Abstract
Background
In traditional North Africa, medicine decoctions of the leaves of Periploca angustifolia are used to treat diarrhea, inflammation, ulcers, edema and diabetes. The aim of the study was to evaluate the phytochemical, anti-inflammatory, anti-ulcerogenic, and hypoglycemic activities of an ethanolic extract of P. angustifolia L. in rats.
Methods
An extract of air-dried powdered P. angustifolia plant was obtained using 96% ethanol. The extract was concentrated and the total phenolic and flavonoids contents were estimated colorimetrically. The phenolic and flavonoid compounds were quantified and identified using high performance liquid chromatography (HPLC). The anti-inflammatory, anti-ulcerogenic and hypoglycemic activities of the extract were evaluated in three rat models respectively: formaldehyde-induced paw edema, ethanol induced gastric damage and alloxan induced hyperglycemia.
Results
The total flavonoids and total phenolics constituted 15% and 2.69% of the extract, respectively and are expressed as quercetin equivalent and μg/mg gallic acid equivalent (GAE). Coumarin, resorcinol, isorhamnetin, quercetin, and naphthalene were isolated from the ethanolic extract of P. angustifolia. Oral administration of the ethanolic extract at 500 mg/kg body weight (b.wt.) significantly reduced paw inflammation, gastric lesions, ulcer index scores and blood glucose levels in normal and diabetic rats.
Conclusion
The crude ethanolic extract of P. angustifolia exhibited promising anti-inflammatory, anti-ulcerogenic, and hypoglycemic activities in accordance with the plant’s uses in folk medicine suggesting that P. angustifolia may be a safe alternative to chemical drugs.
Similar content being viewed by others
Background
Periploca is a genus of plants from the Asclepiadaceae family in the major group of angiosperms. Several species of this family, such as P. angustifolia are widely used in traditional medicine as anti-diabetic, anti-mutagenic, and anti-rheumatic agents [1]. In Egypt, the leaves of P. angustifolia are used to treat rheumatic diseases and the roots are used for hemorrhoids, gastric ulcer and diabetes. Its resin is used as a hypotensive [2] a masticator when burning. P. angustifolia L was used by the Bedouins as animal food and herbal remedies [3]. Different plant extracts of the Asclepiadaceae family have shown significant anti-inflammatory [4] and anti-ulcerogenic properties [5]. In addition, the methanolic extract of P. angustifolia leaves has been shown to have antioxidant effects and exert antidotal effects on cadmium-induced hepatotoxicity [6]. There is a relationship between the antioxidant capacity and anti-hyperglycemic potential of Periploca sylvestre and it may be due to flavonoids and phenolic contents in the plant that impart these properties [7]. Gymnema sylvestre (Asclepiadaceae) has been used since ancient times as a folk medicine for the treatment of diabetes, obesity, and stomach stimulation uses [8]. Aerial parts of P. angustifolia L. collected from southern Tunisia possess antioxidant activity against 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid (ABTS) and 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) [9]. Samples of Hemidesmus indicus var. indicus and var. pubescens during the flowering season possess higher antiulcer and anti- hepato-carcinogenic effects [10, 11]. The chemical composition of the root bark of P. angustifolia, at the flowering stage showed the presence of C-heterosids (anthracenic derivatives), anthocyans, saponins, free quinons and proanthocyanidols [12]. In this study we determined the phytochemical composition and, in particular the phenolic and flavonoids composition of the ethanolic extract of P. angustifolia L., and we assessed anti-inflammatory, anti-ulcerogenic, and hypoglycemic activities.
Methods
Plant material
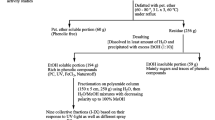
P. angustifolia L. was collected from the Sallum Plateau (northwestern coast of Egypt) during 2012–2013. The plants were air dried at lab-temperature until their weight plateaued, and then ground to a fine powder. The different parts of the plants were identified, confirmed and authenticated by comparing with an authentic specimen at the Plant Taxonomy Unit, Desert Research Center, Cairo, Egypt. The samples were extracted by percolation in ethanol 70%, filtered and this step was repeated several times. The ethanolic extract was concentrated under reduced pressure at temperatures not exceeding 40 °C. The obtained ethanol extract of P. angustifolia L. constituted 10% from the entire dried plant and was used for subsequent investigations. Scheme of separation for flavonoids and phenolics from the whole plant of P. angustifolia L was illustrated in Fig. 1.
Drugs
Diclofenac sodium (Voltarin®) was obtained from Novartis Pharma Co. (Cairo, Egypt) under license from Novartis Pharma AG, (Basle, Switzerland). Ranitidine hydrochloride tablets (Zantac® Batch No. 001716C) were manufactured by Glaxo-Wellcome Egypt (Elsalam City, Cairo, Egypt, each tablet contained 150 mg ranitidine). Glibenclamide (Daonil®) was purchased from Aventis Co., under license from Aventis Pharma Co., West Germany.
Animals
Wistar Albino rats (150–170 g) and Swiss mice (18–22 g) were obtained from the Laboratory Animal Colony, Helwan, Egypt. Animals were maintained in the Animal House of the Pharmacology Department (Faculty of Veterinary Medicine, Cairo University) under controlled conditions [temperature 23 ± 2 °C, humidity 50 ± 5% and 12-h light-dark cycles]. All animals were acclimatized for 7 days before the study. The animals were housed in sanitized polypropylene cages, containing sterile paddy husk as bedding. Animals were habituated to laboratory conditions for 48 h prior to the experimental protocol to minimize non-specific stress. All animals were fed a balanced diet of wheat bran, soybean powder, fish-meal and dietary fibers (manufactured by Cairo Agricultural Development Co.). Water was provided ad libitum. The Institutional Animal Care and Use Committee (IACUC), Cairo University approved this study.
Estimation of Total flavonoids
The flavonoid content in extract was determined spectrophotometrically according to the method described by Djeridane et al. [13], which is based on the formation of a flavonoid–aluminum complex with a maximum absorbance at 430 nm. Total flavonoids are expressed as mg quercetin equivalent.
Estimation of Total phenolic content (TPC)
The Folin-Ciocalteu method [14] was used to determine the TPC spectrophotometrically in the different extracts using gallic acid as standard. The TPC was expressed as μg/mg gallic acid equivalent (GAE).
Identification of phenolic and flavonoids
HPLC was used to identify phenolics and flavonoids. A known weight of air-dried plant powder was soaked in 25 ml sterilized water and agitated on a rotary shaker for 24 h at 200 rpm. Slurry was filtered through Whatmann 3MM filter paper under a vacuum, followed by centrifugation at 12.5 rpm for 30 min at 80 °C. The aqueous extract was acidified to pH 2.5 using diluted phosphoric acid. The sample was sacked three times through a separating funnel with an equal volume of diethyl ether. The combined diethyl ether layer was evaporated to dryness under reduced pressure at 30 °C. The resulting residue was re-dissolved in 3 ml of HPLC-grade methanol and filtered through a sterile membrane with a pore size of a 0.2 μm prior to HPLC analysis [15]. Identification of individual phenolic compounds of the plant sample was performed using a Dionex (Model 3000) HPLC, using a BDS Hypersil C18 reversed-phase column (250 × 4.6 mm) with 10 μm particle size. Injection by means of Rheodyne injection valve (Model 7125) with a 50 μl fixed loop. A constant flow rate of 1 ml/min was used with two mobile phases: distilled water (A), and acetonitrile (B), using a UV detector set at wavelength 254 nm. Phenolic compounds of each sample were identified by comparing their relative retention times with those of the standard mixture chromatogram. The concentration of an individual compound was calculated on the basis of peak area measurements, and then converted to μg/g phenolic dry weight. All chemicals and solvents used were HPLC spectral grade. Standards phenolic compounds were obtained from Sigma (St. Louis, USA) and Merck (Munich, Germany).
Acute toxicity
The acute toxicity (LD50) of ethanolic extract of P. angustifolia administered orally was estimated in mice using Lorke [16] method. Three groups of five animals received 10, 100, 1000 mg/kg of the extract suspended in Tween80 (vehicle 3% v/v). The animals were observed for 72 h for signs of toxicity and death. When no deaths were recorded another four groups of five mice were administrated 2000, 3000, 4000 and 5000 mg/kg of the extract orally. The animals were observed for 72 h for signs of toxicity and the number of deaths was recorded. Control animals were received the equivalent volume of vehicle. The LD50 values were calculated as the geometric mean of the highest non-lethal and the lowest lethal doses mathematically according to the Kerber method [17] using the following formula:
where z is half of the sum animals that died with the two next doses; d is the interval between two next doses and m is the number of animals/group.
Anti-inflammatory activity
The extract was evaluated for its anti-inflammatory activity in rats using the formaldehyde-induced paw edema method [18]. Acute inflammation was produced by sub-plantar injection of 0.2 ml formaldehyde (1% w/v) into the hind paw 1 h after oral administration of ethanolic extract of P. angustifolia (500 mg/kg b.wt.) or diclofenac sodium (50 mg/kg b.wt.) as a standard anti-inflammatory agent. The paw volume was measured in mm by a plethysmometer (Ugo-Basile, Italy) at 1, 2, 3, and 4 h after the formaldehyde injection. Inhibition of inflammation was calculated using the following formula: % inhibition = 100 (1-Vt/Vc), where ‘Vc’ represents edema volume in the control group and ‘Vt’ represents edema volume in the test group.
Anti-ulcerogenic activity
All rats were fasted for 48 h but were given water ad libitum till the start of the experiment. To prevent excessive dehydration during the fasting period, rats were supplied with sucrose (BDH) 8% (w/v) solution in NaCl (BDH) 0.2% (w/v), which was removed 1 h before experiments [19]. The animals were randomly separated into three groups of six rats. One group was pretreated with ethanolic extract of P. angustifolia orally at 500 mg/kg b.wt., another group received ranitidine (100 mg/kg orally) and the control group received equivalent volumes of saline instead of plant extract. One hour later, all groups were treated with ethanol (50%) at a dose of 10 ml/kg. One hour after ethanol administration, all rats were euthanized by an overdose of chloroform and the abdomen was opened. The stomach was removed, opened along the greater curvature, and gently rinsed under running water. The tissues were fixed with 10% formaldehyde in saline. Macroscopic examination was carried out under a hand lens and the presence of ulcer lesions was scored [20]. Lesions in the glandular part of the stomach were measured under an illuminated magnifying microscope (10 x). Long lesions were counted and measured along their greater length. Petechial lesions were counted with the aid of 1-mm squares grid [21]. Each five petechial lesions were considered to represent a 1-mm ulcer. The ulcer index (%) for each group was calculated as the sum of the lengths of long ulcers and petechial lesions divided by its number.
Hypoglycemic effect of P. angustifolia L. extract
Induction of diabetes
Rats were rendered diabetic by subcutaneous injection of alloxan monohydrate (Oxford) at a dose of 150 mg/kg/day for 3 days (early ketosis) and normal feeding was maintained [22]. Five days later, blood samples were drawn and the blood glucose level was measured to establish the occurrence of diabetes. The threshold for diabetes in the present study was a glucose level of ≥225 mg/dl.
Effect of P. angustifolia L. extract on hyperglycemic rats
The hypoglycemic effect of P. angustifolia L. extract was studied in alloxan-induced diabetic rats. The animals were fasted for 8 h but allowed free access to water. The diabetic animals were randomly divided into three groups of 10 rats and received oral P. angustifolia L. extract (500 mg/kg b.wt.), glibenclamide (0.2 mg/kg) or 20% v/v Tween 80 (5 ml/kg b.wt.).
Effect of P. angustifolia L. extract on Normoglycemic rats
Non-diabetic rats were fasted overnight and then, randomly divided into three groups of 10 rats. As with the diabetic rats, the non-diabetic received oral P. angustifolia L. extract (500 mg/kg b.wt.), glibenclamide (0.2 mg/kg b.wt.) or 20% v/v Tween 80 (5 ml/kg b.wt.). One milliliter of blood was collected before and after two hours of treatments from each rat in all groups. Blood glucose was estimated by the glucose oxidase method using the Randox kit (Randox Laboratories Ltd., Ardmore, UK) according to the manufacturer’s instructions.
Statistical analysis
The results are expressed as the mean ± standard error (SE). The differences between the experimental groups were analyzed by one-way analysis of variance (ANOVA), followed by Bonferroni’s test using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). The results were considered statistically significant when p values less than 0.05 and 0.01 were considered significant (*p < 0.05, **p < 0.01).
Results
Acute toxicity
There were no changes in the general behavior of the animals at any dose and, there were no deaths after 72 h at the highest administered dose (5000 mg/kg) of the extract. The safety of the extract was shown by the high LD50 value of the extract (> 5 g/kg).
Total flavonoids and phenolic contents
The total flavonoids and phenolic contents of P. angustifolia L were 3.15 ± 0.7% as quercetin equivalent and 2.69 ± 0.6% as gallic acid equivalent, respectively.
Identification and quantification of phenolic and flavonoid compounds
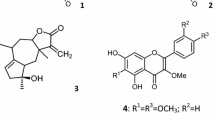
Quantitative and qualitative analysis of the phenolic and flavonoid compounds in the ethanolic extract of P. angustifolia L. was performed using HPLC, where each compound was separated and identified using authentic standard. The compounds were, coumarin, resorcinol, isorhamnetin, quercetin, and naphthalene with different concentration ranges (Table 1 and Fig. 2). Resorcinol reached its maximum value of 56.54% in ethanolic extract of P. angustifolia L. followed by isorhamnetin at 40.53%, while quercetin was estimated to have a minimum value of 0.002%.
Anti-inflammatory activity
The formaldehyde-induced paw edema model showed that sub-plantar injection of formaldehyde in rats caused a time-dependent increase in paw thickness and the maximal increase was observed 4 h after formaldehyde administration (Table 2). However, rats that received the ethanolic extract of P. angustifolia pretreatment showed significantly less (P < 0.05, P < 0.01) formaldehyde-induced inflammation at each time point than the animals that received formaldehyde only as did those that received the reference anti-inflammatory drug diclofenac sodium (P < 0.01). Oral administration of P. angustifolia L. extract at 500 mg/kg resulted in a maximal inhibition of paw inflammation 42.42% that was close to that of diclofenac sodium 45.45% at 50 mg/kg 4 h post formaldehyde administration (Fig. 3).
Anti-ulcer activity
Ethanol caused extensive gastric damage in the mucosa of the control animals. The lesions were characterized by multiple long hemorrhagic red bands of different sizes along the axis of the glandular stomach with petechial patches. By contrast, oral treatment with the ethanolic extract of P. angustifolia L showed significantly fewer (p < 0.01) the gastric lesions and lower ulcer index than those observed in the control animals. The crude ethanolic extract of P. angustifolia L. had a protective index of 44.93%, whereas ranitidine as a reference standard (100 mg/kg) exhibited a protective index of 46.99% indicating a potent anti-ulcerogenic effect of P. angustifolia L. extract (Table 3 and Fig. 4).
Hypoglycemic activity
Subcutaneous injection of rats with alloxan resulted in a significant increase in serum glucose levels. Administration of the crude ethanolic extract of P. angustifolia significantly reduced blood glucose levels at 2 h compared with those untreated diabetic rats. Specifically, the ethanolic extract significantly reduced the postprandial blood glucose level of diabetic rats from 211.16 to 124.33 mg/dl (Table 4 and Fig. 5). Similarly, the extract of P. angustifolia at the same dose significantly reduced blood glucose level in normoglycemic rats (Table 5 and Fig. 6).
Discussion
P. angustifolia extract had a high safety margin in mice as the LD50 was > 5 g/kg. Similarly, Sunil et al. [23] found that alcoholic extract of another plant in the Asclepiadaceae family Holostemma ada kodien was non toxic at a dose of 5 g/kg b.wt.
Phenolic compounds increase a plant biological value because they exhibit a range of pharmacological properties, such as anti-diabetic, anti-allergenic, anti-atherogenic, anti-inflammatory, antioxidant, anti-thrombotic, and vasodilator effects [24, 25]. Oxidative stress activates inflammatory pathways in stem cells and progenitor cells, leading to exhaustion of these cells due to increased levels of reactive oxygen species (ROS) [26]. Cellular exhaustion in turn leads to the development of several diseases, such as gastrointestinal ulcers, hyperglycemia, and hepatic dysfunction [27]. Thus, natural antioxidants provide cellular protection and lead to favorable effects in diabetes mellitus [28] and the majority of inflammatory and cardiovascular diseases [29]. Examples of naturally occurring antioxidants are flavonoids, phenolic acids, coumarin, isorhamnetin and quercetin that were separated and identified from P. angustifolia extract with promising anti-inflammatory agents. This activity may be due to their inhibitory action on neutrophils infiltration, cyclooxygenase-2 activity and inflammatory cytokines release [30,31,32]. The anti-ulcerogenic activity of P. angustifolia L. may also be due to the presence of quercetin, as it prevents gastric mucosal damage by increasing mucus production with a comparable regression of gastric lesions [33]. Hyperglycemia is a metabolic disorder that occurs due to the excess production of ROS, which destroy pancreatic β-cells and is associated with vascular complications including neuropathy, retinopathy, and nephropathy [34]. Herbal medicines have long been used for the treatment of diabetes mellitus and have fewer side-effects of toxicity than other hypoglycemic drugs. In the present study, administration of the ethanolic extract of P. angustifolia significantly reduced blood glucose levels in normoglycemic and diabetic rats. This promising effect may be attributed to the inhibition of aldose reductase which converts glucose to sorbitol and α-amylase and α-glucosidase (key enzymes linked to type-2 diabetes) by the phenols and flavonoids [35, 36].
Because of its basic chemical structure, quercetin is a antioxidant activity and it is now used as a nutritional supplement for a variety of diseases such as diabetes/obesity and circulatory dysfunction, including inflammation as well as mood disorders [37].
Conclusion
The crude ethanolic extract of P. angustifolia exhibited promising anti-inflammatory, anti-ulcerogenic, and hypoglycemic activities, which are in accordance with its use folk medicine. As the extract showed a high, safety profile this study may serve as a guideline for the standardization and validation of natural drugs containing selected medicinal plants ingredients. Moreover, further investigation for the selective activities is required to determine the exact mechanism of action.
Abbreviations
- ABTS:
-
2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid
- DPPH:
-
2,2-diphenyl-1-picrylhydrazyl radical
- GAE:
-
Gallic acid equivalent
- HPLC:
-
High Performance Liquid Chromatography
- LD50:
-
Lethal dose 50
- P. angustifolia :
-
Periploca angustifolia
- ROS:
-
Reactive oxygen species
References
Rabei S, Khalik KA. Conventional keys for Convolvulaceae in the flora of Egypt. Flora Mediterr. 2012;22:45–62.
Hammiche V, Maiza K. Traditional medicine in Central Sahara: pharmacopoeia of Tassili N’ajjer. J Ethnopharmacol. 2006;105:358–67.
Bouhouche N. Conservation and multiplication of an endangered medicinal plant – Caralluma arabica – using tissue culture. Planta Med [Internet]. 2011;77:PB49. Available from: https://www.thieme-connect.com/products/ejournals/abstract/10.1055/s-0031-1282303
Laupattarakasem P, Wangsrimongkol T, Surarit R, Hahnvajanawong C. In vitro and in vivo anti-inflammatory potential of Cryptolepis buchanani. J Ethnopharmacol. 2006;108:349–54.
Pandya D, Anand I. A complete review on Oxystelma esculentum R. Br. Pharmacogn J. 2011;3:87–90.
Athmouni K, Belhaj D, Mkadmini Hammi K, El Feki A, Ayadi H. Phenolic compounds analysis, antioxidant, and hepatoprotective effects of Periploca angustifolia extract on cadmium-induced oxidative damage in HepG2 cell line and rats. Arch Physiol Biochem. 2017:1–14.
Ibrahim A, E O, A. J N, IA U. Combined effect on antioxidant properties of Gymnema Sylvestre and Combretum Micranthum leaf extracts and the relationship to hypoglycemia. Eur Sci J. 2017;13:266–81.
Al-Rejaie SS, Abuohashish HM, Ahmed MM, Aleisa AM, Alkhamees O. Possible biochemical effects following inhibition of ethanol-induced gastric mucosa damage by Gymnema sylvestre in male Wistar albino rats. Pharm Biol. 2012;50:1542–50.
Bouaziz M, Dhouib A, Loukil S. Polyphenols content, antioxidant and antimicrobial activities of extracts of some wild plants collected from the south of Tunisia. African J. Biotechnol. [Internet]. 2009;8:7017–7027. Available from: http://www.ajol.info/index.php/ajb/article/view/68789
Anoop A, Jegadeesan M. Biochemical studies on the anti-ulcerogenic potential of Hemidesmus indicus r.Br. Var. indicus. J Ethnopharmacol. 2003;84:149–56.
Galhena P, Thabrew I, Tammitiyagodage M, Hanna RV. Anti-hepatocarcinogenic Ayurvedic herbal remedy reduces the extent of diethylnitrosamine-induced oxidative stress in rats. Pharmacogn Mag. 2009;5:19–27.
Fairouz D, Sami Z, Mekki B, Mohamed N. Chemical composition of root bark of Periploca angustifolia growing wild in Saharian Tunisia. J Essent Oil-Bearing Plants. 2013;16:338–45.
Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97:654–60.
Li C, Feng J, Huang WY, An XT. Composition of polyphenols and antioxidant activity of rabbiteye blueberry (Vaccinium ashei) in Nanjing. J Agric Food Chem. 2013;61:523–31.
Ma Y, Kosinska-Cagnazzo A, Kerr WL, Amarowicz R, Swanson RB, Pegg RB. Separation and characterization of phenolic compounds from dry-blanched peanut skins by liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr A. 2014;1356:64–81.
Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983;54:275–87.
Dhanarasu S, Selvam M, Al-Shammari NKA. Evaluating the pharmacological dose (Oral ld50) and antibacterial activity of leaf extracts of Mentha piperita Linn. Grown in Kingdom of Saudi Arabia: a pilot study for nephrotoxicity. Int J Pharmacol. 2016;12:195–200.
Nalini GK, Patil VM, Ramabhimaiah S, Patil P, Vijayanath V. Anti-inflammatory activity of wheatgrass juice in albino rats. Biomed Pharmacol J. 2011;4:301–4.
Hironaka A, Susumu O, Yoshihiko I, Masahiro O, Kazuei I, Seiyu H. Polyamine inhibition of gastric ulceration and secretion in rats. Biochem Pharmacol. 1983;32:1733–6.
Nordin N, Salama SM, Golbabapour S, Hajrezaie M, Hassandarvish P, Kamalidehghan B, et al. Anti-ulcerogenic effect of methanolic extracts from Enicosanthellum pulchrum (king) HEUSDEN against ethanol-induced acute gastric lesion in animal models. PLoS One. 2014;9:e111925.
Chen S-H, Liang Y-C, Chao JCJ, Tsai L-H, Chang C-C, Wang C-C, et al. Protective effects of Ginkgo biloba extract on the ethanol-induced gastric ulcer in rats. World J Gastroenterol [Internet]. 2005;11:3746–3750. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15968732
Tang LQ, Wei W, Chen LM, Liu S. Effects of berberine on diabetes induced by alloxan and a high-fat/high-cholesterol diet in rats. J Ethnopharmacol. 2006;108:109–15.
Sunil J, Krishna J, Bramhachari P. Hepatoprotective activity of Holostemma ada Kodien shcult, extract against paracetamol induced hepatic damage in rats. European J Med Plants [Internet]. 2015;6:45–54. Available from: http://www.sciencedomain.org/abstract.php?iid=793&id=13&aid=7471.
Abushouk AI, Ismail A, AMA S, Afifi AM, Abdel-Daim MM. Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity. Biomed Pharmacother. 2017;90:935–46.
Ganguly S, Kumar TG, Mantha S, Panda K. Simultaneous determination of black tea-derived catechins and theaflavins in tissues of tea consuming animals using ultra-performance liquid-chromatography tandem mass spectrometry. PLoS One. 2016;11:e0163498.
Oh J, Lee YD, Wagers AJ. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med. 2014;20:870–80.
Morris G, Maes M. Oxidative and Nitrosative stress and immune-inflammatory pathways in patients with Myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS). Curr Neuropharmacol [Internet]. 2014;12:168–85 Available from: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1570-159X&volume=12&issue=2&spage=168.
Youn J-Y, Siu KL, Lob H, Itani H, Harrison DG, Cai H. Role of vascular oxidative stress in obesity and metabolic syndrome. Diabetes [Internet]. 2014;63:2344–2355. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24550188
Bu J, Dou Y, Tian X, Wang Z, Chen G. The role of Omega-3 polyunsaturated fatty acids in stroke. Oxidative Med Cell Logevity. 2016;2016:1–8.
Nguyen PH, Zhao BT, Kim O, Lee JH, Choi JS, Min BS, et al. Anti-inflammatory terpenylated coumarins from the leaves of Zanthoxylum schinifolium with α-glucosidase inhibitory activity. J Nat Med. 2016;70:276–81.
Antunes-Ricardo M, Gutiérrez-Uribe JA, López-Pacheco F, Alvarez MM, Serna-Saldívar SO. In vivo anti-inflammatory effects of isorhamnetin glycosides isolated from Opuntia ficus-indica (L.) mill cladodes. Ind Crop Prod. 2015;76:803–8.
Wang L, Wang B, Li H, Lu H, Qiu F, Xiong L, et al. Quercetin, a flavonoid with anti-inflammatory activity, suppresses the development of abdominal aortic aneurysms in mice. Eur J Pharmacol. 2012;690:133–41.
De La Lastra CA, Martín MJ, Motilva V. Antiulcer and gastroprotective effects of quercetin: a gross and histologic study. Pharmacology. 1994;48:56–62.
Su S-L, Liao P-Y, Tu S-T, Lin K-C, Tsai D-H, Sia H-K, et al. Correlation analysis of HbAlc and preprandial plasma glucose in diabetes complications. Diabetes [Internet]. 2009:58 Available from: http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L70135710%5Cnhttp://professional.diabetes.org/Abstracts-Display.aspx?TYP=1&CID=73906%5Cnhttp://sfx.library.uu.nl/utrecht?sid=EMBASE&issn=00121797&id=doi:&atitle=Correlation+analysis+.
Lee YS, Lee S, Lee HS, Kim BK, Ohuchi K, Shin KH. Inhibitory effects of isorhamnetin-3-O-beta-D-glucoside from Salicornia herbacea on rat lens aldose reductase and sorbitol accumulation in streptozotocin-induced diabetic rat tissues. Biol Pharm Bull. 2005;28:916–8.
Adedayo BC, Ademiluyi AO, Oboh G, Akindahunsi AA. Interaction of aqueous extracts of two varieties of yam tubers (Dioscorea spp) on some key enzymes linked to type 2 diabetes in vitro. Int J Food Sci Technol. 2012;47:703–9.
D’Andrea G. Quercetin: a flavonol with multifaceted therapeutic applications? Fitoterapia. 2015;106:256–71.
Acknowledgements
Not applicable.
About the authors
The corresponding author.
Prof. Khaled Abo-EL-Sooud.
Professor of Pharmacology, Faculty of Veterinary Medicine, Cairo University from 2005 till now. Ph.D. Canadian-Egyptian Scholarship, Cairo University, 1995 at centre for food and animal research, Agriculture Canada, Ottawa, Canada. Teaching Undergraduate and graduate Courses in University of Science and Technology, Irbid, Jordan (2000–2002) and in Qassim University, Buraidah, Saudi Arabia (2005–2007). Supervising and discussing several Master and Ph.D. theses in Egypt and Arabian Countries. Expertise in Radioisotopes and different types of Chromatography (GC-HPLC-TLC etc.) for detection of drug residues in tissues and food. Publishing about 70 papers in different international journals enclosed list of publications. Member of the promotion committee of Supreme Council committee 100 B for Veterinary Pharmacology, Toxicology and Forensic Medicine from 2013 to 2019. Nowadays the research is shifted to ethnopharmacology. Attained a lot of international conferences and obtained several awards and prizes. Member of veterinary drug administration’s committee, ministry of Health, Egypt.
ASSOCIATE EDITOR.
International Journal of Veterinary Science and Medicine.
GUEST EDITORS IN.
Oxidative Medicine and Cellular Longevity.
https://mts.hindawi.com/guest.editor/journals/omcl/adct/
Evidence-based Complementary and Alternative Medicine.
https://mts.hindawi.com/guest.editor
http://scholar.cu.edu.eg/kasooud
http://scholar.google.com/citations?user=Ww4Vqd8AAAAJ
https://www.scopus.com/authid/detail.uri?authorId=6603356090
http://orcid.org/0000-0001-7636-7018
Funding
Desert Research Center, Medicinal and Aromatic Plants Department, Cairo, Egypt Supporting Ph.D. Study of Dr. Hanan M. ELTantawy.
Author information
Authors and Affiliations
Contributions
KA-E Sooud performed the pharmacological evaluation on animal studies and data collection. Other authors performed the phytochemical analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Animal Care and Use Committee (IACUC), Cairo University approved the animal study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Abo-EL-Sooud, K., Ahmed, F.A., El-Toumy, S.A. et al. Phytochemical, anti-inflammatory, anti-ulcerogenic and hypoglycemic activities of Periploca angustifolia L extracts in rats. Clin Phytosci 4, 27 (2018). https://doi.org/10.1186/s40816-018-0087-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40816-018-0087-6