Abstract
Background
Seniors with recurrent hospitalizations who are taking multiple medications including high-risk medications are at particular risk for serious adverse medication events. We will assess whether an expert Clinical Pharmacology and Toxicology (CPT) medication management intervention during hospitalization with follow-up post-discharge and communication with circle of care is feasible and can decrease drug therapy problems amongst this group.
Methods
The design is a pragmatic pilot randomized trial with 1:1 patient-level concealed randomization with blinded outcome assessment and data analysis. Participants will be adults 65 years and older admitted to internal medicine services for more than 2 days, who have had at least one other hospitalization in the prior year, taking five or more chronic medications including at least one high-risk medication. The CPT intervention identifies medication targets; completes consult, including priorities for improving prescribing negotiated with the patient; starts the care plan; ensures a detailed discharge medication reconciliation and circle-of-care communication; and sees the patient at least twice after hospital discharge via virtual visits to consolidate the care plan in the community. Control group receives usual care. Primary outcomes are feasibility — recruitment, retention, costs, and clinical — number of drug therapy problems improved, with secondary outcomes examining coordination of transitions in care, quality of life, and healthcare utilization and costs. Follow-up is to 3-month posthospital discharge.
Discussion
If results support feasibility of ramp-up and promising clinical outcomes, a follow-up definitive trial will be organized using a developing national platform and medication appropriateness network. Since the intervention allows a very scarce medical specialty expertise to be offered via virtual care, there is potential to improve the safety, outcomes, and cost of care widely.
Trial registration number
ClinicalTrials.gov identifier: NCT04077281.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Current systematic reviews of randomized trials to manage polypharmacy or to manage medications in hospital or in the transitions of care have not consistently shown improvements in important clinical outcomes [1,2,3,4,5]. This is largely because (a) the interventions have been carried out by providers without the requisite combination of diagnostic, therapeutic, and risk management expertise or authority to make requisite changes, (b) the intervention was not sufficiently concentrated (too short, incomplete, or misdirected), (c) the medication focus was misplaced (i.e., not high-risk medications, which are the most associated with adverse clinical outcomes), (d) the outcomes were overly focused on poor quality surrogates for clinical outcomes, or (e) the patient has so much irreversible comorbidity that changes in medications could not have significant impact.
Because of the huge potential burden of harm and the number of affected vulnerable older adults in most countries, the value of more randomized trial inquiry is high. There is enough suggestion of benefit from trials on prescribing appropriateness combined with the potential for major medication safety improvements, to support an expert intervention concentrated on the highest risk group as they transition through a very high-risk period targeting the highest risk medications [4, 6,7,8,9]. In addition, recent advances in digital health make it reasonable to focus on utilizing clinical pharmacologist expertise for this high priority problem within robust communication systems that support patient consultation and follow-up from any geography no matter how remote. Clinical pharmacologists are medical specialists, in this case with internal medicine cross-specialization, who regularly lead the care of hospitalized medically complex patients and formulate their therapeutic priorities.
Transitions in care, particularly in- and out-of-hospital and high alert medications, are two primary high-risk medication safety situations referenced by WHO in their Global Patient Safety Challenge [10]. Even with evidence of under-detection, systematic reviews of the literature conclude that adverse drug events (ADEs) amongst older adults lead to approximately 1 in 10 hospitalizations [11,12,13]. Adverse drug-related hospital admissions appear to be directly correlated with the number of medications taken concurrently, the number of prescribers involved, possibly the number of pharmacies used, and with hospitalization within the previous year [14]. In several studies, female sex was also a risk factor. Once in hospital, patients (n = 46,626) remained at high risk of ADEs, with a mean prevalence of 21.6% (SD 16.7), 20.7% of these judged to be severe or life threatening and 32.3% (SD22.6%) judged to be preventable [15]. In addition to the risk factors above, a complex patient (several comorbidities with several provider experts involved) is significantly more likely to suffer adverse events in hospital [16, 17]. The period immediately following hospital discharge remains high risk with 37% of seniors sustaining medication-related harm (81% serious) within 8 weeks [18]. In this cohort study from the UK, female sex was associated with medication-related harm [18]. The outcomes of ADEs included prolonged length of stay, frequent readmission, emotional trauma, high costs, and death [16, 19, 20].
The prevalence of major polypharmacy defined as the concurrent, regular use of 10 or more medications is high amongst seniors reaching 38.4% in those aged 85 years and older [4, 21]. While the number of medications dispensed remains a useful signal, it is impossible to gauge the quality of medication regimens simply by the number of medications, as this does not account for the patient’s main diagnosis, comorbidities, risk factors, past history of medication use, or the benefit-harm ratio of the drug for that patient. For example, an older patient with diabetes frequently requires two glucose lowering medications, a statin for cholesterol, and two to three medications for blood pressure, just to manage their high cardiovascular risk without treating their other health problems. Thus, the medication safety target is problematic polypharmacy as opposed to appropriate polypharmacy [22].
Medications which frequently lead to harm outweighing benefit in certain situations are termed “potentially inappropriate medications” (PIMs). PIMs that have been associated with ADEs have been grouped together in medication screening lists, with the most evidence-based being the STOPP criteria [23]. Randomized trials in Europe show that use of the STOPP criteria as a trigger for medication review for hospitalized seniors can improve the appropriateness of prescribing, reduce ADEs, and reduce length of stay [24, 25]. A Canadian study found that nearly 40% of seniors fill a prescription for at least one PIM per year, with the highest rates in women > 85 years of age and the most common PIM drug category being sedative-hypnotic drugs [26]. The cost of these PIMS plus the cost of treating their adverse effects was estimated to be more than US $1.8 billion annually [26].
Priority medications
Although STOPP is an excellent screening tool, there are too many alerts (80 in current iteration) to feasibly apply in hospitalized patients where timely discharge is a high priority [25]. Analyses of Canadian and US data on drug-related causes of hospitalization by our group and others suggest recurring groups of very commonly used medications as the main causes of drug-related hospitalizations [14, 27, 28]. These are mostly medication families with proven benefit of varying clinical importance, but all cause clinically important harm when not managed well. Thus, these are medications that should trigger review of the entire medication regimen to consider improvements. We have labelled these the high-risk medications (HRM). A detailed list is shown in Appendix 1, but the main families are as follows:
-
i.
Anticoagulants
-
ii.
Analgesics including opioids, NSAIDs, and colchicine
-
iii.
Antimicrobials — long term or restricted
-
iv.
Antineoplastic agents
-
v.
Glucose-lowering drugs
-
vi.
Cardiac drugs including diuretics and digoxin
-
vii.
Sedative-hypnotics including benzodiazepine receptor antagonists, trazodone, and baclofen
-
viii.
Antipsychotic agents
-
ix.
Other psychoactive medications including lithium, trazodone, and tricyclic antidepressants
-
x.
Dementia medications
-
xi.
Immune-modulating agents such as corticosteroids
In many cases, the high-risk medication is required for the patient, but the dose may require adjustment, a tapering regimen may reduce the potential for harm, or a review for potentially serious drug interactions or medication burden identifies another medication that can be removed to decrease the patient’s overall risk of medication-related harm. We have previously developed an “Appropriateness of Prescribing Evaluation Questionnaire” that has been validated as a comprehensive medication appropriateness assessment tool [29, 30]. Optimization of high-risk medications, of course, opens up other opportunities including removal of medications and supplements with no benefit or with possibility of contamination or substitution of more cost-effective alternatives.
Priority patients
High-cost healthcare users have been an international priority target for quality and cost of care improvements for years [31]. We have shown that 5% of 12 million citizens generate 65% of the entire healthcare costs, and approximately 70% of these are seniors with multiple hospital admissions and problematic polypharmacy [32,33,34]. Use of high-risk medications is very prevalent in this population and strongly predictive of future healthcare utilization and mortality in a dose- and duration-dependent manner compared to nonusers [33,34,35,36].
Priority situations
Senior high-cost users taking high-risk medications who are transitioning into and out of hospital are at very high risk of serious ADEs. Hospitalization is a double-edged sword in that it defines high-risk situations but also houses the expertise required to effectively intervene. This opportunity to optimize medication regimens for these inpatients is widely underutilized worldwide, due to (a) huge pressures to just deal with the main problem requiring admission and get the patient discharged as quickly as possible due to bed shortages and (b) lack of expertise amongst general medicine and surgery admitting services to complete an expert medication assessment quickly. In preparation for this trial, we recently completed a chart review of 100 randomly selected senior high-cost users who were admitted to a Hamilton Hospital (mean age 82 years) and found the mean rate of potentially inappropriate medications to be 2.8 per person [37]. Only 16.6% of these had been addressed by the time of discharge [37].
Methods
Detailed research question
Is it feasible for an expert clinical pharmacology team to coordinate and improve medication management during the high-risk transition period from hospitalization through early post-hospital discharge follow-up for seniors who are high-cost healthcare users and taking high-risk medications, to warrant a large subsequent trial?
Design
This is a single-center pragmatic pilot randomized trial (RCT) with 1:1 patient-level concealed-allocation randomization with blinded outcome assessment and data analysis [38]. Randomization provides the highest quality methods to minimize bias, the pragmatic design ensures relevance to clinical practice essential for implementation, and a pilot RCT addresses feasibility of a large definitive subsequent RCT directly without waste of research dollars [39, 40]. A study flow diagram is shown below in Fig. 1. Study setting is a 700-bed academic hospital with a busy urgent care and emergency department providing medical, surgical, psychiatric, and obstetric-gynecology inpatient and outpatient care in Hamilton, Ontario.
Participants
Adults 65 years and older who are admitted to an internal medicine acute care ward with an expected length of stay of more than 2 days, who are high-cost users defined as at least one other hospitalization within the previous year, who are taking five or more chronic medications including at least one high risk medication, and provide informed consent. Patients will be excluded if they are being discharged to long-term care or other setting where they or their caregiver is not in charge of their medications. It is estimated that at least 10 patients daily meet these eligibility criteria.
Recruitment and randomization
A screening tool in our EMR used by our CPT consult service for older adults taking high-risk medications provides secure messaging to our research staff. Once eligibility is confirmed, the team ensures that a best possible medication history is completed to correct the home medication list side of our discharge medication reconciliation template. Once eligibility is cleared with the most responsible physician team (primary team), research staff will then approach the patient to introduce the study and complete the informed consent process which includes completing a short Capacity to Consent questionnaire (Appendix 2) [41]. Patients with cognitive impairment will not be excluded as they constitute a vulnerable group in need of assistance, but they must have a primary caregiver who assists them with medications and who provides informed consent if the patient is identified as not having capacity of consent [41]. For this pilot trial, either the patient or caregiver must be fluent in English.
Once patients have completed baseline assessments, they will be randomly allocated to the intervention or control arms in a 1:1 ratio in permuted blocks using a statistician-formulated randomization schedule produced in R and implemented in REDCap that will be available online to staff only at the time of randomization [42, 43]. To restrict treatment group imbalance, a maximal tolerable imbalance between treatment groups will be incorporated into the schedule [44].
Intervention
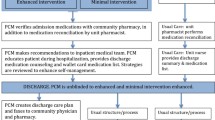
Our intervention follows the general innovative practices framework recommended by Health Quality Ontario for Transitions between Hospital and Home [45]. Fig. 2 shows a step-by-step clinical approach used for this intervention. Randomization to the intervention arm will trigger a request for a CPT (Clinical Pharmacology & Toxicology) consult. The initial CPT consult for each patient includes a comprehensive patient assessment including demographics, social situation, drug coverage insurance, functional status (activities of daily living, instrumental activities of daily living), cognition, frailty markers, level of caregiver involvement, past medical history and current problems, allergies and intolerances, detailed medication history including reminder aids and methods of accessing medication, physical exam, and review of current and historical laboratory and diagnostic imaging results. These details are mostly structured data items in the EMR which populates a customized CPT note template, which ensures some consistency of intervention across the participating consultants. We incorporate an admission medication reconciliation carried out by the ward pharmacist. The CPT consult will document a detailed “circle of care” for each patient (primary caregiver, hospital primary team, primary care team including family physician or nurse practitioner, community physician specialists and community pharmacists) and will identify all potential high-risk medications targets that the patient is taking or is due to resume post-hospitalization. Using patient preference elicitation methods and motivational interviewing, priorities for medication optimization will be negotiated [46,47,48,49,50,51]. Short patient infographics endorsed by the Canadian Medication Appropriateness and Deprescribing Network and largely addressing medication harms will be used as educational materials [52,53,54,55,56,57,58,59,60,61].
While the patient is still hospitalized, the high-risk medications care plan begins, and the team ensures close communication with the primary team, coordinates a detailed discharge medication reconciliation (documenting medication changes with rationale, formulating an accurate discharge prescription including rapid access to new medications), ensures circle-of-care communication, and sees the patient via virtual visit twice in follow-up at 1 week and 1 month after hospital discharge to complete and consolidate the care plan. Since this is a pragmatic trial, concomitant care is not prohibited. Pre-testing of the intervention with several patients has shown that the initial consult and two follow-up visits can be completed in less than 1 h each.
Control
These patients will receive usual care by their primary team. This means that the primary team is responsible for coordinating medication management at discharge and posthospital follow-up, as is currently practiced.
Outcomes
The Core Outcome Set for Interventions to Improve Polypharmacy in Older People was used to inform our selection of outcomes [62]. Core outcome sets are consensus-based guidelines from groups of clinicians, patients, and methodologists on which outcomes with which metrics are the most important to be measured in prospective studies [63]. In addition, we consulted other polypharmacy/deprescribing trials for recommended patient-important outcomes [64,65,66,67,68,69].
For this pilot RCT, we will analyze outcomes according to a set of primary and secondary outcomes (see details in Tables 1 and 2).
Primary outcomes
There are two types of primary outcomes that will be evaluated:
-
a)
The primary feasibility outcomes will be recruitment and retention rate for eligible patients and the estimated resources required per patient to complete the main trial (program delivery costs). We aim for at least 30% recruitment of those eligible, 90% retention of those recruited, and no more than US $1500 per patient spent on running the pilot trial. This figure is based on the limits of peer-reviewed RCT funding for a full trial.
-
b)
The primary clinical outcome will be the number of drug therapy problems remediated including the number of high-risk medications improved (for example, dose adjustment, discontinued, seriously interacting drugs removed) at 3-month posthospital discharge end-study visit, as judged by adjudicators using APEQ.
Secondary outcomes
-
a)
The secondary feasibility outcomes of CPT consultation include recommendation acceptance and adherence by primary team and by patients, consult volume capacity, and potential to apply the intervention entirely through virtual visits including eConsults. Thresholds for success are described in Table 2.
-
b)
The secondary clinical/patient-important outcomes will include the following:
-
1.
Adverse drug events (ADEs) defined as “Harm caused by exposure to a drug” adjudicated using Leape and Bates scale (6 - definitely due to medication, 5 - probably due to medication, 4 - possibly due to medication, 3 - possibly due to disease, 2 - probably due to disease, 1 - definitely due to disease, 5 and 6 considered ADE) [74]. ADE preventability will be evaluated using the recommended “best practice”-based definition [70].
-
2.
Medication errors defined as an error (of commission or omission) at any step along the pathway that begins with prescribing and ends with the patient taking the medication [74]. We will use the standard NCC-MERP classification system [71, 75].
-
3.
Number of medications per patient end study compared to baseline.
-
4.
Patient problems with medications: The COMPETE Medication Problems Questionnaire measures problems with medication access, handling, beliefs, and adherence [76].
-
5.
Medication knowledge assessment: This will be assessed using the Medication Knowledge Assessment form, which tests knowledge of medication name, indication, dosage instructions, and precautions [77].
-
6.
Coordination and continuity of care: Adapted from Health Quality Ontario’s draft guidance and a Rand instrument, the Coordination and Continuity of Care Questionnaire is designed to measure the quality of the transitional and follow-up care. We will focus on medication reconciliations and education and circle-of-care communications [78, 79].
-
7.
Patient quality of life: We will use the general EQ-5D-5L, a five-level measure of health status and utilities well validated in Canada and based on self-reported mobility, self-care, usual activities, pain discomfort, and anxiety/depression [80,81,82]. We will also use a “condition-specific” QOL measure, the medication-related quality-of-life measure which is designed for people living with polypharmacy [83].
-
8.
Satisfaction with care: Satisfaction reported by patients and by key health professionals is one of the recommended outcomes to report in medical research, as it may influence adherence [84, 85]. This outcome will be assessed by the Patient/Caregiver Study Satisfaction Survey and by the Provider Study Satisfaction Survey [86].
-
9.
Health resource utilization: This is a key outcome to determine cost-effectiveness and cost-utility which then determines whether healthcare systems might pay for this type of care [87, 88]. Using questionnaire and chart review, we will capture emergency department visits, hospitalizations, unplanned physician visits, and medication costs, including out-of-pocket medication costs in follow-up [79, 88].
-
1.
Outcome data will be collected by research staff through a mix of patient interview and chart review. All adjudication will be carried out blinded to group allocation of the patient. Completeness of data for variables including sex, gender, age, social support, socioeconomic status, cognition, number of medications, and comorbidities will be examined as these are potential predictors of outcomes. Sensitivity analyses will assist with determination of potential for cost-effectiveness of the intervention overall and in selected subgroups.
Geographic wards in the hospital allow for evaluation of the intra-cluster correlation (ICC), which based on past experience, we expect to be low [76]. Additionally, barriers and facilitators to the success of the primary outcomes and potential for scalability to a larger trial will be assessed in weekly team meetings.
Follow-up
Patients will be followed until 3-month posthospital discharge or until death or admission to long-term care home, whichever occurs first. Follow-up visits will be conducted by videoconference via Epic EMR or Ontario Telehealth Network, or by phone call, depending on the patient’s digital technology capability.
Sample size
Since this is a pilot RCT, we will aim for 30 patients per group or 60 in total as this number is likely to provide adequate evidence regarding feasibility for ramp-up to a definitive trial.
Blinding
As a pragmatic RCT layered on routine clinical care, it will not be possible to completely blind patients or their providers; however, outcome data collectors, adjudicators, and statisticians will be blinded to group allocation until analysis is completed at the end of the study.
Data collection methods
Trained research staff will conduct interviews with the patients or caregivers, entering data electronically on study laptops directly into REDCap case report forms. The participants’ medical records will be reviewed to abstract data on baseline characteristics, medical history, and medication information. Strategies to promote participant retention and complete follow-up include reminding participants in advance of their end-of-study visit and communicating by email if email address is provided at baseline. Participants who drop out of the study will have their data to that point retained in the study, as approved by REB, to avoid bias. The reasons for study non-completion will be recorded. The SPIRIT figure outlining the schedule of enrolment, intervention, and assessment (as per evidence-based recommendations for the minimum content of a clinical trial protocol) can be found in Table 3 [89].
Data management, privacy, and confidentiality
REDCap’s secure, web-based platform used widely internationally, providing interfaces for validated data capture, role-specific access, audit trails for tracking data manipulation and exports, automated export procedures to SAS, and encrypted transmissions [90, 91]. Paper study documents such as signed informed consent forms will be stored in our secure research office once they are scanned into REDCap study files. Regular data quality checks, such as automatic range checks, will be performed by the study team to identify data that appear inconsistent, incomplete, or inaccurate.
Patients will not be identifiable in the project results database. The identifying information required for the clinical team to deliver the intervention will be kept in a separate database. Access to the final dataset will be restricted to the core research team.
Statistical analyses
The reporting of the results of this trial will follow the CONSORT extension for pilot trials [92]. We will use descriptive statistics for presentation of baseline variables and adequacy of follow-up. Feasibility analysis, including recruitment rate (at least 30% of those eligible is considered success), participant retention rate (≥ 90% to end of study is considered success), and study resource utilization required (less than US $1500 CAD per patient recruited), will be descriptive.
Analysis will use intention-to-treat methods with censoring only if the patient dies or drops out of the study with refusal of negotiated further assessments. Costs and quality-adjusted life-years (QALYs) associated with the two interventions will be determined from a payer and societal perspective. Healthcare resource utilization collected as part of the trial will be costed using public data sources. Using information from the EQ-5D-5L, quality-adjusted life-years (QALYs) will be determined under an area under the curve approach.
Research staff and statisticians will review outcome data and analysis blinded to group identification. Given the short follow-up, low risk of the trial, and pilot design, no interim analysis or imputation for missing data is planned. All statistical analyses will be performed using SAS V9.4 software (SAS Institute Inc., Cary, NC, USA).
Data monitoring
Any serious adverse event will be reviewed by our Trial Steering Committee (TSC) within 48 h of detection, to discern any attribution to our procedures. If found to be due to our coordination procedures, the Trial Steering Committee will recommend whether modifications are indicated. The TSC will be composed of individuals with expertise in clinical trials, chaired by the lead statistician, and include the PI, the operational statistician, plus a methodologist independent of the study team. Similarly, since this is a short pilot pragmatic RCT where no harm is expected and adjustment of trial procedures may be necessary for feasibility, no formal external auditing of trial conduct is planned. There is no requirement for additional ancillary and posttrial care for those who might come to harm while in the trial, as usual medical care which covers this eventuality is already in place.
Impact of research
By creating a network of clinicians, patients, researchers, drug policy advisors, information technology advisors, quality improvement advisors, and hospital and national medication safety administration, we hope to strengthen and broaden the usual dissemination of research to practice We expect the pilot study to inform a future definitive trial by identifying solutions to overcome potential limitations including the following:
-
a)
The CPT team is a scarce resource which limits eventual generalizability. Based on pilot results, we will plan to study how physician assistants and clinical pharmacists can be trained to do this work.
-
b)
The short follow-up of 3 months is typical of transitional care initiatives but may be too short to show impact on clinical outcomes.
-
c)
The single-center design aids feasibility of the pilot but limits generalizability. A larger trial, if feasible, will aim to recruit other hospitals using the Epic EMR or members of the national deprescribing network.
-
d)
A definitive trial will highlight clinical outcomes as primary outcomes.
Availability of data and materials
An anonymized dataset will be shared in accordance with future requirements of our funders, the Canadian Institutes of Health Research.
Abbreviations
- ADE:
-
Adverse drug event
- APEQ:
-
Appropriateness of Prescribing Evaluation Questionnaire
- CPT:
-
Clinical pharmacology and toxicology
- EMR:
-
Electronic medical records
- EQ-5D-5L:
-
European Quality-of-Life Five Dimension
- HRM:
-
High-risk medication
- NCC-MERP:
-
National Coordinating Council for Medication Error Reporting and Prevention
- PIM:
-
Potentially inappropriate medication
- QOL:
-
Quality of life
- RCT:
-
Randomized control trial
- REDCap:
-
Research Electronic Data Capture
- SD:
-
Standard deviation
- STOPP:
-
Screening Tool of Older People’s Prescriptions
- WHO:
-
World Health Organization
References
Rankin A, C C, Patterson SM, Kerse N, Cardwell CR, Bradley MC, et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2018;9(9):CD008165.
Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
Redmond P, Grimes TC, McDonnell R, Boland F, Hughes C, Fahey T. Impact of medication reconciliation for improving transitions of care. Cochrane Database Syst Rev. 2018;8:CD010791.
Anderson LJ, Schnipper JL, Nuckols TK, Shane R, Sarkisian C, Le MM, et al. A systematic overview of systematic reviews evaluating interventions addressing polypharmacy. Am J Health Syst Pharm. 2019;76(21):1777–87.
Johansson T, Abuzahra ME, Keller S, Mann E, Faller B, Sommerauer C, et al. Impact of strategies to reduce polypharmacy on clinically relevant endpoints: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(2):532–48.
Gray SL, Hart LA, Perera S, Semla TP, Schmader KE, Hanlon JT. Meta-analysis of interventions to reduce adverse drug reactions in older adults. J Am Geriatr Soc. 2018;66(2):282–8.
Le Berre M, Maimon G, Sourial N, Gueriton M, Vedel I. Impact of transitional care services for chronically ill older patients: a systematic evidence review. J Am Geriatr Soc. 2017;65(7):1597–608.
Kastner M, Cardoso R, Lai Y, Treister V, Hamid JS, Hayden L, et al. Effectiveness of interventions for managing multiple high-burden chronic diseases in older adults: a systematic review and meta-analysis. CMAJ. 2018;190(34):E1004–12.
Ravn-Nielsen LV, Duckert ML, Lund ML, Henriksen JP, Nielsen ML, Eriksen CS, et al. Effect of an in-hospital multifaceted clinical pharmacist intervention on the risk of readmission: a randomized clinical trial. JAMA Intern Med. 2018;178(3):375–82.
WHO. Medication without harm: WHO; 2017. Available from: https://www.who.int/initiatives/medication-without-harm. Cited 2023.
Oscanoa TJ, L F, Carvajal A. Hospital admissions due to adverse drug reactions in the elderly: a meta-analysis. Eur J Clin Pharmacol. 2017;6:759–70.
Alhawassi TM, Krass I, Bajorek BV, Pont LG. A systematic review of the prevalence and risk factors for adverse drug reactions in the elderly in the acute care setting. Clin Interv Aging. 2014;9:2079–86.
Hohl CM, de Lemos J, Abu Laban RB. Emergency hospitalizations for adverse drug events. N Engl J Med. 2012;366(9):858–9. author reply 9-60.
Canadian Institute for Health Information. Adverse drug reaction–related hospitalizations among seniors, 2006 to 2011: CIHI; 2013. Available from: www.cihi.ca.
Laatikainen O, Miettunen J, Sneck S, Lehtiniemi H, Tenhunen O, Turpeinen M. The prevalence of medication-related adverse events in inpatients-a systematic review and meta-analysis. Eur J Clin Pharmacol. 2017;73(12):1539–49.
Chan B CD. Measuring patient harm in Canadian hospitals. With what can be done to improve patient safety? Canadian Institute for Health Information (CIHI); 2016. Available from: www.cihi.ca.
Institue for Safe Medication Practices Canada. Ontario Hospital critical incidents related to medications or IV fluids analysis report: October 2011 to December 2012: ISMP; 2013. Available from: www.ismp-canada.org.
Parekh N, Ali K, Stevenson JM, Davies JG, Schiff R, Van der Cammen T, et al. Incidence and cost of medication harm in older adults following hospital discharge: a multicentre prospective study in the UK. Br J Clin Pharmacol. 2018;84(8):1789–97.
Amelung S, Meid AD, Nafe M, Thalheimer M, Hoppe-Tichy T, Haefeli WE, et al. Association of preventable adverse drug events with inpatients' length of stay-a propensity-matched cohort study. Int J Clin Pract. 2017;71(10).
Parameswaran Nair N, Chalmers L, Bereznicki BJ, Curtain CM, Bereznicki LR. Repeat adverse drug reaction-related hospital admissions in elderly Australians: a retrospective study at the Royal Hobart Hospital. Drugs Aging. 2017;34(10):777–83.
Canadian Institute for Health Information. Drug use among seniors in Canada: CIHI; 2016. Available from: https://www.cihi.ca/sites/default/files/document/drug-use-among-seniors-2016-en-web.pdf.
Duerden M, A T, Payne R. Polypharmacy and medicines optimisation: making it safe and sound. UK: Kings Fund; 2013.
Hamilton H, Gallagher P, Ryan C, Byrne S, O’Mahony D. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med. 2011;171(11):1013–9.
Gallagher PF, O’Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89(6):845–54.
O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–8.
Morgan SG, Hunt J, Rioux J, Proulx J, Weymann D, Tannenbaum C. Frequency and cost of potentially inappropriate prescribing for older adults: a cross-sectional study. CMAJ Open. 2016;4(2):E346–51.
Bayoumi I, Dolovich L, Hutchison B, Holbrook A. Medication-related emergency department visits and hospitalizations among older adults. Can Fam Physician. 2014;60(4):e217–22.
Budnitz DS, Shehab N, Lovegrove MC, Geller AI, Lind JN, Pollock DA. US emergency department visits attributed to medication harms, 2017–2019. JAMA. 2021;326(13):1299–309.
Holbrook A, J V, Goldsmith CH, Shcherbatykh IY, COMPETE Investigators. A comprehensive appropriateness of prescribing questionnaire was validated by nominal consensus group. J Clin Epidemiol. 2007;10(60):1022–8.
Holbrook A KK, Troyan S, Goldmith C, Smiley T, Nabzdyk K. Measuring the appropriateness of prescribing. Can it be explicit and evidence-based? Can J Clin Pharmacol. 1999;6(44):533-9.
Lee JY. The quality of prescribing and medication use and its impact on older adult high-cost healthcare users. Hamilton: McMaster University; 2021.
Lee JMS, M S, Tarride J-E, Paterson JM, Thavorn K, Mbuagbaw L, Gomes T, Khuu W, Seow H, Thabane L, Holbrook A. Medication use and its impact on high-cost health care users among older adults: protocol for the population-based matched cohort HiCOSTT study. CMAJ Open. 2011;9(1):E44–52.
Muratov S, Lee J, Holbrook A, Paterson JM, Guertin JR, Mbuagbaw L, et al. Unplanned index hospital admissions among new older high-cost health care users in Ontario: a population-based matched cohort study. CMAJ Open. 2019;3(7):E537–45.
Muratov S, Lee J, Holbrook A, Guertin JR, Mbuagbaw L, Paterson JM, et al. Incremental healthcare utilisation and costs among new senior high-cost users in Ontario, Canada: a retrospective matched cohort study. BMJ Open. 2019;9(10):e028637.
Lee J, M S, Tarride J, Paterson J, Thavorn K, Mbuagbaw L, et al. Medication predictors of high cost healthcare use among older adults. Montreal: Canadian Association for Health Services and Policy Research (CAHSPR) Conference; 2018.
J L. The quality of prescribing and medication use and its impact on older adult high-cost healthcare users. Hamilton: McMaster University; 2021.
Sanh M, H A, Macdonald P, Lee J. Potentially inappropriate prescribing in hospitalized older adult high-cost health care users: a pilot study. Can J Hosp Pharm. 2022;75(3):219.
Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147.
Thabane L, Hopewell S, Lancaster GA, Bond CM, Coleman CL, Campbell MJ, et al. Methods and processes for development of a CONSORT extension for reporting pilot randomized controlled trials. Pilot Feasibility Stud. 2016;2:25.
Thabane L, Lancaster G. A guide to the reporting of protocols of pilot and feasibility trials. Pilot Feasibility Stud. 2019;5:37.
COACHeD RCT Capacity to Consent Questionnaire. MacSphere. 2021. Available from: http://hdl.handle.net/11375/27712.
Lj W. The adaptive biased coin design for sequential experiments. Ann Stat. 1978;6(1):92–100.
Markaaryan T, R W. Exact properties of Efron’s biased coin randomization procedure. Ann Statist. 2010;38(3):1546–67.
Soares JF, JW C. Some restricted randomization rules in sequential designs. Commun Stat. 1983;12(17):2017–34.
Health Quality Ontario. Transitions between hospital and home: HQO; 2022. Available from: https://www.hqontario.ca/Quality-Improvement/Quality-Improvement-in-Action/Health-Links/Health-Links-Resources/Transitions-between-Hospital-and-Home.
Stacey D, Legare F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431.
Fatima S, Holbrook A, Schulman S, Park S, Troyan S, Curnew G. Development and validation of a decision aid for choosing among antithrombotic agents for atrial fibrillation. Thromb Res. 2016;145:143–8.
Holbrook AM, P E, Troyan SM, Nikitovic M, Crowther MA. Personalized benefit-harm information influences patient decisions regarding warfarin. J Popul Ther Clin Pharmacol. 2013;20(3):e229.
Hong C, K S, Curnew G, Schulman S, Pullenayegum E, Holbrook A. Validation of a patient decision aid for choosing between dabigatran and warfarin for atrial fibrillation. J Popul Ther Clin Pharmacol. 2013;20(3):e229–37.
Holbrook A, Labiris R, Goldsmith CH, Ota K, Harb S, Sebaldt RJ. Influence of decision aids on patient preferences for anticoagulant therapy: a randomized trial. CMAJ. 2007;176(11):1583–7.
Lundahl B, Moleni T, Burke BL, Butters R, Tollefson D, Butler C, et al. Motivational interviewing in medical care settings: a systematic review and meta-analysis of randomized controlled trials. Patient Educ Couns. 2013;93(2):157–68.
Canadian Medication Appropriateness and Deprescribing Network. How to get a good night's sleep without medication. EMPOWER brochures: Montreal: Canadian Medication Appropriateness and Deprescribing Network; 2014. Accessed Jan 2023. www.deprescribingnetwork.ca/sleep.
Canadian Medication Appropriateness and Deprescribing Network. Is it time to review your medications? Deprescribing handouts: Montreal: Canadian Medication Appropriateness and Deprescribing Network; 2022. Accessed Jan 2023. www.deprescribingnetwork.ca/medication-review-handout/.
Canadian Medication Appropriateness and Deprescribing Network. You May be at Risk (sedative-hypnotics). Patient Handouts: Montreal: Canadian Medication Appropriateness and Deprescribing Network; 2014. Accessed Jan 2023. www.deprescribingwork.ca/patient-handouts/.
Canadian Medication Appropriateness and Deprescribing Network. Do I Still Need This Medication? Patient Handouts: Montreal: Canadian Medication Appropriateness and Deprescribing Network; 2014. Accessed Jan 2023. www.deprescribingnetwork.ca/patient-handouts/.
Canadian Medication Appropriateness and Deprescribing Network. You may be at risk if you are taking opioids/narcotics for chronic pain. Patient Handouts: Montreal: Canadian Medication Appropriateness and Deprescribing Network; 2018. Accessed Jan 2023. www.deprescribingnetwork.ca/patient-handouts/.
Canadian Medication Appropriateness and Deprescribing Network. You May be at Risk (antihistamines). Patient Handouts: Montreal: Canadian Medication Appropriateness and Deprescribing Network; 2014. Accessed Jan 2023. www.deprescribingnetwork.ca/patient-handouts/.
Canadian Medication Appropriateness and Deprescribing Network. You May be at Risk (antipsychotics). Patient Handouts: Montreal: Canadian Medication Appropriateness and Deprescribing Network; 2014. Accessed Jan 2023. www.deprescribingnetwork.ca/patient-handouts/.
Canadian Medication Appropriateness and Deprescribing Network. You May be at Risk (gabapentin/pregabalin). Patient Handouts: Montreal: Canadian Medication Appropriateness and Deprescribing Network; 2020. Accessed Jan 2023. www.deprescribingnetwork.ca/patient-handouts/.
Canadian Medication Appropriateness and Deprescribing Network. You May be at Risk (NSAIDS). Patient Handouts: Montreal: Canadian Medication Appropriateness and Deprescribing Network; 2014. Accessed Jan 2023. www.deprescribingnetwork.ca/patient-handouts/.
Canadian Medication Appropriateness and Deprescribing Network. You May be at Risk (sulfonylureas). Patient Handouts: Montreal: Canadian Medication Appropriateness and Deprescribing Network; 2014. Accessed Jan 2023. www.deprescribingnetwork.ca/patient-handouts/.
Rankin A, C C, Ryan CI, Clyne B, Smith SM, Hughes CM. Core outcome set for trials aimed at improving the appropriateness of polypharmacy in older people in primary care. J Am Geriatr Soc. 2018;66(6):1206–12.
Prinsen CA, V S, Rose MR, Boers M, Tugwell P, Clarke M, et al. How to select outcome measurement instruments for outcomes included in a “core outcome set” - a practical guidline. Trials. 2016;17(1):449.
McDonald EG, Wu PE, Rashidi B, Wilson MG, Bortolussi-Courval E, Atique A, et al. The MedSafer study-electronic decision support for deprescribing in hospitalized older adults: a cluster randomized clinical trial. JAMA Intern Med. 2022;182(3):265–73.
Mangin D, Lamarche L, Agarwal G, Banh HL, Dore Brown N, Cassels A, et al. Team approach to polypharmacy evaluation and reduction: study protocol for a randomized controlled trial. Trials. 2021;22(1):746.
Blum MR, Sallevelt B, Spinewine A, O’Mahony D, Moutzouri E, Feller M, et al. Optimizing therapy to prevent avoidable hospital admissions in multimorbid older adults (OPERAM): cluster randomised controlled trial. BMJ. 2021;374:n1585.
O’Mahony D, Gudmundsson A, Soiza RL, Petrovic M, Jose Cruz-Jentoft A, Cherubini A, et al. Prevention of adverse drug reactions in hospitalized older patients with multi-morbidity and polypharmacy: the SENATOR* randomized controlled clinical trial. Age Ageing. 2020;49(4):605–14.
Riceckert A, R D, Altiner A, Drewelow E, Esmail A, Flamm M, Hann M, Johansson T, Klaassen-Mielke R, Kunnamo I, Loffler C, Piccoliori G, Sommerauer C, Trampisch U, Vogele A, Woodham A, Sonnichsen A. Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: cluster randomised controlled trial. BMJ. 2020;369:m1822.
Vasilevskis E, S AS, Hollingsworth EK, Shotwell S, Kripalani S, Mixon A, Simmons S. Deprescribing medications among older adults from end of hospitalization through postacute care: a Shed-MEDS randomized clinical trial. JAMA Intern Med. 2023;183:223.
Woo SA, Cragg A, Wickham ME, Peddie D, Balka E, Scheuermeyer F, et al. Methods for evaluating adverse drug event preventability in emergency department patients. BMC Med Res Methodol. 2018;18(1):160.
Patient Safety Network. Patient safety 101: medication errors and adverse drug events Rockville, Maryland: agency for healthcare research and quality; 2019. Available from: https://psnet.ahrq.gov/primer/medication-errors-and-adverse-drug-events.
Holbrook A, V K, Yoo L, Troyan S, Schulman S, Douketis J, Thabane L, Giilck S, Koubaesh Y, Hyland S, Keshavjee K, Ho J, Tarride J-E, Ahemd A, Talman M, Leonard B, Ahemd K, Refai M, Siegal D. Coordination of Oral Anticoagulant Care at Hospital Discharge (COACHeD): protocol for a pilot randomised controlled trial. Pilot Feasibility Stud. 2022;8:166.
Ross MCSB, Wu PE, Atique Md Candidate A, Papillon-Ferland L, Tamblyn R, Lee TC, et al. Adverse drug events in older adults: review of adjudication methods in deprescribing studies. J Am Geriatr Soc. 2020;68(7):1594–602.
Leape LL, Brennan TA, Laird N, Lawthers AG, Localio AR, Barnes BA, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324(6):377–84.
National Coordinating Council for Medication Error Reporting and Prevention. About medication errors: MERP: National Coordinating Council for Medication Error Reporting and Prevention; 2020. Available from: https://www.nccmerp.org/about-medication-errors.
Holbrook A, Pullenayegum E, Thabane L, Troyan S, Foster G, Keshavjee K, et al. Shared electronic vascular risk decision support in primary care: Computerization of Medical Practices for the Enhancement of Therapeutic Effectiveness (COMPETE III) randomized trial. Arch Intern Med. 2011;171(19):1736–44.
American Society on Aging and American Society of Consultant Pharmacists Foundation. Medication knowledge assessment: adult medication; 2012. Available from: http://adultmeducation.com/AssessmentTools_2.html.
Ontario HQ. Transitions between hospital and home: care for people of all ages: HQO; 2020. Available from: https://www.hqontario.ca/Portals/0/documents/evidence/quality-standards/qs-transitions-between-hospital-and-home-quality-standard-en.pdf.
Wenger NS, Roth CP, Shekelle P, Investigators A. Introduction to the assessing care of vulnerable elders-3 quality indicator measurement set. J Am Geriatr Soc. 2007;55(Suppl 2):S247–52.
Euroqol. EQ-5D Instruments: EuroQol; 2018. Available from: https://euroqol.org/eq-5d-instruments/.
Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–36.
Xie F, Pullenayegum E, Gaebel K, Bansback N, Bryan S, Ohinmaa A, et al. A time trade-off-derived value set of the EQ-5D-5L for Canada. Med Care. 2016;54(1):98–105.
Tseng HM, Lee CH, Chen YJ, Hsu HH, Huang LY, Huang JL. Developing a measure of medication-related quality of life for people with polypharmacy. Qual Life Res. 2016;25(5):1295–302.
Williamson PR, Altman DG, Bagley H, Barnes KL, Blazeby JM, Brookes ST, et al. The COMET Handbook: version 1.0. Trials. 2017;18(Suppl 3):280.
Perino AC, Shrader P, Turakhia MP, Ansell JE, Gersh BJ, Fonarow GC, et al. Comparison of patient-reported care satisfaction, quality of warfarin therapy, and outcomes of atrial fibrillation: findings from the ORBIT - AF registry. J Am Heart Assoc. 2019;8(9):e011205.
COACHeD RCT Additional Files 2021 (CCCQ, OAC Knowledge Test, Patient/Caregiver Study Satisfaction Survey). MacSphere, McMaster University. 2021. Available from: http://hdl.handle.net/11375/27213.
Carrasquillo O. Health care utilization. Encyclopedia of behavioral medicine. Gellman MD, Turner JR, editors. New York: Springer; 2013. p. 909–10.
Canadian Agency for Drug and Technogolies in Health (CADTH). Canadian Agency for Drug and Technogolies in Health Canada: Canadian Journal of Health Technologies; 2021. Available from: https://www.cadth.ca/.
SPIRIT Group. SPIRIT: Schedule of Enrolment, Interventions and Assessments: Standard Protocol Items: Recommendations for Interventional Trials 2020. Available from: https://www.spirit-statement.org/schedule-of-enrolment-interventions-and-assessments/.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibility Stud. 2016;2:64.
Acknowledgements
The authors acknowledge the ongoing advice and support from our Patient Advisory Committee — Melissa Sheldrick, Jeanette Smith, and Dave Patterson.
Funding
This work was supported by Canadian Institutes of Health Research (funding reference number TEG-165595). The study funders had no role in the study design; collection, management, analysis, or interpretation of data; writing of the report; or the decision to submit the report for publication.
Author information
Authors and Affiliations
Contributions
AH is the principal investigator and conceived the study idea, wrote the protocol, wrote the grant for funding, and leads the project team. All authors have made contributions to the conception or design of the work, have reviewed drafts for important intellectual content, and have approved this submitted version. No professional writers or AI-powered writing assistants were used to write this manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study has been approved (study no. 7598) by Hamilton Integrated Research Ethics Board. Significant protocol modifications will be proactively communicated to the research ethics boards through study amendments to obtain approval prior to the changes being implemented. Each modification will be assessed to determine whether it warrants communication with trial participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 2: Appendix 2.
IMPROVE-IT HRM Capacity to Consent Questionnaire.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Holbrook, A., Perri, D., Levine, M. et al. Improving medication prescribing-related outcomes for vulnerable elderly in transitions on high-risk medications (IMPROVE-IT HRM): a pilot randomized trial protocol. Pilot Feasibility Stud 10, 60 (2024). https://doi.org/10.1186/s40814-024-01484-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40814-024-01484-6