Abstract
The dried fruits of the chaste tree Vitex agnus castus (VAC) were traditionally used by monks as a substitute for pepper and was therefore also called Monk’s pepper. For the last 50 years it is commercially provided for the treatment of premenstrual symptoms, particularly to prevent premenstrual mastodynia (mastalgia). Most studies were performed with the preparation containing an aqueous/ethanolic ectract BNO 1095. A number of placebo controlled studies gave proof that extracts of VAC had beneficial effects on premenstrual breast pain. This breast sensation is induced by latent hyperprolactinemia which is characterized by secretory episodes of prolactin release by the pituitary in response to stress and deep sleep phases. This latent hyperprolactinemia induces also often a corpus luteum insufficiency which is a common reason for infertilty.
It is well accepted that prolactin release can be reduced by dopamine and dopaminergic drugs. The efficacy of VAC extracts to ameliorate prolactin induced premenstrual mastodynia was therefore suggestive that VAC may contain dopaminergic compounds. Indeed, a number of diterpenes were identified that bound to recombinant Dopamine receptors of the 2 subtype (D2 receptors) which are present in pituitary lactotropes and which mediate the inhibitory effects of dopamine and dopaminergic drugs on pituitary prolactin release. Consequently, prolactin release in vitro from dispersed pituitary cells and in vivo in rats and postmenopausal women was inhibited by VAC 1095. Placebo controlled studies proved also the efficacy of VAC extracts to ameliorate premenstrual symptoms. In several placebo-controlled studies a clear relation between reduction of breast pain and reduction of serum prolactin levels could be established. In addition VAC extracts was also highly effective in women suffering from fibrocystic mastopathy. In many of these women serum prolactin levels were also elevated and reduced by VAC extracts.
The results from all trials suggested that VAC extracts ameliorated premenstrual symptoms including mastodynia, premenstrual dysphoric disorder and latent hyperprolactinemia. Cystic mastopathy and sterility due to corpus luteum insufficiencies were also beneficially influenced.
Adverse events with VAC were mild and generally infrequent.
Similar content being viewed by others
Introduction
The Premenstrual Syndrome (PMS) also called premenstrual disorders (PMDs) affect up to 70% of women and between 10–20% of them have a severe form, the Premenstrual Dysphoric disorder (PMDD). The definition, diagnosis and management have always been challenging. These conditions affect reproductive aged women and can have a substantial impact on quality of life, with resultant impairment of education/work. The definition of PMSD is somehow weak but there is consensus that symptoms of the core menstrual disorders occur pemenstrually, may occur already during the luteal phase but exacerbate premenstrually and ease during the menstrual period. The symptoms may be primarily psychic or somatic with a wide overlap. The most common symptoms of PMS are listed in Table 1.
Premenstrual syndrome (PMS), mastodynia, prolactin and infertility
One of the most common and frightening symptoms for women is premenstrual breast pain - premenstrual mastodynia - which occurs in more than 20% of the female population. When this discomfort is extreme it is classified as mastalgia (severe mastodynia) [1–3]. There is evidence that in women suffering from premenstrual mastodynia or mastalgia a latent hyperprolactinemia is one of the major reasons for the development of the complaints [3, 4]. Patients suffering from premenstrual mastodynia have under resting conditions often normal serum prolactin levels, under stress situations however, pituitary prolactin release seems to be augmented. The spontaneous release of prolactin occurs in pulses and in the luteal phase these pulses occur synchronously with LH pulses [5]. In women with a latent hyperprolactinemia these prolactin pulses are higher than in women with normal prolactin release (Fig. 1). Hence, highest prolactin pulses in women suffering from PMS are observed in the late luteal and the premenstrual phase (Fig. 1) and also stress and deep sleep induced prolactin release is exaggerated during the luteal phase. This was studied in some more detail and results are shown in Fig. 2. These results stem from a study comprising women with PMdy and women without breast discomfort. They were investigated during the late luteal phase. Prolactin levels in women suffering of premenstrual mastodyniay were in a pathologic range i.e. > 500 μU. Such effects were not observed in the women who did not suffer from premenstrual breast pain. Interesting, in comparison to women without breast complaints the women with premenstrual mastodynia had significantly lower serum progesterone values and these low levels were in the pathologic range indicating a corpus luteum insufficiency (Fig. 2) which is a common reason for infertility. These findings confirm and extend earlier results demonstrating increased prolactin and low progesterone levels in women suffering from premenstrual mastodynia [3, 6, 7].
In women who do not suffer from premenstrual mastodynia LH pulses during the luteal phase occur relatively regular at 2–4 h intervals. These pulses are accompanied by prolactin pulses and are stimulatory to progesterone secretion by the corpus luteum (upper graph). In women suffering from premenstrual mastodynia the prolactin pulses are often of such a height that-at their peak time-one could suspect a pituitary prolactinoma. In the woman show in the lower graph LH pulses are of normal height gut the corpus luteum fails to respond with increade progesterone secretion
Infertile patients with premenstrual mastodynia (PMDY) have often a moderate hyperprolacinemia (i.e. values > 500 μU/ml) during the midluteal phase and this is associated with low serum progesterone. Women who do not suffer under PMDY have normal prolactin and progesterone values This indicates that latent hyperprolactinemia is often associated with a corpus luteum insufficiency
It is currently generally accepted that high and frequently occurring prolactin episodes stimulate proliferation of mammary gland tissue causing the uncomfortable breast sensations, i.e. mastodynia (mastalgia) [8]. Hence, the latent hyperprolactinemia mimicks effects seen during early pregnancy [9].
Taken together it appears quite clear that latent hyperprolactinemia is a, if not the major cause of premenstrual mastodynia, mastalgia and infertility due to insufficient function of the corpus luteum. It was therefore very early attempted to cure the painful breast sensations by drugs which inhibit pituitary prolactin release. Under physiologic conditions the biogenic amine dopamine inhibits pituitary prolactin release. This has led to development of synthetic drugs with dopaminergic activity which were all derivatives of ergot alkaloids. It became soon clear that decreased prolactin levels under treatment with the dopamine receptor agonist bromocriptine ameliorated the severity of premenstrual mastodynia, an effect not seen in the placebo treated patients [3, 10]. Also, a study addressing the question whether the synthetic dopamine agonist lisuride ameliorates also other PMS symptoms yielded positive results [11]. But these systemic dopaminergic drugs had all severe side effects such as nausea and orthostatic dysregulation [12].
Treatment options with Vitex agnus castus (VAC)
As mentioned above synthetic dopaminergic drugs ameliorated premenstrual symptoms including mastodynia. Due to severe side effects of these drugs many women do refuse to use conventional treatment with hormones or psychopharmaceuticals and seek often for plant derived alternatives which become therefore increasingly prevalent in the Western world. In this context, the extracts of Vitex agnus-castus fruit (VAC, chaste tree, chasteberry; family: Verbenaceae) are commonly used for the treatment of premenstrual syndrome which-as elaborated above-is often associated by premenstrual mastodynia, premenstrual dysphoric disorder (PMDD) and corpus luteum insufficiency.
Pharmacology of VAC
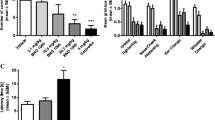
A number of plants produce substances which bind to one or both estrogen receptors (ERα and ERβ). Most adverse effects of estrogens in the mammary gland and uterus are exerted via the ERα. The uterotrophic assay in ovariectomized rats is the OECD recommended test system for ERα activity [13]. Administration of the VAC extract BNO 1095 over a period of 3 months even at high doses neither stimulated uterine weights nor were estrogen stimulated genes affected (Fig. 3a, b and c, unpublished data). In earlier studies we demonstarted the presence of apigenin in VAC BNO 1095 which binds to ERß [14]. In another study [15] linoleic acid was isolated from a VAC extract which stimulated some ERα specific events in ERα expressing cells and recently some substances have been isolated with affinities to both ER subtypes [16].
Particularly the beneficial effects of VAC extract on mastalgia were suggestive that extracts of the dried fruit may contain dopaminergic compounds which inhibit pituitary prolactin release. Consequently it was indeed shown that VAC extracts may fulfill these prolactin inhibiting conditions [17–19]. Dopaminergic activity via binding to dopamine-2 (DA-2) receptors [17] was proven by several experimental approaches:
-
1.
Radioreceptorassays
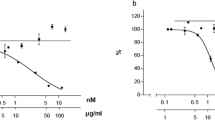
The D2 receptor is nowadays available as recombinant protein. When incubated with radioactively labeled dopamine a large fraction of this labeled amine binds to the receptor and this bound radioactive dopamine can be dose dependently displaced by non labeled dopamine (Fig. 4). When the VAC extract BNO 1095 was added instead of the non labeled dopamine this resulted also in a dose dependent displacement of the labeled dopamine (Fig. 4) indicating binding to the recombinant dopamine receptor and thereby displacing the radioactively labeled dopamine from the receptor.
Fig. 4 Radioactively labeled dopamine (DA) binds to recombinant DA receptors and increasing amounts of non labeled DA displaces the radioactively labeled DA. After separation of bound from non bound DA this results in a standard curve. Any substance which also displaces the radioactively labeled DA indicates that it binds also to the DA receptor. The VAC extract BNO 1095 displaces the radioactively labeled DA from the DA receptor which is evidence for the presence of dopaminergic compounds
-
2.
In vitro studies
Rat pituitary cells, kept under culture conditions secrete large amounts of prolactin and this can be effectively inhibited by dopamine and dose dependently by the VAC extract BNO 1095 which is used commercially for the production of Mastodynon and Agnucaston (Fig. 5). The receptor specificity of the extract was proven by the fact that haloperidol, a specific dopamine receptor antagonist prevented the inhibitory effects of dopamine as well as of the VAC extract (Fig. 5).
Fig. 5 Dispersed rat pituitary cells secrete large amounts of prolactin which are profoundly inhibited by DA. The VAC extract BNO 1095 inhibits prolactin release which is further evidence for dopaminergic activity of this extract. The DA 2 receptor specificity is proven by the reduction of the inhibitory effects of both, DA and VAC BNO 1095 by haloperidol, a DA 2 receptor blocker
-
3.
In vivo studies
-
a.
In rats
It was already mentioned above that stress is a potent stimulus for pituitary prolactin release in women. Also rats react to stress with massive release of prolactin (Fig. 6). When rats were pretreated with the VAC extract BNO 1095 prior to application of an ether stress this prevented prolactin release significantly (Fig. 6).
Fig. 6 In female rats the response to a stressful stimulus (in this case ether stress) results in marked prolactin release. Application of the VAC extract BNO 1095 ameliorated this stress induced prolactin release significantly giving further evidence to the presence of dopaminergic compounds in this extract
-
b.
In women
Also women suffering from premenstrual mastodynia have supraphysiologic high prolactin pulses resulting premenstrually in supraphysiologic mean prolactin levels (Fig. 1 and 2). Such high prolactin levels can be normalized by the treatment with the BNO 1095 containing extract in Mastodynon and Agnucaston (Fig. 7) [19]. In this and in another trial [20] the effects of a VAC BNO 1095 containing preparation on premenstrual mastodynia were significantly better than those of placebo (Fig. 7).
-
a.
In attempts to identify the dopaminergic substances 2 different VAC extracts (one of them was BNO 1095) were shown to contain a number of diterpenes with dopaminergic activities [18, 19]. In addition to these prolactin inhibiting substances VAC extracts were shown to contain flavonoids which bound to μ- and δ-opioid receptors and it was suggested that such compound may also ease premenstrual symptoms [21].
Clinical studies
Most publications specified several different VAC preparations with VAC BNO 1095 present in Mastodynon® and Agnucaston®. The PMS studies as well as studies on mastodynia/mastalgia, latent hyperprolactinemia and fibrocystiv mastopathy are specified in Table 2. The most commonly prescribed dose was 40 mg/day dried fruit which is equivalent to 4.0 mg extract.
Fourteen of 21 PMS studies were placebo-controlled while 7 studies compared the effects of VAC with the serotonin reuptake inhibiting antidepressant fluoxetine, or with other plant derived non-estrogenic extracts or with pyridoxine (vitamin B6) and magnesium, respectively.
Mastodynia and fertility
As outlined above stress- and sleep related increased prolactin levels appear to inhibit corpus luteum function and to reduce the secretion of progesterone in the luteal phase of the menstrual cycle [22] and this has led to clinical studies in latent hyperprolactinemic women with premenstrual mastodynia (Fig. 2). Improved fertility was later confirmed in a trial involving 44 infertile patients due to luteal phase defects, treatment with 40 mg of a dried Vitex agnus castus (VAC BNO 1095) preparation increased both, serum progesterone and estradiol [23, 24]. Following this treatment ovulatory cycles were present in 93% and fertility rate was restored in 71.4% of the patients. These results are comparable to those shown in Figs. 1 and 2 and indicate that VAC extracts may indeed be helpful in cases of infertility. The same group demonstrated a significant reduction of serum prolactin levels under a VAC preparation in patients suffering from polycystic ovarian disease [24].
How can the effects of VAC on luteal function be explained? Continuously high prolactin levels inhibit the hypothalamic pulse generator [25]. This results in infertility because normal gonadotropin release is inhibited. In latent hyperprolactinemic patients the hypothalamic GnRH pulse generator appears to function normally because LH pulses of normal height are present (Fig. 1). Hence we face the possibility that the exaggerated height of prolactin pulses during the luteal phase (Fig. 1) have a direct inhibitory effect on luteal progesterone secretion. It appears therefore safe to conclude that VAC preparations contain dopaminergic substances which normalize the exaggerated height of prolactin pulse which allows normal luteal progesterone secretion, thereby preventing luteal insufficiency and promoting fertility. This suggestion is graphically outlined in Fig. 8.
In one of the studies shown in Fig. 7 (Wuttke et al. 2003) treatment of PMDY patients with the VAC 1095 containing preparations resulted in significantly lower midluteal serum prolactin levels. Such effects were not seen in the placebo treated patients
Another additional positive effect of the dopaminergic effects in VAC may be the demonstration that dopamine receptors of the 1, 2, 4 and 5 subtype are expressed in follicular granulosa and in luteal cells which opens the possibility that the dopaminergic compounds in VAC promote follicular development and luteal function [26–28].
The issue of a relation between premenstrual symptoms, particularly of premenstrual mastodynia and the effectiveness of VAC preparations was recently reviewed by van Die et al. [8]. This review lacks, however, a number of European, particularly East European studies. A wider review of placebo controlled studies utilizing VAC extract are shown in Table 2a-c. In all, but one of a total of 21 well conducted, placebo- or comparator controlled clinical trials such effects were well documented.
Results of studies not included in the recent review by van Die [8] are briefly discussed in the following: Women who start contraception with combined oral contraceptives (COC) frequently experience mastodynia which may last several months. Of particular interest is therefore the large study involving patients who suffer from mastodynia under COC [29]. In this study BNO 1095 (Mastodynon) proved highly effective to reduce the COC induced painful sensations.
In a solid comparator controlled trial the effects of a treatment with 40 mg of a dried VAC fruit preparation for 3 months on premenstrual mastalgia, other PMS symptoms an on latent hyperprolactinemia in 2×24 patients were compared with effects of 2×2.5 mg bromocriptine [30]. Although bromocriptine was more effective to reduce serum prolactin levels into the normal range, the VAC preparation reduced mastalgia and PMS symptoms more efficiently than the synthetic dopamine agonist. While a small number of VAC treated women reported mild side effects (8.3% experienced headache and 5.8% nausea), such effects occurred more frequently in the bromocriptine treated women (21% headache, 15.8% nausea and 12.3% constipation).
In a large study, comprising 129 women, the effects of 40 mg dried VAC fruit on latent hyperprolactinemia in patients suffering from mastodynia were compared to those of a treatment with bromocriptine or Dostinex. The latent hyperprolactinemia was successfully treated, i.e. prolactin levels were normalized in all 3 treatment groups which caused cessation of the painful breast sensations. Interestingly mammary duct thickness was reduced by the 2 synthetic but not by the VAC preparation.
In another trial severity of PMS and of premenstrual mastodynia was evaluated in 31 subjects with latent hyperprolactinemia prior to and after treatment with 40 mg of a VAC dried fruit preparation, which resulted in a significant reduction of both parameters [31].
In summary for the efficacy of VAC preparations and premenstrual PMS and mastodynia: Almost all placebo controlled studies published hitherto agree that extracts of the fruit of this plant have beneficial effects on PMS, particularly on premenstrual mastodynia (Table 2).
Fibrocystic mastopathy (FCM)
Fibrocystic mastopathy is the most common mammary pathology and affects 60–80% of women in the reproductive age [32–34]. Many women are frightened that the development of fibrous nodes and/or cysts indicates malignity and there is evidence that the incidence of mammary cancers is indeed higher in women with fibrocystic mastopathy [35]. These fibrocystic phenomena increase mammographic breast density which represents a risk for the development of mammary carcinomas (for review s.36). The etiology of FCM is not totally clear: Estrogens and progestins in combination with latent hyperprolactinemia are the most commonly accused hormones which cause proliferation of mammary gland epithelia and connective tissue and which results in enlargement of ducts into cysts and proliferated connective tissue cause the nods [32]. There is also evidence that patients with high mammographic density have elevated prolactin levels [36] On the other hand many patients with FCM have normal prolactin levels. A possible explanation for this phenomenon is a mutation of prolactin receptors which results in their hypersensitivity [37]. There is also some recently described evidence that such mutated prolactin receptors may be constitutively active [38]. Under these conditions a number of paracrine acting factors are locally secreted which stimulate further proliferation of surrounding cells [39]. These abnormalities seem often to be accompanied increased local by production of proinflammatory cytokines and/or higher susceptibility of cytokine receptors due to gene polymorphisms [39] causing local, often painful inflammatory processes and consequently high local oxidative stress. In each case, it appears from early studies that dopaminergic drugs are able to reduce both subjectively felt pain and objectively measured fibrocystic structures. The most detailed and recent information of effects of VAC preparations on FCM with or without latent hyperprolactinemia or mastodynia stem from East European studies. A brief overview of these studies is given in Table 2 and further detailed in the following text.
Two studies addressed alternatives to the disturbed estradiol/progesterone relation as causative factor for FCM. In one trial 90 women with FCM had significantly higher serum prolactin levels in comparison to 20 women without mammary gland symptoms [40]. In this study differences in E2 metabolites in women with primarily glandular or cystic or fibrotic components were found. Similarly, in another study 60 patients with FCM were either treated with Mastodynon or received a placebo preparation [41]. In 30 of the patients the VAC preparation BNO 1095 normalized the 16-α-hydroxy metabolites and increased the 2-α-hydroxyderivatives of E2. Such effects were not seen in the placebo group and. The authors claim that this disturbance of E2 metabolism is the major reason for the development of FCM.
In aother study 120 women with latent hyperprolactinemia and mastalgia-some with nipple discharge, some without-were either treated with 2.5 mg bromocriptine or 0.24 mg Dostinex or with 40 mg of a dried VAC fruit preparation used to produce the VAC extract BNO 1095. Pain was improved and serum prolactin levels reduced in all groups. Severe side effects such as nausea and orthostatic dysregulation occurred only in the bromocriptine group. The size of mammary gland ducts, as measured by ultrasound sonography became thinner in the bomocriptine and the Dostinex group but not in the VAC group. Authors conclude that AC BNO 1055 should be the treatment of first choice in patients with mastodynia whereas Dostinex should be given in more severe forms of FCM. Elevated serum prolactin levels were also published for a group of women suffering from FCM in combination with premenstrual mastodynia [40].
It is also of interest that blockade of prolactin release by bromocriptine prevented stimulation of lobulo-alveolar tissue in women with FCM. This observation confirms and extends earlier reports which demonstrated also a higher prolactin release in women with fibrocystic mastopathy in response to a TRH stimulus [3].
Women with a latent or manifest hypothyroidism suffer often from mastodynia [41–43] and are often hyperprolactinemic [44], a phenomenon which is easily explainable: In an attempt to stimulate the thyroid function the hypothalamus releases high amounts of thyroid hormone releasing hormone (TRH) which is also stimulatory to prolactin release.
Relation to breast cancer
A mammary cancer promoting effects of prolactin was already suggested more than 50 years ago but it was for a long time almost a dogma that this effect occurs only in rodents. Indeed in rats high prolactin levels stimulate mammary cancers. It is however, now well established that in women local production of prolactin occurs in the mammary gland and prolactin receptor mRNA and its protein has also been demonstrated in mammary gland tissue and is overexpressed in malignant breast epithelia. Both, prolactin mRNA and the protein appear to be overexpressed and to exert paracrine effects which may be causally linked to development end growth of mammary cancers. In addition high circulating prolactin levels are present in most mammary cancer patients and are indicators for progression of the tumor. Such high prolactin levels in 2250 mammary cancer patients were recently confirmed.
Taken together it appears now well established that prolactin is indeed involved in generation and promotion of breast cancer. This makes it highly likely that such cancers may be prevented and their progression slowed down by dopamine agonists. Particularly VAC extracts with their low side effects seem favorable for the prevention of breast cancer by reducing mastodynia and fibrocystic mastopathy.
Conclusion
Despite small sample and often poorly defined patient populations sizes in most studies, randomised, controlled trials support the efficacy and tolerability of Vitex agnus-castus extracts in the treatment of premenstrual syndrome, premenstrual dysphoric disorder, premenstrual mastodynia and mastalgia accompanied by latent hyperprolactinaemia and of fibrocystic mastopathy. Future investigations with Vitex agnus castus extracts would benefit from use of tightly defined patient populations and common endpoints.
References
Halbreich U, Ben-David M, Assael M, Bornstein R. Serum-prolatic in women with premenstrual syndrome. Lancet. 1976;2:654–6.
Reid RL, Yen SS. Premenstrual syndrome. Am J Obstet Gynecol. 1981;139:85–104.
Schulz KD, Del Pozo E, Lose KH, Kunzig HJ, Geiger W. Successful treatment of mastodynia with the prolactin inhibitor bromocryptine (CB 154). Arch Gynakol. 1975;220:83–7.
Schwibbe MH. Multivariate relationship analysis of personality, speech and EEG. Z Exp Angew Psychol. 1983;30:133–52.
Muhlenstedt D, Bohnet HG, Hanker JP, Schneider HP. Short luteal phase and prolactin. Int J Fertil. 1978;23:213–8.
del Pozo E, Wyss H, Tollis G, et al. Prolactin and deficient luteal function. Obstet Gynecol. 1979;53:282–6.
Wuttke W, Pitzel L, Seidlova-Wuttke D, Hinney B. LH pulses and the corpus luteum: the luteal phase deficiency LPD). Vitam Horm. 2001;63:131–58.
van Die MD, Burger HG, Teede HJ, Bone KM. Vitex agnus-castus extracts for female reproductive disorders: a systematic review of clinical trials. Planta Med. 2013;79:562–75.
Wuttke W, Jarry H, Knoke I, Pitzel L, Spiess S. Luteotropic and luteolytic effects of oxytocin in the porcine corpus luteum. Adv Exp Med Biol. 1995;395:495–506.
Wuttke W, Duker EM, Demajo M, Mansky T, Lira S. Postnatal development of hypothalamic neurotransmitters. Monogr Neural Sci. 1983;9:225–33.
Guy PL, Webster DE, Davis L, Forster RL. Pests of non-indigenous organisms: Hidden costs of introduction. Trends Ecol Evol. 1998;13:111.
Jarry H, Spengler B, Porzel A, et al. Evidence for estrogen receptor beta-selective activity of Vitex agnus-castus and isolated flavones. Planta Med. 2003;69:945–7.
Liu J, Burdette JE, Sun Y, et al. Isolation of linoleic acid as an estrogenic compound from the fruits of Vitex agnus-castus L. (chaste-berry). Phytomedicine. 2004;11:18–23.
Powers CN, Setzer WN. A molecular docking study of phytochemical estrogen mimics from dietary herbal supplements. In Silico Pharmacol. 2015;3:4.
Jarry H, Leonhardt S, Gorkow C, Wuttke W. In vitro prolactin but not LH and FSH release is inhibited by compounds in extracts of Agnus castus: direct evidence for a dopaminergic principle by the dopamine receptor assay. Exp Clin Endocrinol. 1994;102:448–54.
Meier B, Berger D, Hoberg E, Sticher O, Schaffner W. Pharmacological activities of Vitex agnus-castus extracts in vitro. Phytomedicine. 2000;7:373–81.
Wuttke W, Jarry H, Christoffel V, Spengler B, Seidlova-Wuttke D. Chaste tree (Vitex agnus-castus)-pharmacology and clinical indications. Phytomedicine. 2003;10:348–57.
Chen SN, Friesen JB, Webster D, et al. Phytoconstituents from Vitex agnus-castus fruits. Fitoterapia. 2011;82:528–33.
Webster DE, He Y, Chen SN, et al. Opioidergic mechanisms underlying the actions of Vitex agnus-castus L. Biochem Pharmacol. 2011;81:170–7.
Milewicz A, Gejdel E, Sworen H, et al. Vitex agnus castus extract in the treatment of luteal phase defects due to latent hyperprolactinemia. Results of a randomized placebo-controlled double-blind study. Arzneimittelforschung. 1993;43:752–6.
Mayerhofer A, Fritz S, Grunert R, et al. D1-Receptor, DARPP-32, and PP-1 in the primate corpus luteum and luteinized granulosa cells: evidence for phosphorylation of DARPP-32 by dopamine and human chorionic gonadotropin. J Clin Endocrinol Metab. 2000;85:4750–7.
Mayerhofer A, Hemmings Jr HC, Snyder GL, et al. Functional dopamine-1 receptors and DARPP-32 are expressed in human ovary and granulosa luteal cells in vitro. J Clin Endocrinol Metab. 1999;84:257–64.
Rey-Ares V, Lazarov N, Berg D, et al. Dopamine receptor repertoire of human granulosa cells. Reprod Biol Endocrinol. 2007;5:40.
Vorherr H. Fibrocystic breast disease: pathophysiology, pathomorphology, clinical picture, and management. Am J Obstet Gynecol. 1986;154:161–79.
Murta EF, de Freitas MM, Velludo MA. Histologic changes in fibrocystic breast disease before and after treatment with bromocriptine. Rev Paul Med. 1992;110:251–6.
Courtillot C, Plu-Bureau G, Binart N, et al. Benign breast diseases. J Mammary Gland Biol Neoplasia. 2005;10:325–35.
Warner E, Lockwood G, Tritchler D, Boyd NF. The risk of breast cancer associated with mammographic parenchymal patterns: a meta-analysis of the published literature to examine the effect of method of classification. Cancer Detect Prev. 1992;16:67–72.
Ho JM, Jafferjee N, Covarrubias GM, Ghesani M, Handler B. Dense breasts: a review of reporting legislation and available supplemental screening options. AJR Am J Roentgenol. 2014;203:449–56.
Walker K, Fletcher O, Johnson N, et al. Premenopausal mammographic density in relation to cyclic variations in endogenous sex hormone levels, prolactin, and insulin-like growth factors. Cancer Res. 2009;69:6490–9.
Laud K, Gourdou I, Belair L, Peyrat JP, Djiane J. Characterization and modulation of a prolactin receptor mRNA isoform in normal and tumoral human breast tissues. Int J Cancer. 2000;85:771–6.
Courtillot C, Chakhtoura Z, Bogorad R, et al. Characterization of two constitutively active prolactin receptor variants in a cohort of 95 women with multiple breast fibroadenomas. J Clin Endocrinol Metab. 2010;95:271–9.
Sirotkovic-Skerlev M, Cacev T, Krizanac S, et al. TNF alpha promoter polymorphisms analysis in benign and malignant breast lesions. Exp Mol Pathol. 2007;83:54–8.
Bhargav PR, Mishra A, Agarwal G, et al. Prevalence of hypothyroidism in benign breast disorders and effect of thyroxine replacement on the clinical outcome. World J Surg. 2009;33:2087–93.
Bazyka DA, Lytvynenko O, Bugaistov S. Structural and functional thyroid abnormalities in patients with dyshormonal breast disorders and tumors. Probl Radiac Med Radiobiol. 2013;18:156–68.
Adashi EY, Katz E. Diagnostic work-up of hyperprolactinemic disorders. Gynecol Endocrinol. 1988;2:339–57.
McCann SM, Ono N, Khorram O, Kentroti S, Aguila C. The role of brain peptides in neuroimmunomodulation. Ann N Y Acad Sci. 1987;496:173–81.
Reichlin S. Neuroendocrinology of the pituitary gland. Toxicol Pathol. 1989;17:250–5.
Korbonits M, Morris DG, Nanzer A, Kola B, Grossman AB. Role of regulatory factors in pituitary tumour formation. Front Horm Res. 2004;32:63–95.
Fernandez I, Touraine P, Goffin V. Prolactin and human tumourogenesis. J Neuroendocrinol. 2010;22:771–7.
Meites J, Lu KH, Wuttke W, et al. Recent studies on functions and control of prolactin secretion in rats. Recent Prog Horm Res. 1972;28:471–526.
Damiano JS, Wasserman E. Molecular pathways: blockade of the PRLR signaling pathway as a novel antihormonal approach for the treatment of breast and prostate cancer. Clin Cancer Res. 2013;19:1644–50.
Touraine P, Martini JF, Zafrani B, et al. Increased expression of prolactin receptor gene assessed by quantitative polymerase chain reaction in human breast tumors versus normal breast tissues. J Clin Endocrinol Metab. 1998;83:667–74.
Tworoger SS, Eliassen AH, Sluss P, Hankinson SE. A prospective study of plasma prolactin concentrations and risk of premenopausal and postmenopausal breast cancer. J Clin Oncol. 2007;25:1482–8.
Tikk K, Sookthai D, Johnson T, et al. Circulating prolactin and breast cancer risk among pre- and postmenopausal women in the EPIC cohort. Ann Oncol. 2014;25:1422–8.
Gumenyuk, EG. Some problems of premenstrual syndrome and alternative therapy. Jounal of Obstetrics and gynecological diseases. 2010;:38-45.
Binita G, Suprava P, Mainak C, Koner BC, Alpana S. Correlation of prolactin and thyroid hormone concentration with menstrual patterns in infertile women. J Reprod Infertil. 2009;10:207–12.
Authors’ contributions
Both authors read and approved the final manuscript.
Competing interests
Both authors are advisors of the Bionorica SE, Neumarkt, Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Seidlova-Wuttke, D., Wuttke, W. The premenstrual syndrome, premenstrual mastodynia, fibrocystic mastopathy and infertility have often common roots: effects of extracts of chasteberry (Vitex agnus castus) as a solution. Clin Phytosci 3, 6 (2017). https://doi.org/10.1186/s40816-016-0038-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40816-016-0038-z