may occur in LT2D, when it may lead to mitochondrial dysfunction and enhanced ROS production. Meanwhile, the attenuation of free radical scavenging mechanisms would lead to fatty liver, oxidative stress, fibrosis, and liver damage. CCAE was able to fight against hyperglycemia and fatty liver in many frontlines, including free radical scavenging and lowering ROS levels, inhibiting CPT1, activating the expression of PPARα, and directing PEPCK activity more towards glyceroneogenesis than towards gluconeogenesis. Another vital function of PEPCK, not shown here, is to carry out cataplerosis, the removal of citric acid cycle intermediates to prevent their accumulation in the mitochondrial matrix [66, 88]
PPARα upregulates fatty acid oxidative enzymes in the liver and skeletal muscle [94]. The reduction in PPARα mRNA levels in the diabetic liver, in this study, was in contrast to some reports [95,96,97] but consistent with other reports that attributed its decrease to such circumstances as a mitochondrial failure and oxidative stress [64], hyperglycemia [98], and low lipoprotein lipase (LpL) activity in the skeletal muscle [66]. The discrepancy may be related to the primary root cause of diabetes and whether it is due to obesity (high-fat-diet-induced diabetes) [99] or beta-cell failure (STZ-induced diabetes) [98, 100, 101] at the onset. Nevertheless, activation of hepatic expression of PPARα by agonists as fenofibrate and berberine is commonly regarded as a remedy for hypertriglyceridemia and hyperglycemia and is known to ameliorate markers of diabetes, such as hepatic insulin resistance and steatosis [91, 94, 101,102,103,104,105].
The arrangement of microvesicular LD in the liver of LT2D rats may be the net effect of the high inflow of old fat and a disproportionate presence of oxidative stress [92, 93]. “Old fat” tends to accumulate in the liver as it is unable to activate PPARα and, therefore, mitochondrial β-oxidation (black boxes in Fig. 8) [66, 91]. Meanwhile, increased FA flow into the mitochondria of hepatocytes due to a more prominent up-regulation in LT2D of CPT1 expression (Fig. 5) overwhelms the mitochondrial β-oxidation; and activates the peroxisomal β-oxidation and cytochrome P450-dependent ω-oxidation in the ER leading to ROS formation, lipid peroxidation, oxidative stress, inflammation, fibroblast accumulation, fibrosis, and liver injury, as seen in the histology of liver tissue in LT2D rats (Fig. 7) [64, 106] and as evidenced by the high levels of ROS (PCC) in LT2D liver (Fig. 6). The up-regulation of CPT1 expression in diabetes arises from the diminished malonyl-CoA concentration and elimination of its inhibitory effect on CPT1 activity [58, 67, 68]. The function of CPT1 is to mediate the transfer of FAs into mitochondria from the cytosol. The presence of higher levels of CPT1 in STZ-rats implied a greater level of hepatic β-oxidation compared to NIA/STZ-rats as manifested, in the present study, by a 2.5 times larger ratio of β-oxidation to lipogenesis (considered as CPT1: SREBP1c ratio) in LT2D (STZ group) compared to ET2D (NIA/STZ group) (Fig. 5). Therefore, the simultaneous presence of β-oxidation and ROS formation may lead to microvesicular LD in LT2D (Fig. 8). Accordingly, the activation of PPARα expression and the inhibition of the expression of CPT1 in the liver (Fig. 5) can explain CCAE’s protective action against hepatic lipid accumulation in LT2D.
Despite their reported parallel relationship [96, 97, 107], we noticed an inverse association between the hepatic PPARα and CPT1 mRNA expressions, both after diabetes induction and after treatment with CCAE. As inhibition of the CPT1 gene may present a way to improve T2D [108], the inconsistency may convey the presence of PPARα-independent pathways of CPT1 gene regulation [109].
Macrovesicular steatosis may be more benign than microvesicular steatosis because large LDs can fuse with lysosomes into autophagosomes. After fusion, β-oxidation catabolizes and gets rid of the resulting FAs [110,111,112]. The insulin-resistant state greatly diminishes autophagy, but we could not comment further as we did not measure autophagy markers in the present study.
Further insight into the contrasting metabolic aberrations and the discriminating mechanisms of hypoglycemic actions of CCAE in ET2D and LT2D came from the differential expressions of gluconeogenic enzymes, G6Pase and PEPCK. The reaction catalyzed by G6Pase serves as the final rate-limiting step in both glycogenolysis and gluconeogenesis pathways. However, the presence of low and high mRNA expressions for PEPCK and G6Pase, respectively, in the liver of ET2D rats (Fig. 4) along with relatively low glycogen levels in the liver of ET2D relative to LT2D rats (Supplement 4) suggested that mild diabetes (ET2D) was comparable to short-term fasting state with the enhanced glycogenolysis being the source of extra blood sugar in ET2D. Shafaei et al. detected low glycogen levels in alloxan-induced diabetic rabbits (without mentioning the stage of disease) [44], and Bandsma et al. observed higher glycogen levels in the liver during 15 or 24 h (long) fasting periods in mice with PPARα deficiency [113]. Meanwhile, the low and high mRNA expression of G6Pase and PEPCK, respectively, in the liver of LT2D rats (Fig. 4) implied that the advanced stage of diabetes was analogous to prolonged fasting, the enhanced gluconeogenesis being the source of extra sugar in the blood.
According to the results, CCAE’s sugar-lowering capacity in ET2D stemmed from its inhibitory action on G6Pase. In LT2D, however, CCAE bypassed a significant suppression of PEPCK mRNA expression to reroute its activity toward G3P production. This diversion of PEPCK activity would reduce glucose production via gluconeogenesis but allow the continuous operation of the TG/FA cycle, which could also partially account for the significant reduction of TG levels in the sera of LT2D rats (CCAE-STZ group) (Fig. 6). The purpose of the TG/FA cycle is to create a balance between lipolysis and re-esterification and to prevent an overt release of FA into the blood via increasing the uptake of TG into the adipose tissue [114,115,116].
The diminished levels of PCC in this study confirmed CCAE’s antioxidant and free radical scavenging potential in agreement with studies that have reported a reduction of other oxidative stress markers, such as Thiobarbituric acid reactive substance (TBARS), TNF-α, and IL-6, and an augmentation of antioxidant enzymes as superoxide dismutase (SOD) and catalase (CAT) in diabetic animals treated with various C. colocynthis extracts [117, 118].
Plant extracts’ antioxidant, free radical scavenging, and glucose-lowering properties stem from their constituent phytochemicals. Secondary metabolites such as phenolics, flavonoids, terpenes, and alkaloids are abundant in fruit pulps, leaves, and seeds of C. colocynthis [15, 50, 119,120,121]. Some of the glucose-lowering components in the seeds are saponosides [43], and Shahin-Kaleybar et al. recently identified several cysteine-rich peptides in C. colocynthis [122].
Our HPLC-PDA library search suggested the presence of phenolics, such as Fer A, Chl A, GA, Van A, and Chi A (Fig. 1A and B), but CCAE appeared to be richer in flavonoids in agreement with Hussain et al. [50] because the UV spectra of the most prominent peaks contain two major absorption bands (λmax) in the UV region [123]. Quercetin, catechin, myricetin, and kaempferol are the major flavonoids in the roots, leaves, and fruits of C. colocynthis [50]. Rutin is also a flavonoid and a quercetin glycoside found in green tea, buckwheat seeds, and some fruits and vegetables, but its encounter in CCAE disagrees with previous reports [124]. We could not investigate the presence of myricetin and kaempferol in CCAE as we did not have the corresponding standards.
Also, it is worth mentioning that harsher methods of hydrolysis [125] resulted in a large (98.82% similar to Cin A) and a smaller peak (99.32% similar to formestane) with 1% H2SO4; and only one peak with 5% H2SO4 (99.48% similar to Cin A) (Supplement 5, Fig. S5A and B). The similarity of this lone peak (tR = 11.8) to Cin A was despite tR ≅ 47.90 for Cin A pure standard (Supplement 5); nevertheless, the finding of Cin A as the succinct elemental compound in CCAE may be valid as it is the primary organic chemical from which many phenolics and flavonoids are synthesized [126]. The results of an attempted HPLC-MS analysis on CCAE by the Medicinal Plants and Drugs Research Institute at Shahid Beheshti University of Medical Sciences are presented in Supplement 6.
The common notion about secondary plant metabolites, phenolics, and flavonoids, is that they act as natural anti-inflammatory agents due to their innate free radical scavenging capacity. However, the antioxidant activity of plant phytochemicals could relate to their ability to act as PPARα ligands. PPARα has anti-inflammatory effects, and PPARα ligands are known to inhibit IL-1-induced IL-6 and prostaglandin production and cyclooxygenase-2 expression [45]. Future studies may show that due to containing a variety of phytochemicals that can act in concert, plant extracts, such as CCAE, may function as a dual PPARα/γ or even trial PPAR-α, PPAR-γ, and PPAR-δ agonists [127, 128]. The unexpected detection of formetanate, formestane, and canrenoic acid-like structures in CCAE (Fig. 1 A and B) further illustrates the versatility of natural remedies’ domain of action.
Conclusion
The present study showed a significant degree of glucose intolerance in both mild and advanced stages of diabetes. CCAE showed acute (in ET2D) and chronic (in both ET2D and LT2D) hypoglycemic effects. ET2D and LT2D seemed to be analogous to short- and long-term fasting states, respectively. The enhanced glycogenolysis appeared to be the source of extra blood sugar in ET2D, whereas enhanced gluconeogenesis seemed to be the major contributor to high blood sugar in LT2D. CCAE affected both the sugar and fat metabolic pathways. CCAE inhibited the expression of G6Pase, and therefore glycogenolysis, in the liver of ET2D rats. However, its glucose-lowering activity in LT2D was not due to a reduction of PEPCK gene expression; instead, it decreased the rate of gluconeogenesis pathway mainly by rerouting PEPCK activity toward glyceroneogenesis. Low blood and hepatic TG were the outcomes of the enhanced hepatic FA utilization due to an upregulated PPARα expression and down-regulated CPT1 expression and the improved intracellular (within WAT) and systemic (between WAT and liver) TG/FA recycling as a result of improved glyceroneogenesis. A deeper understanding of the effect of CCAE on the liver-adipose tissue axis can follow the three-dimensional co-culturing of different cell types and a more profound analysis of CCAE constituents. Finally, evaluating medicinal plants’ healing properties and therapeutic mechanisms at different stages of a disease can help provide more personalized drug recipes for patients.
Availability of data and materials
The dataset(s) supporting the conclusions of this article is (are) included within the article (and its additional file(s). We can present the raw data files if requested.
Abbreviations
- ACC:
-
Acetyl-CoA Carboxylase
- ALP:
-
Alkaline Phosphatase
- ALT:
-
Alanine Transaminase
- AST:
-
Aspartate Transaminase
- BCA:
-
Bicinchoninic Acid
- CAT:
-
Catalase
- CCAE:
-
Citrullus colocynthis Aqueous Extract
- CPT1:
-
Carnitine Palmitoyltransferase
- Chi A:
-
Chicoric acid
- Chl A:
-
Chlorogenic acid
- Cin A:
-
Cinnamic acid
- DNL:
-
De Novo Lipogenesis
- DNPH:
-
2,4-Dinitrophenylhydrazine
- ET2D:
-
Early-Stage Type 2 Diabetes
- FA:
-
Fatty Acid
- FAS:
-
Fatty Acid Synthase
- FBS:
-
Fasting Blood Sugar
- FCR:
-
Folin-Ciocalteu Reagent
- Fer A:
-
Ferulic acid
- GA:
-
Gallic Acid
- G6Pase:
-
Glucose 6-Phosphatase
- HbA1c:
-
Glycosylated Hemoglobin
- HDL:
-
High-Density Lipoprotein
- HGP:
-
Hepatic glucose production
- IL-6:
-
Interleukin 6
- IP:
-
Intraperitoneal
- IGT:
-
Impaired Glucose Tolerance
- IPGTT:
-
Intraperitoneal Glucose Tolerance Test
- LR:
-
Linear Range
- LD:
-
Lipid Droplet
- LDL:
-
Low-Density Lipoprotein
- LOD:
-
Limit of Detection
- LOQ:
-
Limit of Quantitation
- LT2D:
-
Late-Stage Type 2 Diabetes
- NIA:
-
Niacinamide (Nicotinamide)
- PCC:
-
Protein Carbonyl Content
- PEPCK:
-
Phophoenlpyruvate Carboxykinase
- PMSF:
-
Phenylmethylsulfonyl Fluoride
- PPARa:
-
Peroxisome Proliferator-Activated Receptor Alpha
- QC:
-
Quality Control
- RBC:
-
Red Blood Cell
- RH:
-
Rutin Hydrate
- SOD:
-
Superoxide Dismutase
- SREBP-1c:
-
Sterol Regulatory Element Binding Protein-1c
- STZ:
-
Streptozotocin
- T2D:
-
Type 2 Diabetes
- TBARS:
-
Thiobarbituric Acid Reactive Substance
- TG:
-
Triacylglycerol
- TG/FA Cycle:
-
Triglyceride/Fatty acid Cycle
- TNFα:
-
Tumour Necrosis Factor Alpha
- Van A:
-
vanillic acid
- WAT:
-
White Adipose Tissue
References
Ghasemi A, Khalifi S, Jedi S. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes (review). Acta Physiol Hung. 2014;101(4):408–20. https://doi.org/10.1556/APhysiol.101.2014.4.2.
Kishore L, Kajal A, Kaur N. Role of nicotinamide in streptozotocin induced diabetes in animal models. J Endocrinol Thyroid Res. 2017;2(1):555577. https://doi.org/10.19080/JETR.2017.02.555577.
Rahimi R, Amin G, Ardekani MR. A review on Citrullus colocynthis Schrad.: from traditional Iranian medicine to modern phytotherapy. J Altern Complement Med. 2012;18(6):551–4. https://doi.org/10.1089/acm.2011.0297.
Akbar S. Malva sylvestris L. (Malvaceae). In: Handbook of 200 Medicinal Plants: A Comprehensive Review of Their Traditional Medical Uses and Scientific Justifications; 2020:1129-1136. Springer International Publishing.https://doi.org/10.1007/978-3-030-16807-0_121.
Sanei M, Mokaberinejad R, Roozafzai F, Abousaidi SR. Citrullus colocynthis: the most suggested herb in Persian medicine for management of low-back pain. Res J Pharmacogn. 2020;7(1):77–84. https://doi.org/10.22127/rjp.2019.185587.1496.
Gurudeeban S, Satyavani K, Ramanathan T. Bitter apple (Citrullus colocynthis): an overview of chemical composition and biomedical potentials. Asian J Plant Sci. 2010;9(7):394–401. https://doi.org/10.3923/ajps.2010.394.401.
Hussain AI, Rathore HA, Sattar MZ, Chatha SA, Sarker SD, Gilani AH. Citrullus colocynthis (L.) Schrad (bitter apple fruit): a review of its phytochemistry, pharmacology, traditional uses and nutritional potential. J Ethnopharmacol. 2014;155(1):54–66. https://doi.org/10.1016/j.jep.2014.06.011.
Al-Hwaiti MS, Alsbou EM, Abu Sheikha G, Bakchiche B, Pham TH, Thomas RH, et al. Evaluation of the anticancer activity and fatty acids composition of “Handal” (Citrullus colocynthis L.) seed oil, a desert plant from south Jordan. Food Sci Nutr. 2021;9(1):282–9. https://doi.org/10.1002/fsn3.1994.
Ayyad SE, Abdel-Lateff A, Alarif WM, Patacchioli FR, Badria FA, Ezmirly ST. In vitro and in vivo study of cucurbitacins-type triterpene glucoside from Citrullus colocynthis growing in Saudi Arabia against hepatocellular carcinoma. Environ Toxicol Pharmacol. 2012;33(2):245–51. https://doi.org/10.1016/j.etap.2011.12.010.
Saeed MEM, Boulos JC, Elhaboub G, Rigano D, Saab A, Loizzo MR, et al. Cytotoxicity of cucurbitacin E from Citrullus colocynthis against multidrug-resistant cancer cells. Phytomedicine. 2019;62:152945. https://doi.org/10.1016/j.phymed.2019.152945.
Perveen S, Ashfaq H, Ambreen S, Ashfaq I, Kanwal Z, Tayyeb A. Methanolic extract of Citrullus colocynthis suppresses growth and proliferation of breast cancer cells through regulation of cell cycle. Saudi J Biol Sci. 2021;28(1):879–86. https://doi.org/10.1016/j.sjbs.2020.11.029.
Tannin-Spitz T, Grossman S, Dovrat S, Gottlieb HE, Bergman M. Growth inhibitory activity of cucurbitacin glucosides isolated from Citrullus colocynthis on human breast cancer cells. Biochem Pharmacol. 2007;73(1):56–67. https://doi.org/10.1016/j.bcp.2006.09.012.
Abdulridha MK, Al-Marzoqi AH, Ghasemian A. The anticancer efficiency of Citrullus colocynthis toward the colorectal cancer therapy. J Gastrointest Cancer. 2020;51(2):439–44. https://doi.org/10.1007/s12029-019-00299-6.
Asadi-Samani M, Kooti W, Aslani E, Shirzad H. A systematic review of Iran’s medicinal plants with anticancer effect. Evid Based Complement Alternat Med. 2015;21(2):143–53. https://doi.org/10.1177/2156587215600873.
Bourhia M, Messaoudi M, Bakrim H, Mothana RA, Sddiqui NA, Almarfadi OM, et al. Citrullus colocynthis (L.) Schrad: chemical characterization, scavenging and cytotoxic activities. Open Chem. 2020;18(1):986–94. https://doi.org/10.1515/chem-2020-0124.
Abu-Odeh AM, Talib WH. Middle East medicinal plants in the treatment of diabetes: a review. Molecules. 2021;26(3):742. https://doi.org/10.3390/molecules26030742.
El-Abhar HS, Schaalan MF. Phytotherapy in diabetes: review on potential mechanistic perspectives. World J Diabetes. 2014;5(2):176–97. https://doi.org/10.4239/wjd.v5.i2.176.
Shi C, Karim S, Wang C, Zhao M, Murtaza G. A review on antidiabetic activity of Citrullus colocynthis Schrad.Acta Pol Pharm. 2014;71(3):363–7.
Alhawiti NM. Antiplatelets and profibrinolytic activity of Citrullus colocynthis in control and high-fat diet-induced obese rats: mechanisms of action. Arch Physiol Biochem. 2018;124(2):156–66. https://doi.org/10.1080/13813455.2017.1369999.
Jeyanthi KA, Mary V, Christy A. Hypolipidemic Effect of Citrullus colocynthis Seed Powder in Alloxan Induced Diabetic Rats. J Int Dent Medical Res. 2009;2:105–9.
Rahbar AR, Nabipour I. The Hypolipidemic Effect of Citrullus colocynthis on Patients with Hyperlipidemia. Pak J Biol Sci. 2010;13(24):1202–7. https://doi.org/10.3923/pjbs.2010.1202.1207.
Zamani M, Rahimi AO, Mahdavi R, Nikbakhsh M, Jabbari MV, Rezazadeh H, et al. Assessment of anti-hyperlipidemic effect of Citrullus colocynthis. Rev Bras Farmacognosia. 2007;17(4):492–6. https://doi.org/10.1590/S0102-695X2007000400003.
Kim MG, Lee SE, Yang JY, Lee HS. Antimicrobial potentials of active component isolated from Citrullus colocynthis fruits and structure-activity relationships of its analogues against foodborne bacteria. J Sci Food Agric. 2014;94(12):2529–33. https://doi.org/10.1002/jsfa.6590.
Keikhaie KR, Ghorbani S, Hosseinzadeh Z, Hassanshahian M. Antimicrobial activity of methanol extract of Citrullus colocynthis against antibiotic-resistant Staphylococcus aureus. Adv Herb Med. 2018;4:64–72.
Ponsankar A, Sahayaraj K, Senthil-Nathan S, Vasantha-Srinivasan P, Karthi S, Thanigaivel A, et al. Toxicity and developmental effect of cucurbitacin E from Citrullus colocynthis L. (Cucurbitales: Cucurbitaceae) against Spodoptera litura Fab. and a non-target earthworm Eisenia fetida Savigny. Environ Sci Pollut Res Int. 2020;27(19):23390–401. https://doi.org/10.1007/s11356-019-04438-1.
Ahmed M, Peiwen Q, Gu Z, Liu Y, Sikandar A, Hussain D, et al. Insecticidal activity and biochemical composition of Citrullus colocynthis, Cannabis indica and Artemisia argyi extracts against cabbage aphid (Brevicoryne brassicae L.). Sci Rep. 2020;10(1):522. https://doi.org/10.1038/s41598-019-57092-5.
Ostovar M, Akbari A, Anbardar MH, Iraji A, Salmanpour M, Hafez Ghoran S, et al. Effects of Citrullus colocynthis L. in a rat model of diabetic neuropathy. J Integr Med. 2020;18(1):59–67. https://doi.org/10.1016/j.joim.2019.12.002.
Li Y, Zheng MIN, Zhai X, Huang Y, Khalid A, Malik A, et al. Effect of Gymnema sylvestre, Citrullus colocynthis and Artemisia absinthium on blood glucose and lipid profile in diabetic human. Acta Pol Pharm. 2015;72(5):981–5.
Ghauri AO, Ahmad S, Rehman T. In vitro and in vivo anti-diabetic activity of Citrullus colocynthis pulpy flesh with seeds hydro-ethanolic extract. J Complement Integr Med. 2020;17(2). https://doi.org/10.1515/jcim-2018-0228.
Karimabad MN, Niknia S, Golnabadi MB, Poor SF, Hajizadeh MR, Mahmoodi M. Effect of Citrullus colocynthis extract on glycated hemoglobin formation (in vitro). Eurasian J Med. 2020;52(1):47–51. https://doi.org/10.5152/eurasianjmed.2020.19223.
Rajizadeh MA, Aminizadeh AH, Esmaeilpour K, Bejeshk MA, Sadeghi A, Salimi F. Investigating the effects of Citrullus colocynthis on cognitive performance and anxiety-like behaviors in STZ-induced diabetic rats. Int J Neurosci. 2021:1–13. https://doi.org/10.1080/00207454.2021.1916743.
Ahangarpour A, Oroojan AA. Effect of Crust and Seed Hydro-Alcoholic and aqueous Extract and Pulp Hydro-Alcoholic Extract of Citrullus colocynthis on Glucose Level in Insulin Resistance Male Rats. OFOGH-E-DANESH 2013;19(3):149-54.Q Horiz Med Sci. 2013;19:149–54.
Sebbagh N, Cruciani-Guglielmacci C, Ouali F, Berthault MF, Rouch C, Sari DC, et al. Comparative effects of Citrullus colocynthis, sunflower and olive oil-enriched diet in streptozotocin-induced diabetes in rats. Diabetes Metab. 2009;35(3):178–84. https://doi.org/10.1016/j.diabet.2008.10.005.
Shafaei H, Rad JS, Delazar A, Behjati M. The effect of pulp and seed extract of Citrullus Colocynthis, as an antidaibetic medicinal herb, on hepatocytes glycogen stores in diabetic rabbits. Adv Biomed Res. 2014;3(1):258. https://doi.org/10.4103/2277-9175.148230.
Agarwal V, Sharma AK, Upadhyay A, Singh G, Gupta R. Hypoglycemic effects of Citrullus colocynthis roots. Acta Pol Pharm. 2012;69(1):75–9.
Lahfa FB, Azzi R, Mezouar DR, Djaziri R. Hypoglycemic effect of Citrullus colocynthis extracts. Phytothérapie. 2017;15(2):50–6. https://doi.org/10.1007/s10298-015-0997-4.
Albokhary K, Aljaser F, Abudawood M, Tabassum H, Bakhsh A, Alhammada S, et al. Role of oxidative stress and severity of diabetic retinopathy in type 1 & type 2 diabetes. Ophthalmic Res. 2021;64(4):613–21. https://doi.org/10.1159/000514722.
Victor P, Umapathy D, George L, Juttada U, Ganesh GV, Amin KN, et al. Crosstalk between endoplasmic reticulum stress and oxidative stress in the progression of diabetic nephropathy. Cell Stress Chaperones. 2021;26(2):311–21. https://doi.org/10.1007/s12192-020-01176-z.
Ji LL, Yeo D. Oxidative stress: an evolving definition. Fac Rev. 2021;10:1-13. https://doi.org/10.12703/r/10-13.
Iova GM, Calniceanu H, Popa A, Szuhanek CA, Marcu O, Ciavoi G, et al. The antioxidant effect of curcumin and rutin on oxidative stress biomarkers in experimentally induced periodontitis in hyperglycemic Wistar rats. Molecules. 2021;26(5):1332. https://doi.org/10.3390/molecules26051332.
Pashmforosh M, Rajabi Vardanjani H, Rajabi Vardanjani H, Pashmforosh M, Khodayar MJ. Topical anti-inflammatory and analgesic activities of Citrullus colocynthis extract cream in rats. Medicina (Kaunas). 2018;54(4):51. https://doi.org/10.3390/medicina54040051.
Rizvi TS, Khan AL, Ali L, Al-Mawali N, Mabood F, Hussain J, et al. In vitro oxidative stress regulatory potential of Citrullus colocynthis and Tephrosia apollinea. Acta Pharma. 2018;68(2):235–42. https://doi.org/10.2478/acph-2018-0012.
Benmehdi H, Azzi R, Djaziri R, Lahfa F, Benariba N, Tabti B. Effect of saponosides crude extract isolated from Citrullus Colocynthis (L.) seeds on blood glucose level in normal and streptozotocin induced diabetic rats. J Med Plant Res. 2011;5(31):6864–8. https://doi.org/10.5897/JMPR11.11369.
Mojaz Dalfardi N, Ghodrati Azadi H, Fathi HB. Comparison of the effect of edible Citrullus colocynthis fruit powder with metformin on the level of blood glucose in streptozotocin-induced diabetic male rats. Q Horiz Med Sci. 2015;21(1):7–12. https://doi.org/10.18869/acadpub.hms.21.1.7.
Blaschke F, Takata Y, Caglayan E, Law RE, Hsueh WA. Obesity, peroxisome proliferator-activated receptor, and atherosclerosis in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2006;26(1):28–40. https://doi.org/10.1161/01.ATV.0000191663.12164.77.
Ghamarian A, Abdollahi M, Su X, Amiri A, Ahadi A, Nowrouzi A. Effect of chicory seed extract on glucose tolerance test (GTT) and metabolic profile in early and late stage diabetic rats. DARU. 2012;20(1):56. https://doi.org/10.1186/2008-2231-20-56.
Rub RA, Sidiqqi A, Ali AM, Shaikh A, Mukadam M. Screening of antioxidant & antidiabetic potential of polyphenol rich fraction from Cichorium intybus. Pharmacogn J. 2014;6(4):1–9. https://doi.org/10.5530/pj.2014.4.1.
Heimler D, Isolani L, Vignolini P, Romani A. Polyphenol content and antiradical activity of Cichorium intybus L. from biodynamic and conventional farming. Food Chem. 2009;114(3):765–70. https://doi.org/10.1016/j.foodchem.2008.10.010.
Kumar S, Kumar D, Saroha K, Singh N, Vashishta B. Antioxidant and free radical scavenging potential of Citrullus colocynthis (L.) Schrad. methanolic fruit extract. Acta Pharm. 2008;58(2):215–20. https://doi.org/10.2478/v10007-008-0008-1.
Hussain AI, Rathore HA, Sattar MZA, Chatha SAS, Ahmad F, Ahmad A, et al. Phenolic profile and antioxidant activity of various extracts from Citrullus colocynthis (L.) from the Pakistani flora. Ind Crops Prod. 2013;45:416–22. https://doi.org/10.1016/j.indcrop.2013.01.002.
Rezagholizadeh L, Pourfarjam Y, Nowrouzi A, Nakhjavani M, Meysamie A, Ziamajidi N, et al. Effect of Cichorium intybus L. on the expression of hepatic NF-kappaB and IKKbeta and serum TNF-alpha in STZ- and STZ+ niacinamide-induced diabetes in rats. Diabetol Metab Syndr. 2016. https://doi.org/10.1186/s13098-016-0128-6.
Dehghani F, Panjehshahin MR. The toxic effect of alcoholic extract of Citrullus colocynthis on rat liver. Iran J Pharmacol Ther. 2006;5:117–9.
Sun X, Han F, Yi J, Han L, Wang B. Effect of aspirin on the expression of hepatocyte NF-κB and serum TNF-α in streptozotocin-induced type 2 diabetic rats. J Korean Med Sci. 2011;26(6):765–70. https://doi.org/10.3346/jkms.2011.26.6.765.
Olson BJ, Markwell J. Assays for determination of protein concentration. Curr Protoc Protein Sci. 2007;48(1):3–4. https://doi.org/10.1002/0471140864.ps0304s48.
Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. 1994. https://doi.org/10.1016/s0076-6879(94)33041-7.
Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226(1):497–509. https://doi.org/10.1016/S0021-9258(18)64849-5.
Abdel-Hassan IA, Abdel-Barry JA, Mohammeda ST. The hypoglycaemic and antihyperglycaemic effect of Citrullus colocynthis fruit aqueous extract in normal and alloxan diabetic rabbits. J Ethnopharmacol. 2000;71(1-2):325–30. https://doi.org/10.1016/S0378-8741(99)00215-9.
Liu F, Xie M, Chen D, Li J, Ding W. Effect of V (IV) O (dipic-cl)(H2O)2 on lipid metabolism disorders in the liver of STZ-induced diabetic rats. J Diabetes Res. 2013;2013:1–10. https://doi.org/10.1155/2013/956737.
Ohno T, Horio F, Tanaka S, Terada M, Namikawa T, Kitoh J. Fatty liver and hyperlipidemia in IDDM (insulin-dependent diabetes mellitus) of streptozotocin-treated shrews. Life Sci. 2000;66(2):125–31. https://doi.org/10.1016/s0024-3205(99)00570-6.
Esmail OEA. A possible protective effect of Citrullus colocynthis Melon against diabetes mellitus type 2 associated with non-alcoholic fatty liver syndrome in rats. J Am Sci. 2012;8:1054–61.
Daradka H, Almasad MM, Qazan W, El-Banna NM, Samara OH. Hypolipidaemic effects of Citrullus colocynthis L. in rabbits. Pak J Biol Sci. 2007;10(16):2768–71. https://doi.org/10.3923/pjbs.2007.2768.2771.
Gurudeeban S, Ramanathan T. Invent Rapid Ethnopharmacol. 2010;1.
Shimomura I, Bashmakov Y, Ikemoto S, Horton JD, Brown MS, Goldstein JL. Insulin selectively increases SREBP-1c mRNA in the livers of rats with streptozotocin-induced diabetes. Proc Natl Acad Sci. 1999;96(24):13656–61. https://doi.org/10.1073/pnas.96.24.13656.
Ziamajidi N, Khaghani S, Hassanzadeh G, Vardasbi S, Ahmadian S, Nowrouzi A, et al. Amelioration by chicory seed extract of diabetes- and oleic acid-induced non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) via modulation of PPARalpha and SREBP-1. Food Chem Toxicol. 2013;58:198–209. https://doi.org/10.1016/j.fct.2013.04.018.
Cumaoglu A, Cevik C, Rackova L, Ari N, Karasu C. Effects of antioxidant stobadine on protein carbonylation, advanced oxidation protein products and reductive capacity of liver in streptozotocin-diabetic rats: role of oxidative/nitrosative stress. BioFactors. 2007;30(3):171–8. https://doi.org/10.1002/biof.5520300304.
Willecke F, Scerbo D, Nagareddy P, Obunike JC, Barrett TJ, Abdillahi ML, et al. Lipolysis, and not hepatic lipogenesis, is the primary modulator of triglyceride levels in streptozotocin-induced diabetic mice. Arterioscler Thromb Vasc Biol. 2015;35(1):102–10. https://doi.org/10.1161/ATVBAHA.114.304615.
Jourdan T, Djaouti L, Demizieux L, Gresti J, Verges B, Degrace P. Liver carbohydrate and lipid metabolism of insulin-deficient mice is altered by trans-10, cis-12 conjugated linoleic acid. J Nutr. 2009;139(10):1901–7. https://doi.org/10.3945/jn.109.111062.
Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab. 2011;22(9):353–63. https://doi.org/10.1016/j.tem.2011.04.007.
Khalil M, Mohamed G, Dallak M, Al-Hashem F, Sakr H, Eid RA, et al. The effect of Citrullus colocynthis pulp extract on the liver of diabetic rats a light and scanning electron microscopic study. Am J Biochem Biotechnol. 2010;6(3):155–63. https://doi.org/10.3844/ajbbsp.2010.155.163.
Oryan A, Hashemnia M, Hamidi AR, Mohammadalipour A. Effects of hydro-ethanol extract of Citrullus colocynthis on blood glucose levels and pathology of organs in alloxan-induced diabetic rats. Asian Pac J Trop Dis. 2014;4(2):125–30. https://doi.org/10.1016/s2222-1808(14)60328-5.
Benariba N, Djaziri R, Zerriouh BH, Bellakhdar W, Hupkens E, Boucherit Z, et al. Short- and long-term effects of various Citrullus colocynthis seed extracts in normal and streptozotocin-induced diabetic rats. Int J Mol Med. 2012;30(6):1528–36. https://doi.org/10.3892/ijmm.2012.1127.
Benariba N, Djaziri R, Zerriouh BH, Boucherit Z, Louchami K, Senner A, et al. Antihyperglycemic effect of Citrullus colocynthis seed aqueous extracts in streptozotocin-induced diabetic rats. Met Funct Res Diab. 2009;2:71–7.
Savaj S, Ghaffari M, Abbasi MA, Azar J. Acute Interstitial Nephritis Induced by Citrullus Colocynthis. Iran J. Kidney Dis. 2017;11(5):385–7.
Matthews DR, Rudenski AS, Burnett MA, Darling P, Turner RC. The half-life of endogenous insulin and C-peptide in man assessed by somatostatin suppression. Clin Endocrinol. 1985;23(1):71–9. https://doi.org/10.1111/j.1365-2265.1985.tb00185.x.
Guildford L, Crofts C, Lu J. Can the molar insulin: c-peptide ratio be used to predict hyperinsulinaemia? Biomedicines. 2020;8(5):108. https://doi.org/10.3390/biomedicines8050108.
Amin A, Tahir M, Lone KP. Effect of Citrullus colocynthis aqueous seed extract on beta cell regeneration and intra-islet vasculature in alloxan induced diabetic male albino rats. J Pak Med Assoc. 2017;67(5):715–21.
Benariba N, Djaziri R, Hupkens E, Louchami K, Malaisse WJ, Sener A. Insulinotropic action of Citrullus colocynthis seed extracts in rat pancreatic islets. Mol Med Rep. 2013;7(1):233–6. https://doi.org/10.3892/mmr.2012.1151.
Ebrahimi E, Bahramzadeh S, Hashemitabar M, Mohammadzadeh G, Shirali S, Jodat J. Effect of hydroalcoholic leaves extract of Citrullus colocynthis on induction of insulin secretion from isolated rat islets of Langerhans. Asian Pac J Trop Dis. 2016;6(8):638–41. https://doi.org/10.1016/S2222-1808(16)61101-5.
Nmila R, Gross R, Rchid H, Roye M, Manteghetti M, Petit P, et al. Insulinotropic effect of Citrullus colocynthis fruit extracts. Planta Med. 2000;66(5):418–23. https://doi.org/10.1055/s-2000-8586.
Drissi F, Lahfa F, Gonzalez T, Peiretti F, Tanti JF, Haddad M, et al. A Citrullus colocynthis fruit extract acutely enhances insulin-induced GLUT4 translocation and glucose uptake in adipocytes by increasing PKB phosphorylation. J Ethnopharmacol. 2021;270:113772. https://doi.org/10.1016/j.jep.2020.113772.
Barghamdi B, Ghorat F, Asadollahi K, Sayehmiri K, Peyghambari R, Abangah G. Therapeutic effects of Citrullus colocynthis fruit in patients with type II diabetes: a clinical trial study. J Pharm Bioallied Sci. 2016;8(2):130–4. https://doi.org/10.4103/0975-7406.171702.
Huseini HF, Darvishzadeh F, Heshmat R, Jafariazar Z, Raza M, Larijani B. The clinical investigation of Citrullus colocynthis (L.) schrad fruit in treatment of type II diabetic patients: a randomized, double blind, placebo-controlled clinical trial. Phytother Res. 2009;23(8):1186–9. https://doi.org/10.1002/ptr.2754.
Al-Ghaithi F, El-Ridi MR, Adeghate E, Amiri MH. Biochemical effects of Citrullus colocynthis in normal and diabetic rats. Mol Cell Biochem. 2004;261(1):143–9. https://doi.org/10.1023/B:MCBI.0000028749.63101.cc.
Arkkila PE, Koskinen PJ, Kantola IM, Ronnemaa T, Seppanen E, Viikari JS. Diabetic complications are associated with liver enzyme activities in people with type 1 diabetes. Diabetes Res Clin Pract. 2001;52(2):113–8. https://doi.org/10.1016/s0168-8227(00)00241-2.
Dallak M. In vivo, hypolipidemic and antioxidant effects of Citrullus colocynthis pulp extract in alloxan-induced diabetic rats. Afr J Biotechnol. 2011;10(48):9898–903. https://doi.org/10.5897/ajb11.268.
Meziane RK, Khemmar L, Amamou F, Yazit M, Didi A, Chabane-Sari D. Ann Biol Res. 2012;3:2486–90.
Gluchowski NL, Becuwe M, Walther TC, Farese RV Jr. Lipid droplets and liver disease: from basic biology to clinical implications. Nat Rev Gastroenterol Hepatol. 2017;14(6):343–55. https://doi.org/10.1038/nrgastro.2017.32.
Yang J, Kalhan SC, Hanson RW. What is the metabolic role of phosphoenolpyruvate carboxykinase. J Biol Chem. 2009;284(40):27025–9. https://doi.org/10.1074/jbc.R109.040543.
Wilfling F, Wang H, Haas JT, Krahmer N, Gould TJ, Uchida A, et al. Triacylglycerol synthesis enzymes mediate lipid droplet growth by relocalizing from the ER to lipid droplets. Dev Cell. 2013;24(4):384–99. https://doi.org/10.1016/j.devcel.2013.01.013.
Matsuzaka T, Shimano H, Yahagi N, Amemiya-Kudo M, Okazaki H, Tamura Y, et al. Insulin-independent induction of sterol regulatory element-binding protein-1c expression in the livers of streptozotocin-treated mice. Diabetes. 2004;53(3):560–9. https://doi.org/10.2337/diabetes.53.3.560.
Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, et al. “New” hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 2005;1(5):309–22. https://doi.org/10.1016/j.cmet.2005.04.002.
Mashek DG. Hepatic fatty acid trafficking: multiple forks in the road. Adv Nutr. 2013;4(6):697–710. https://doi.org/10.3945/an.113.004648.
Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, et al. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem. 2002;277(36):32571–7. https://doi.org/10.1074/jbc.M201692200.
Koh EH, Kim MS, Park JY, Kim HS, Youn JY, Park HS, et al. Peroxisome proliferator–activated receptor (PPAR)-alpha activation prevents diabetes in OLETF rats. Diabetes. 2003;52(9):2331–7. https://doi.org/10.2337/diabetes.52.9.2331.
Im SS, Kim MY, Kwon SK, Kim TH, Bae JS, Kim H, et al. Peroxisome proliferator-activated receptor {alpha} is responsible for the up-regulation of hepatic glucose-6-phosphatase gene expression in fasting and db/db mice. J Biol Chem. 2011;286(2):1157–64. https://doi.org/10.1074/jbc.M110.157875.
Memon RA, Tecott LH, Nonogaki K, Beigneux A, Moser AH, Grunfeld CA, et al. Up-regulation of peroxisome proliferator-activated receptors (PPAR-alpha) and PPAR-gamma messenger ribonucleic acid expression in the liver in murine obesity: Troglitazone induces expression of PPARg-responsive adipose tissue-specific genes in the liver of obese diabetic mice. Endocrinology. 2000;141(11):4021–31. https://doi.org/10.1210/endo.141.11.7771.
Zhang F, Xu X, Zhang Y, Zhou B, He Z, Zhai Q. Gene expression profile analysis of type 2 diabetic mouse liver. PLoS One. 2013;8(3):e57766. https://doi.org/10.1371/journal.pone.0057766.
Zhou JY, Zhou SW, Zhang KB, Tang JL, Guang LX, Ying Y, et al. Chronic effects of berberine on blood, liver glucolipid metabolism and liver PPARs expression in diabetic hyperlipidemic rats. Biol Pharm Bull. 2008;31(6):1169–76. https://doi.org/10.1248/bpb.31.1169.
Lu KL, Xu WN, Wang LN, Zhang DD, Zhang CN, Liu WB. Hepatic beta-oxidation and regulation of carnitine palmitoyltransferase (CPT) I in blunt snout bream Megalobrama amblycephala fed a high fat diet. PLoS One. 2014;9(3):e93135. https://doi.org/10.1371/journal.pone.0093135.
Hu Y, Chen Y, Ding L, He X, Takahashi Y, Gao Y, et al. Pathogenic role of diabetes-induced PPAR-α down-regulation in microvascular dysfunction. Proc Natl Acad Sci. 2013;110(38):15401–6. https://doi.org/10.1073/pnas.1307211110.
Kanie N, Matsumoto T, Kobayashi T, Kamata K. Relationship between peroxisome proliferator-activated receptors (PPAR alpha and PPAR gamma) and endothelium-dependent relaxation in streptozotocin-induced diabetic rats. Br J Pharmacol. 2003;140(1):23–32. https://doi.org/10.1038/sj.bjp.0705414.
Chan SM, Sun RQ, Zeng XY, Choong ZH, Wang H, Watt MJ, et al. Activation of PPARalpha ameliorates hepatic insulin resistance and steatosis in high fructose-fed mice despite increased endoplasmic reticulum stress. Diabetes. 2013;62(6):2095–105. https://doi.org/10.2337/db12-1397.
Charbonnel B. PPAR-alpha and PPAR-gamma agonists for type 2 diabetes. Lancet. 2009. https://doi.org/10.1016/S0140-6736(09)61040-0.
Peeters A, Baes M. Role of PPARalpha in hepatic carbohydrate metabolism. PPAR Res. 2010;2010:1–12. https://doi.org/10.1155/2010/572405.
Xu KZ-Y, Zhu C, Kim MS, Yamahara J, Li Y. Pomegranate flower ameliorates fatty liver in an animal model of type 2 diabetes and obesity. J Ethnopharmacol. 2009;123(2):280–7. https://doi.org/10.1016/j.jep.2009.03.035.
Tang CC, Huang HP, Lee YJ, Tang YH, Wang CJ. Hepatoprotective effect of mulberry water extracts on ethanol-induced liver injury via anti-inflammation and inhibition of lipogenesis in C57BL/6J mice. Food Chem Toxicol. 2013;62:786–96. https://doi.org/10.1016/j.fct.2013.10.011.
Song S, Attia RR, Connaughton S, Niesen MI, Ness GC, Elam MB, et al. Peroxisome proliferator activated receptor alpha (PPARalpha) and PPAR gamma coactivator (PGC-1alpha) induce carnitine palmitoyltransferase IA (CPT-1A) via independent gene elements. Mol Cell Endocrinol. 2010;325(1-2):54–63. https://doi.org/10.1016/j.mce.2010.05.019.
Chiodi P. Diabetes mellitus: could the inhibition of a single enzyme (CPT-1) involved in the beta-oxidation process improve this complex Disease. Curr Res Diabetes Obes J. 2017. https://doi.org/10.19080/CRDOJ.2016.13.555563.
Louet JF, Chatelain F, Decaux JF, Park EA, Kohl C, Pineau T, et al. Long-chain fatty acids regulate liver carnitine palmitoyltransferase I gene (L-CPT I) expression through a peroxisome-proliferator-activated receptor alpha (PPARalpha)-independent pathway. Biochem J. 2001;354(1):189–97. https://doi.org/10.1042/0264-6021:3540189.
Allaire M, Rautou PE, Codogno P, Lotersztajn S. Autophagy in liver diseases: time for translation? J Hepatol. 2019;70(5):985–98. https://doi.org/10.1016/j.jhep.2019.01.026.
Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56(4):952–64. https://doi.org/10.1016/j.jhep.2011.08.025.
Yan H, Gao YQ, Zhang Y, Wang H, Liu GS, Lei JY. Chlorogenic acid alleviates autophagy and insulin resistance by suppressing JNK pathway in a rat model of nonalcoholic fatty liver disease. J Biosci. 2018;43(2):287–94. https://doi.org/10.1007/s12038-018-9746-5.
Bandsma RH, Van Dijk TH, Harmsel At A, Kok T, Reijngoud DJ, Staels B, et al. Hepatic de novo synthesis of glucose 6-phosphate is not affected in peroxisome proliferator-activated receptor alpha-deficient mice but is preferentially directed toward hepatic glycogen stores after a short term fast. J Biol Chem. 2004;279(10):8930–7. https://doi.org/10.1074/jbc.M310067200.
Beale EG, Antoine B, Forest C. Glyceroneogenesis in adipocytes: another textbook case. Trends Biochem Sci. 2003;28(8):402–3. https://doi.org/10.1016/S0968-0004(03)00163-4.
Kalhan SC, Bugianesi E, McCullough AJ, Hanson RW, Kelley DE. Estimates of hepatic glyceroneogenesis in type 2 diabetes mellitus in humans. Metabolism. 2008;57(3):305–12. https://doi.org/10.1016/j.metabol.2007.10.003.
Reshef L, Olswang Y, Cassuto H, Blum B, Croniger CM, Kalhan SC, et al. Glyceroneogenesis and the triglyceride/fatty acid cycle. J Biol Chem. 2003;278(33):30413–6. https://doi.org/10.1074/jbc.R300017200.
Dallak M, Jaliah BI. Antioxidant activity of Citrullus colocynthis pulp extract in the RBCs of alloxan-induced diabetic rats. Pak J. Physiol. 2010;6(1):1–5.
Sanadgol N, Najafi S, Ghasemi LV, Motalleb G, Estakhr J. J Pharmacognosy Phytother. 2011;3:81–8.
Kumar MV, Shimokawa T, Nagy TR, Lane MD. Differential effects of a centrally acting fatty acid synthase inhibitor in lean and obese mice. Proc Natl Acad Sci U S A. 2002;99(4):1921–5. https://doi.org/10.1073/pnas.042683699.
Parveen R, Khan N, Zahiruddin S, Ibrahim M, Anjum V, Parveen B, et al. TLC-bioautographic evaluation for high-throughput screening and identification of free radical scavenging and antidiabetic compounds from traditional Unani medicinal plant: Citrullus colocynthis Schrad. J AOAC Int. 2020;103(3):669–77. https://doi.org/10.5740/jaoacint.19-0287.
Rani A, Goyal A, Arora S. A brief review on Citrullus colocynthis- bitter apple. Arch Curr Res Int. 2017;8(4):1–9. https://doi.org/10.9734/ACRI/2017/35158.
Shahin-Kaleybar B, Niazi A, Afsharifar A, Nematzadeh G, Yousefi R, Retzl B, et al. Isolation of cysteine-rich peptides from Citrullus colocynthis. Biomolecules. 2020;10(9):1326. https://doi.org/10.3390/biom10091326.
Figueirinha A, Paranhos A, Pérez-Alonso JJ, Santos-Buelga C, Batista MT. Cymbopogon citratus leaves: characterization of flavonoids by HPLC–PDA–ESI/MS/MS and an approach to their potential as a source of bioactive polyphenols. Food Chem. 2008;110(3):718–28. https://doi.org/10.1016/j.foodchem.2008.02.045.
Mohamed MSM, Saleh AM, Abdel-Farid IB, El-Naggar SA. Growth, hydrolases and ultrastructure of fusarium oxysporum as affected by phenolic rich extracts from several xerophytic plants. Pestic Biochem Physiol. 2017;141:57–64. https://doi.org/10.1016/j.pestbp.2016.11.007.
Jang S-S. Production of mono sugar from acid hydrolysis of seaweed. Afr J Biotechnol. 2012;11(8). https://doi.org/10.5897/AJB10.1681.
De P, Baltas M, Bedos-Belval F. Cinnamic acid derivatives as anticancer agents-a review. Curr Med Chem. 2011;18(11):1672–703. https://doi.org/10.2174/092986711795471347.
Chen KC, Chang SS, Huang HJ, Lin TL, Wu YJ, Chen CY. Three-in-one agonists for PPAR-alpha, PPAR-gamma, and PPAR-delta from traditional Chinese medicine. J Biomol Struct Dyn. 2012;30(6):662–83. https://doi.org/10.1080/07391102.2012.689699.
Jain N, Bhansali S, Kurpad AV, Hawkins M, Sharma A, Kaur S, et al. Effect of a dual PPAR alpha/gamma agonist on insulin sensitivity in patients of type 2 diabetes with hypertriglyceridemia- randomized double-blind placebo-controlled trial. Sci Rep. 2019;9(1):19017. https://doi.org/10.1038/s41598-019-55466-3.
Acknowledgments
We want to thank the Diabetes Center of Shariati Hospital, Tehran, Iran, for blood analysis, the Histogenotech Research Laboratory, Tehran, Iran, for preparing and staining the various liver tissue sections, and the Medicinal Plants and Drugs Research Institute of Shahid Beheshti University of Medical Sciences for conducting an HPLC-MS analysis on CCAE.
Funding
The financial support was provided by the Tehran University of Medical Sciences and Health Services through Grants No. 94–01–30-28678 and 95–04–30-33306.
Author information
Authors and Affiliations
Contributions
AA and FS participated equally in designing the study, carrying out the lab work, and drafting the manuscript. AN conceived, designed, and coordinated the study, performed the HPLC-DAD experiments and phytochemical analysis, wrote and revised the manuscript. MBK supervised the Real-Time PCR experiments; SB participated in conceptualization and data analysis; GH was responsible for micrograph interpretation. MS prepared the samples for Oil-red O staining. AA prepared and lyophilized the extract, and MF helped with the phytochemical analysis. All authors gave final approval for publication and agreed to be held accountable for the work performed therein and declare no conflict of interest. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study does not involve human participants. The ethical committee approved the animal experiments of Tehran University of Medical Sciences under ethical-code number IR.TUMS.MEDICINE.REC.1396.2639.3.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Fig. S1A. Determination of CCAE dosage. Fig. S1B. Study groups for long-term (28-day) experimental process. Table S1. Area under curve (AUC) for data in the above graph. Fig. S1C. The status of glucose tolerance in diabetic rats
Additional file 2.
Table S2-1. Names of the standard compounds and their retention times when injected as separate solutions (alone) and all mixed. Figure S2-1. When the standards in the above Table were injected as a mixture, retention times changed. Fig S2-2. Chromatograms corresponding to CCAE (5 μl, green), a mixture of two ferulic acid and rutin hydrate standards (Fer A/RH, 5 μl, blue), and a combined sample of CCAE and Fer A/RH standards (50/50 v/v, 10 μl, brown). Fig S2-3. The results of spiking either the CCAE or the hydrolyzed CCAE with the standards. GA, gallic acid; RH, rutin hydrate; Fer A, ferulic acid; Cin A, Cinnamic acid; Van A, vanillic acid
Additional file 3.
Supplement 3. Masson's Trichrome Staining
Additional file 4.
Supplement 4. Periodic acid–Schiff (PAS)
Additional file 5.
Figure S5. HPLC profiles of CCAE after harsh hydrolysis using 1% (A) and 5% H2SO4 (B)
Additional file 6.
Fig. S6. HPLC-MS analysis (A) HPLC chromatogram; (B) TIC; and (C) full scan mass spectra
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Afshari, A., Salimi, F., Nowrouzi, A. et al. Differential expression of gluconeogenic enzymes in early- and late-stage diabetes: the effect of Citrullus colocynthis (L.) Schrad. Seed extract on hyperglycemia and hyperlipidemia in Wistar-Albino rats model. Clin Phytosci 7, 88 (2021). https://doi.org/10.1186/s40816-021-00324-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40816-021-00324-x