Abstract
Background
Cineole has documented anti-inflammatory, expectorant, and mucolytic properties and has shown to be a valuable treatment option in different airway diseases. Our study examined whether a therapy with Cineole as add on to an antiviral therapy can relieve symptoms of acute bronchitis, and accelerate recovery in everyday practice.
Methods
In an open-label, randomized, parallel-group phase III clinical trial, 132 patients diagnosed with “acute bronchitis” or “acute tracheobronchitis” were included and treated with 3 × 200 mg of Cineole on top of antiviral treatment, or antiviral treatment alone (Ingavirin® 90 mg), per day for 4–9 days. The primary outcome measure was the change in cough frequency assessed in a Cough Frequency Assessment Scale, secondary outcomes were the total Bronchitis Severity Scale (BSS), as well as individual symptoms of the BSS score. Adverse events were collected for safety analysis. The study sites were located in Russia.
Results
After 4 days of therapy, there was a significant difference between the groups in favour of the patients treated with Cineole which persisted until the end of the study. At that time, cough during the day, assessed by the Cough Frequency Assessment Scale, was absent in 14 patients in the Cineole group (21.5%), compared to 4 (6.2%) patients in the control arm (p = 0.0203), which was replicated using the BSS individual cough score. In addition, significant improvements in the individual symptoms of the BSS in patients taking Cineole were documented.
The study drug showed good tolerability without differences to antiviral treatment and results were in line with previous experiences with this drug.
Conclusions
Assessment after 4 days of treatment with additional Cineole showed a significant reduction of cough frequency and other symptoms of acute bronchitis compared to antiviral treatment alone. In addition, patients recovered faster from the disease. Additional treatment with Cineole is a valuable treatment option in acute bronchitis.
Trial registration
Ministry of Health, Russia, No. 592. Registered 19 October 2015.
Similar content being viewed by others
Introduction
Acute respiratory tract infections are often induced by viral infections leading to the classic “common cold”. Symptoms include rhinitis, sinusitis, pharyngitis, laryngitis, bronchitis and belong to the most common medical conditions, reasons for sick certificates and days of loss of work [1]. They are responsible for considerable direct and indirect health care costs, and therefore have a high socio-economic relevance [2].
Acute bronchitis as a clinical diagnosis is part of common colds [3], characterized by acute cough being the predominant symptom, with or without sputum production, in the absence of chronic lung disease [4]. According to a publication of the “European Lung Foundation (ELF)” and the “European Respiratory Society (ERS)”, 16.500.000 cases of acute bronchitis are seen in Europe every year [2].
Up to 95% of acute bronchitis are caused by viruses [5]. Therefore, antibiotics are inappropriate in the vast majority of cases. According to observations in general practice, the median time for symptoms associated with acute bronchitis to resolve following consultation varies between 5 days (for dyspnea) and 11 days for cough [6]. Therefore, evidence based cough guidelines define acute cough - mostly caused by common cold - as lasting 2 to 3 weeks of duration [7, 8]. A reduction of the duration of illness is highly desirable.
Globally increasing drug resistance rates due to inappropriate use of antibiotics present an increasing and serious health burden [9]. In the outpatient setting most antibiotics are inappropriately prescribed for treating common cold/acute bronchitis [10]. The integration of safe and effective alternative treatment possibilities such as herbal remedies is a welcome addition to conventional treatment in acute bronchitis and might help to limit the use of antibiotics.
Eucalyptus oil is traditionally used for common colds and acute respiratory tract infections. Its main ingredient 1,8-Cineole has shown to have mucolytic, expectorant, and anti-inflammatory properties [11]. Cineole inhibits proinflammatory cytokine production in human lymphocytes and lipopolysaccharide-stimulated monocytes, and thus is able to control airway mucus hypersecretion and inflammation processes [12]. In previous clinical studies, Cineole has shown to be effective in the treatment of rhinosinusitis, asthma, COPD, and acute bronchitis [5, 13,14,15]. In Russia for treatment of acute viral respiratory tract infections Imidazolyl Ethanamide Pentandioic Acid (Ingavirin®) is approved and research has documented an antiviral effect of the substance against human influenza and parainfluenza-viruses as well as adenoviruses [16,17,18,19,20,21,22,23].
The study was performed to evaluate the efficacy and safety of add on Cineole in patients with acute bronchitis treated with an antiviral therapy as well to support the launch of Cineole in the Russian Federation.
Materials and methods
Study design and population
This open-label, randomized, parallel-group phase III clinical trial was performed at seven study sites in Russia. It was conducted in compliance with the principles of the Declaration of Helsinki approved by the 64th WMA General Assembly, Fortaleza, Brazil, October 2013 and the rules of Good Clinical Practice that are applied in Russia and approved by the Ethics Council of the Ministry of Health of the RF as well as the local ethical committees. Both investigator and patients signed an informed consent after the investigator had informed the patient about all relevant aspects of the clinical study.
All participants suffered from acute bronchitis or tracheobronchitis with a symptom duration of less than 12 h. The study was limited to adult patients, aged 18–70 years. Inclusion criteria were an established clinical diagnosis of acute bronchitis or acute tracheobronchitis (ICD-10 code J20), accompanied by subfebrile body temperature below 39.0 °С and a total score according to the “Bronchitis Severity Scale (BSS)” of 5–15 points out of a maximum score of 20. Patients were excluded, if they had a history of underlying chronic bronchial and pulmonary diseases, fever > 39 °С, known hypersensitivity to any component of the study drug or any contraindication to the antiviral therapy.
Patients were randomly assigned by a computerized random member generation to treatment with enteric-coated Cineole capsules plus one capsule Ingavirin® [16], or to Ingavirin® capsules alone (antiviral treatment group) for 4–9 days. If necessary, patients were allowed to use antipyretics or nasal decongestants.
Medication
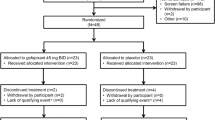
Every patient received Ingavirin® capsules 90 mg once daily, in addition, patients in the Cineole group received 1,8-Cineole enteric-coated capsules, 200 mg (Soledum®) daily doses according to recommendations in the product information (3 capsules a day) for 4–9 days. In both groups 66 patients were included. Figure 1 shows the study flow.
Outcome measures
The primary efficacy endpoint was the change in cough frequency compared to visit 0 in the two groups according to the “Cough Frequency Assessment Scale” [13]. This 5-point scale is a non-validated scoring questionnaire and assesses cough frequency, ranging from score 0 (no cough during a day), score 1 (single episode of cough during a day), score 2 (2–3 episodes of cough during a day), score 3 (4–5 episodes of cough during a day), score 4 (5–6 episodes of cough during a day), to score 5 (≥ 10 episodes of cough during a day).
Change in the total score of the Bronchitis Severity Scale (BSS), a standardized and validated questionnaire to assess the severity of acute bronchitis, was evaluated as a secondary endpoint: The BSS comprises the following five symptoms typical for AB: cough, sputum production, cough induced chest pain, rales on auscultation, and dyspnea [24]. These symptoms are each assessed according to a 5-point Likert scale: 0 = absent, 1 = mild, 2 = moderate, 3 = severe and 4 = very severe. The points allocated to each of the symptoms are added to make the total score, which can therefore vary between 0 and 20 points (see Table 1) [1, 24]. In addition to the total score, the individual scores of each symptom were assessed in the study. Complete recovery was defined as a BSS Total Score ≤ 3.
Adverse events were systematically collected, assessed and evaluated by the treating physicians at the different visits.
Visits
At the screening visit (visit 0), patients were evaluated including family history, physical examination, total score according to the “Cough Frequency Assessment Scale”, and the BSS. In addition, a complete blood count, metabolic panel, urine analysis, pregnancy test and ECG were performed.
At visit one, participants were randomized to treatment. On day 2, the investigator contacted the patient via phone. At day four and day seven (visit 3 and 4), physical examination, assessment of vital signs, respiratory examination, measurement of body temperature and assessment of complaints were repeated. At the end of the study after 9 days (visit 5), in addition, a complete blood count, metabolic panel, urine analysis, pregnancy test and ECG were repeated.
From visit 3 on, the investigator decided whether patients should discontinue the study, if they fulfilled all criteria for clinical recovery (BSS-Score ≤ 3, body temperature normalization, and no need to use antimicrobial agents).
Statistical methods
An analysis of efficacy was performed both with the intention-to-treat (ITT)-population and the per protocol (PP) population. The ITT population included all eligible patients, who received at least one dose of medication and had at least one evaluation of efficacy following the initiation of the study (visit 3). The PP population comprised patients for whom there were no significant protocol deviations affecting the primary efficacy parameters and who were adherent by at least 80%.
Regarding the primary outcome measure, the “Cough Frequency Assessment Scale”, Mann-Whitney U-test was used to establish superiority over the control group. In order to compare mean changes in the BSS scale, the analysis of covariance (ANCOVA) was used where symptoms observed at baseline were applied as a covariate.
All data are expressed as mean values (with SD) and all tests were two-tailed. P-values of 0.05 or less were considered to indicate statistical significance.
Results
One hundred thirty-two patients with “acute bronchitis” or “acute tracheobronchitis” were enrolled in the study and randomized 1:1 to the cineole group or the control group (n = 66). At the end of the trial, 130 patients could be included in the ITT population (Fig. 1). Compliance with the study requirements was high, and no patients were withdrawn from the study due to non-compliance.
At enrollment in the study, all patients suffered from ≥10 cough episodes a day. The groups were comparable with regard to baseline characteristics, BSS was 8,4 in the Cineole group and 8,7 in the group with antiviral therapy only, respectively (see Table 2).
Primary endpoint
Starting from visit 3, there was a significant difference between the groups in favor of the patients that were treated with cineole in all efficacy endpoints. This difference continued until the final visit: At that time, cough during the day, assessed by the “Cough Frequency Assessment Scale”, was absent in 14 patients in the cineole group (21.5%), compared to 4 (6.2%) patients in the control arm (p = 0.0203) (Fig.2).
Mean of the scores in CG (control group = standard antiviral): visit 0 = 5.0 SD(0.0), visit 1 = 5.0 SD(0.0), visit 2 = 4.9 SD(0.4), visit 3 = 3.9 SD(0.9), visit 4 = 2.7 SD(1.3), visit 5 = 2.2 SD(1.1). Mean of the scores in MG (main group = standard antiviral + cineole): visit 0 = 5.0 SD(0.0), visit 1 = 5.0 SD(0.0), visit 2 = 4.8 SD(0.4), visit 3 = 3.5 SD(0.9), visit 4 = 2.3 SD(1.5), visit 5 = 1.5 SD(0.9)
Secondary endpoints
Similarly, the absence of cough based on the BSS assessment was registered at the final visit in 14 patients in the cineole group (21.5%), compared to only 4 (6.2%) patients in the control group (p = 0.0203). From visit 3 onward, there was a statistically significant difference in favor of the Cineole group in the BSS total score: at least 50% of the patients in this group had a score of 3 or less. Distribution of the total scores of study subjects in the control and the Cineole group is shown in Fig. 3.
Mean of the scores in CG (control group = standard antiviral): visit 0 = 8.7 SD(2.0), visit 1 = 8.7 SD(2.0), visit 3 = 6.2 SD(2.1), visit 4 = 3.8 SD(1.9), visit 5 = 3.3 SD(2.0). Mean of the scores in MG (main group = standard antiviral + cineole): visit 0 = 8.4 SD(2.1), visit 1 = 8.2 SD(2.1), visit 3 = 5.3 SD(1.8), visit 4 = 2.9 SD(1.8), visit 5 = 1.6 SD(1.1).). From visit 3 onward, there was a statistically significant difference in favor of the Cineole group in the BSS total score (p < 0.05)
In addition, patients in the Cineole group experienced advantages in the individual scores of the BSS: Compared to baseline coughing, sputum production, cough-induced chest pain, wheezing were significantly reduced as compared to the control arm from visit 3 onwards. At the final visit, there was absence of sputum production in the BSS assessment in 36 patients in the Cineole group (55.4%), compared to 21 patients in the control group (32.3%; p = 0.0130). Additionally, at the final visit 60 patients treated with Cineole were free of chest pain (92.3%) compared to 36 patients in the control group (55.4%; p < 0.0001). Absence of rales during auscultation was registered at the final visit in 64 patients in the Cineole group (98.5%) compared to 51 (78.5%) of patients in the control arm (p = 0.0005). Concerning dyspnea, there could be seen a slight advance for cineole: At the end of the study, no patient treated with Cineole suffered from dyspnea, compared to 3 patients in the control group (p = 0.2442). Distribution of BSS at the end of study also indicated that more patients with Cineole compared to the antiviral therapy alone had mild or no relevant symptoms anymore (Fig. 4).
In the ITT population, 63 of 65 patients (96.9%) treated with Cineole compared to 30 out of 65 patients in the control group (46.2%) had completely recovered (p < 0.0001) at the final visit (95% CI: 35,3-66,3%). The corresponding numbers in the PP population were similar: 63 patients in the Cineole group (96.9%) compared to 30 in the control group (48.4%) had completely recovered (p < 0.0001).
The efficacy of the study drug was also demonstrated by the reduced use of additional, symptomatic medication: At the final visit, 69.7% patients in the control group compared to 39.4% of patients taking Cineole used additional symptomatic medications (p = 0.0008).
Cineole was generally well tolerated with only a few mild side effects (nausea and smell of eucalyptus in the nose) (see Table 2). Concerning the smell of eucalyptus, this is not an undesirable side effect but represents the pharmacokinetic properties of cineole which is partially exhaled. Thus, cineole comes into contact with the respiratory epithelium of the upper and lower airways, the destination where cineole can exert its secretolytic and anti-inflammatory effects. There were no severe side effects, nor changes in blood tests related to the study medication. Only one patient discontinued the study due to his complaints of nausea after the intake of the study drug.
Discussion
Effective mucus clearance is essential for lung health, and infections and inflammatory processes as in acute bronchitis may impair mucociliary activity [25]. Newer studies have shed light on the molecular mode of action of Cineole: Anti-inflammatory activities are mediated by influence on different cytokines, as well as prostaglandins and leukotrienes. Additionally, inhibiting effects have been described on mucus hypersecretion [12]. Its anti-inflammatory effect is based on an inhibition of nuclear NF-κB p65 translocation that results in decreased levels of proinflammatory NF-κB target genes [26]. A recent publication showed that Cineole potentiates interferon regulatory factor 3, thus enhancing the antiviral response in human stem cells and in an ex vivo model of rhinosinusitis [27]. The antitussive effect of Cineole can probably be explained by an amelioration of inflammation and mucociliary clearance in patients with acute bronchitis [13].
In this randomized open label study, add-on therapy with Cineole led to a rapid improvement in bronchitis symptoms which was significant if compared to the Ingavirin-only group starting from visit 3 (day 4) compared to a standard antiviral treatment in adult patients under conditions of everyday clinical practice. Acute bronchitis is a self-limiting disease, with a usual duration of up to 14 days [4]. Thus, an accelerated improvement at day 3 is the most important effect of every intervention in common cold. The last assessment at day 9 also shows the acceleration of symptom relief in the Cineole group. Due to the natural course of the disease, no such difference can be expected after 14 days.
Cough is one of the predominant and defining symptom of acute bronchitis and common cold [4]. In addition, the number of coughing fits is the most relevant parameter with regard to the symptom burden of acute bronchitis [13]. Therapy with Cineole reduced the number of coughing fits, as well as symptoms of acute bronchitis in the BSS score. The treatment effects of Cineole and the onset of efficacy seen in this study are in the same order of magnitude to effects seen in a previous clinical trial with this drug in the same indication [13]. In that placebo-controlled randomized clinical trial including 242 patients with acute bronchitis, therapy with Cineole led to a significant reduction of the frequency of cough fits, assessed in the BSS score, starting after 4 days of treatment. After this time, the mean decrease in the BSS was 3.55 score-points in the Cineole group, and 2.91 score-points in the placebo-group (p = 0.0383). Acute rhinosinusitis is an important contributor to the symptomatic load including cough in common cold, comprising usually not only the bronchi but also nose, sinuses and throat.
In the present study, the Cineole preparation reduced not only cough frequency, the key symptom of acute bronchitis, but also other symptoms as depicted in the BSS symptom score. Results with Cineole in this open label study are in line with clinical data from double-blind trials.
Cineole has not only shown therapeutic efficacy in acute bronchitis, but also in diseases of the concomitant upper respiratory tract, e.g. in patients with acute non purulent rhinosinusitis: In a placebo controlled trial, treatment differences between patients in the Cineole group and placebo in a symptom sum score of rhinosinusitis became also significant after 4 days [14]. Another double-blind, placebo-controlled trial assessed the efficacy of add on cineole in asthma [5]. In this trial, cineole improved lung function, asthma symptoms including dyspnea and quality of life. The documented anti-inflammatory effect of the substance may have a significant role in these settings as well. Viral infections, which can induce asthma exacerbations, as well as postinfectious bronchial hyperresponsiveness [13] may play an important role in the course of the disease.
The good tolerability seen in this trial also is in line with previous experiences with this drug: Only few adverse events occurred in both groups, all assessed as mild. Three adverse events were observed in the group “cineole + antiviral therapy” and one adverse event was observed in the group “antiviral therapy” (Table 3).
Limitations of the study
Besides the open label design, the inclusion of patients only if they were subfebrile and a Bronchitis Severity Scale not over 15 might be regarded as limitations. On the other hand, the aim of this study was to investigate the effectiveness of cineole in acute bronchitis, an infection of the airways which can be subsumed under the umbrella term “common cold” [1]. Fever over 38.5 °C is not usual in adults with common cold resp. acute bronchitis, but patients in both groups of this study, although included under the preamble “fever”, only had a slightly higher body temperature (37.8 °C), thus fitting to the real life situation of patients with acute bronchitis. Also a Bronchitis Severity Scale not over 15, excluding patients with very severe acute bronchitis, can be seen as reasonable as it is questionable whether this very severe stage still belongs to the symptom picture of acute bronchitis as a manifestation of common cold.
Conclusion
Addition of Cineole to antiviral therapy of acute bronchitis or tracheobronchitis as symptoms of common cold shortens duration of symptoms and reduces their intensity. It reduces cough frequency, the key symptom of acute bronchitis, as well as dyspnea and chest pain, and accelerates recovery. Therefore, the cineole preparation studied above is a valuable and cost-effective therapeutic option in acute bronchitis that might help to reduce the widespread inappropriate use of antibiotics in a primarily virus borne disease.
Availability of data and materials
Original data will not be shared due to confidentiality reasons.
References
Kardos P, Malek FA. Common cold – an umbrella term for acute infections of nose, throat, Larynx and Bronchi. Pneumologie. 2017;71(4):221–6. https://doi.org/10.1055/s-0042-116112.
Gibson J, Loddenkemper R, Sibille Y, Lundbäck B, Fletcher M. Lunge und Gesundheit in Europa - Fakten & Zahlen: Zum besseren Verständnis von Lungenkrankheiten und ihrer Versorgung in Europa. Broschüre. Herne: FRISCHTEXTE Verlag; 2014.
Worrall G. Acute Bronchitis. Can Fam Physician. 2008;54(2):238–9.
Kinkade S, Long NA. Acute bronchitis. Am Fam Physician. 2016;94(7):560–5.
Worth H, Dethlefsen U. Patients with asthma benefit from concomitant therapy with cineole. J Asthma. 2012;49(8):849–53. https://doi.org/10.3109/02770903.2012.717657.
Moore M, Little P, Rumsby K, Kelly J, Watson L, Warner G, et al. Predicting the duration of symptoms in lower respiratory tract infection. Br J Gen Pract. 2008;58(547):88–92. https://doi.org/10.3399/bjgp08X264045.
Irwin RS, French CL, Chang AB, Altman KW, CHEST Expert Cough Panel. Classification of Cough as a symptom in adults and management algorithms: CHEST guideline and Expert Panel report. Chest. 2018;153(1):196–209. https://doi.org/10.1016/j.chest.2017.10.016.
Kardos P, Dinh QT, Fuchs KH, Gillissen A, Klimek L, Koehler M, et al. Leitlinie der Deutschen Gesellschaft für Pneumologie und Beatmungsmedizin zur Diagnostik und Therapie von erwachsenen Patienten mit Husten [guidelines of the German respiratory Society for Diagnosis and Treatment of adults suffering from acute, subacute and chronic Cough]. Pneumologie. 2019;73(03):143–80. https://doi.org/10.1055/a-0808-7409.
McCaig LF, Besser RE, Hughes JM. Trends in antimicrobial prescribing rates for children and adolescents. JAMA. 2002;287(23):3096–102. https://doi.org/10.1001/jama.287.23.3096.
Butler CC, Hood K, Verheij T, Little P, Melbye H, Nutall J, et al. Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care: prospective study in 13 countries. BMJ. 2009;338(jun23 2):b2242. https://doi.org/10.1136/bmj.b2242.
Juergens UR. Anti-inflammatory properties of the monoterpene 1.8-cineole: current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014;64(12):1–9. https://doi.org/10.1055/s-0034-1372609.
Juergens UR, Engelen T, Racké K, Stöber M, Gillissen A, Vetter H. Inhibitory activity of 1,8-cineol (eucalyptol) on cytokine production in cultured human lymphocytes and monocytes. Pulm Pharmacol Ther. 2004;17(5):281–7. https://doi.org/10.1016/j.pupt.2004.06.002.
Fischer J, Dethlefsen U. Efficacy of cineole in patients suffering from acute bronchitis: a placebo-controlled double-blind trial. Cough. 2013;9(1):25. https://doi.org/10.1186/1745-9974-9-25.
Kehrl W, Sonnemann U, Dethlefsen U. Therapy of acute nonpurulent rhinosinusitis with cineole: results of a double-blind, randomized, placebo-controlled trial. Laryngoscope. 2004;114(4):738–42. https://doi.org/10.1097/00005537-200404000-00027.
Worth H, Schacher C, Dethlefsen U. Concomitant therapy with cineole (eucalyptole) reduces exacerbations in COPD: a placebo-controlled double-blind trial. Respir Res. 2009;10(1):69. https://doi.org/10.1186/1465-9921-10-69.
ClinicalTrials.gov: Study of Post-Exposure Ingavirin® Prophylaxis of Influenza and Acute Respiratory Viral Infections. https://clinicaltrials.gov/ct2/show/NCT03189537. Accessed 09 Oct 2019.
Kolobukhina L, Merkulova LN, Mlu S, Burtseva EI, Isaeva EI, Malyshev NA, et al. Ter Arkh. 2009;81(3):51–4.
Sla L, Borisevich SV, Shkliaeva OM, Maksimov VA, Bondarev VP, Nebols’sin VE. [Prophylactic and therapeutic efficacies of Ingavirin, a novel Russian chemotherapeutic, with respect to influenza pathogen A (H5N1)] (Article in Russian). Antibiot Khimother. 2010;55(7–8):10–2.
Lvov DK, Kolobukhina LV, Burtseva EI, Kruzhkova IS, Malyshv NA, Fedyakina IT, et al. [The 2015-2016 Epidemic season in Russia and the world: circulation of influenza viruses, trends in incidence, clinical aspects and treatment algorithm] (article in Russian). Ter Arkh. 2016;88(11):112–20. https://doi.org/10.17116/terarkh20168811112-120.
Shishkina LN, Nebol’sin VE, Kabanov AS, Skarnovich MO, Mazurkova NA, Sergeev AA, et al. [In vitro and in vivo efficacy of Ingavirin against strains of pandemic influenza virus A(H1N1/09)v] (Article in Russian). Zh Mikrobiol Epidemiol Immunobiol. 2011;2:93–6.
Zarubaev VV, Krivitskaia VZ, Nebol’sin VE, Kiselev OI. [Experimental investigation of Ingavirin antiviral activity against human parainfluenza virus] (Article in Russian). Antibiot Khimoter. 2010;55(7–8):13–6.
Zarubaev VV, Sirotkin AK BSV, Anfimov PM, Nebol’sin VE, Kiselev OI, Reikhart DV. [In vitro and in vivo effects of Ingavirin on the ultrastructure and infectivity of influenza virus] (Article in Russian). Vopr Virusol. 2011;56(5):21–5.
Zarubaev VV, Slita AV, Sirotkin AK, Beliavskaia SV, Nebol’sin VE, Reikhart DV, et al. [Effect of Ingavirin on the ultrastructure of the morphogenesis of adenovirus infection in vivo]. (Article in Russian). Vopr Virusol. 2012;57(3):17–23.
Lehrl S, Matthys H, Kamin W, Kardos P. The BSS – a valid clinical instrument to measure the severity of acute bronchitis. J Lung Pulm Respir Res. 2014;1(3):00016. https://doi.org/10.15406/jlprr.2014.01.00016.
Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363(23):2233–47. https://doi.org/10.1056/NEJMra0910061.
Greiner JF-W, Müller J, Zeuner M-T, Hauser S, Seidel T, Klenke C, et al. 1,8-cineol inhibits nuclear translocation of NF-κB p65 and NF-κB-dependent transcriptional activity. Biochim Biophys Acta. 1833;2013(12):2866–78. https://doi.org/10.1016/j.bbamcr.2013.07.001.
Müller J, Greiner JFW, Zeuner M, Brotzmann V, Schäfermann J, Wieters F, et al. 1,8-cineole potentiates IRF3-mediated antiviral response in human stem cells and in an ex vivo model of rhinosinusitis. Clin Sci (Lond). 2016;130(15):1339–52. https://doi.org/10.1042/CS20160218.
Acknowledgements
The authors would like to acknowledge the preparational work by Brigitte Welbers-Joop and additional statistical analyses by Petra Weyrauch, and Susanne Kammerer for preparing the first draft of the manuscript.
Funding
This work has been sponsored by MCL-Pharma LLC, Russia. Legal address: 17 Gogolevsky pkwy., building 1, Moscow 119019. Actual address: 8 Nauchny passage, building 1, office 235, Moscow 117246.
Author information
Authors and Affiliations
Contributions
PK is the author of the manuscript. OK was principal investigator of the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in compliance with the principles of the Declaration of Helsinki approved by the 64th WMA General Assembly, Fortaleza, Brazil, October 2013 and the rules of Good Clinical Practice that are applied in Russia. The investigators committed not to disclose personal or medical data of the volunteers. The conduct of this clinical study was approved by the Ethics Council of the Ministry of Health of the Russian Federation and local ethical committees of healthcare institutions where the study was to be conducted.
Consent for publication
Not applicable.
Competing interests
PK was reimbursed for advising another Soledum study by Klosterfrau, Germany, in the last three years he also had honoraria for advisory boards, steering committees and presentations from following companies: AstraZeneca, Bionorica, Boehringer Ingelheim, Chiesi, Engelhardt, GSK, Menarini, MSD, Novartis, Schwabe. OK was principal investigator of the Soledum study. OK: The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kardos, P., Khaletskaya, O. & Kropova, O. Efficacy and safety of Cineole (Soledum®) in the treatment of patients with acute bronchitis: results of an open-label randomized clinical phase III study. Clin Phytosci 7, 83 (2021). https://doi.org/10.1186/s40816-021-00319-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40816-021-00319-8