Abstract
Background
Lower-limb osteoarthritis (OA) causes high levels of pain and disability in adults over 45 years of age. Adopting and maintaining appropriate levels of physical activity (PA) can help patients with lower-limb OA self-manage their symptoms and reduce the likelihood of developing secondary noncommunicable diseases. However, patients with lower-limb OA are less active than people without musculoskeletal pain. This single-arm feasibility trial seeks to determine the feasibility and acceptability of a complex multicomponent physiotherapy behaviour change intervention that aims to aid patients with lower-limb OA to adopt and maintain optimal levels of PA.
Methods
This trial will be conducted at one site in a National Health Service physiotherapy outpatient setting in the West Midlands of England. Up to thirty-five participants with lower-limb OA will be recruited to receive a physiotherapy intervention of six sessions that aims to optimise their PA levels during phases of behavioural change: adoption, routine formation and maintenance. The intervention is underpinned by self-determination theory (and other motivational frameworks) and seeks to foster a motivationally optimal (empowering) treatment environment and implement behaviour change techniques (BCTs) that target PA behaviours across the three phases of the intervention. Physiotherapists (n = 5–6) will receive training in the why and how of developing a more empowering motivational environment and the delivery of the intervention BCTs. Participants will complete patient-reported and performance-based outcome measures at baseline and 3-month (to reflect behavioural adoption) and 6-month (maintenance) post-baseline. Feasibility and acceptability will be primarily assessed through semi-structured interviews (purposively recruiting participants) and focus groups (inviting all physiotherapists and research staff). Further evaluation will include descriptive analysis of recruitment rates, loss of follow-up and intervention fidelity.
Discussion
A novel complex, multicomponent theoretical physiotherapy behaviour change intervention that aims to create a more empowering motivational treatment environment to assist patients with lower-limb OA to adopt and maintain optimal PA levels has been developed. Testing the feasibility and acceptability of the intervention and its associated physiotherapist training and related trial procedures is required to determine whether a full-scale parallel group (1:1) randomised controlled trial to evaluate the interventions effectiveness in clinical practice is indicated.
Trial registration
Trial register: International Standard Randomised Controlled Trial identification number: ISRCTN12002764.
Date of registration: 15 February 2022.
Similar content being viewed by others
Background
Osteoarthritis (OA) is the leading cause of individual level disability for elderly adults [1] and a significant socioeconomic burden [2] which is anticipated to continue to increase [3] and engulf global healthcare systems within 10 years [4]. Lower-limb (hip and knee) OA is of primary concern, with over 300 million global cases reported, which represents an increase of over 9% from figures detailed in 1990 [5]. In the United Kingdom (UK), approximately one-in-three people over 45 years, equating to 6.6 million individuals, experience lower-limb OA symptoms [1].
Strategies to promote physical activity (PA), including planned exercises and lifestyle activities [6], are fundamental aspects of non-pharmacological interventions to help patients reduce pain and optimise function in several international OA guidelines [1, 7,8,9]. Physiotherapists are specialists in the assessment and management of musculoskeletal conditions and the primary healthcare provider of PA interventions within the National Health Service (NHS) in the UK [10].However, physiotherapy PA interventions help patients with lower-limb OA to moderate their clinical symptoms only over short time periods (≤ 3-month post-baseline) with symptom recurrence about 6-month post-baseline [11, 12]. Therefore, patients with lower-limb OA may seek further treatment, which places additional strain on the healthcare services. Clinical symptom regression and subsequent re-referral are likely associated with difficulties that patients with lower-limb OA experience when maintaining their prescribed PA behaviours post-discharge [13].
The Medical Research Council (MRC) [14] recommends that behaviour change theory should be utilised when developing complex interventions. Using theory enables specific behavioural determinants (e.g. self-efficacy for PA) to be targeted by the interventions’ treatment techniques [i.e. behaviour change techniques (BCTs)] [15]. Therefore, the interventions active ingredients can be modified over time, leading to potential increased clinical effectiveness on the target behaviour [15]. However, there is a lack of theoretical behaviour change interventions tested on patients with musculoskeletal pain [15].
Researchers postulate that individuals undergo several ‘phases’ of behaviour change when incorporating new behaviours into their lifestyle, with the most important phases for intervention delivery being adoption/initiation and maintenance [16]. In regard to the promotion of PA by physiotherapists, it is assumed that patients will be adopting PA behaviours, while receiving physiotherapy treatment and maintenancewill be manifested and hopefully ongoing post-discharge. While it is likely that several BCTs are consistently relevant to the behaviour change process overall, others are more pertinent to either the adoption or maintenance of PA phase [17].
Randomised controlled trials (RCTs) are considered the gold standard procedure to evaluate the clinical effectiveness of an intervention. However, due to the complex processes and costs that occur when delivering and evaluating behaviour change interventions, robust scoping work prior to a definitive RCT is essential [18]. Feasibility trials are advocated to test implementation of the interventions’ procedures [19] and trial design [18], thus informing whether a full-scale RCT is warranted [20]. To date, one theoretical physiotherapy behaviour change feasibility trial has been undertaken on patients with lower-limb OA. Hurley et al. (2020) [21] evaluated the feasibility and acceptability of a complex group intervention that aimed to promote self-management behaviours in patients with musculoskeletal pain. Although the intervention was highly acceptable, eligible participants preferred to be managed individually (i.e. one to one). Low recruitment rates and delays in commencing the group intervention led to a full-scale trial being unfeasible to deliver [21]. These issues have also been highlighted in research where patients and physiotherapists did not feel they received, or were unable to deliver, effective personalised treatment within the current time slots employed in the National Health Service (NHS) [22]. Furthermore, physiotherapists most commonly treat patients with lower-limb OA 1 to 1 in clinical practice [23], and this delivery maybe more effective than group classes at aiding patients’ self-management of their levels of pain and function [24].
To address these issues, an individually delivered, complex, multicomponent theoretical behaviour change physiotherapy intervention has been developed. Briefly, the intervention and associated training programme (Empowering Physio™) are underpinned by self-determination theory (SDT) which focuses on the underlying reasons for individuals’ behavioural engagement [25]. The overarching aim of the intervention is to create a more motivationally empowering treatment climate to deliver BCTs that target the determinants of PA adoption and maintenance in patients with lower-limb OA.
Aim
The trial has two primary aims:
-
1)
To investigate the feasibility and acceptability of a complex, multicomponent theoretical behaviour change physiotherapy intervention in the view of patients with lower-limb OA and treating physiotherapists
-
2)
To evaluate the feasibility and acceptability of trial-related procedures to participants and research-related staff
Objectives
Intervention feasibility and acceptability
To determine the feasibility and acceptability of the complex and multicomponent behaviour change intervention to participants and treating physiotherapists
To determine the acceptability of the bespoke Empowering Physio training programme to treating physiotherapists
To evaluate fidelity of intervention delivery by treating physiotherapists
Trial feasibility and acceptability
-
To measure the recruitment rates of participants
-
To measure the completeness of data collection of performance-based and patient-reported outcome measures at baseline, 3- and 6-month post-baseline
-
To determine the feasibility and acceptability of trial-related procedures (recruitment, outcome assessment) to participants and research staff
-
To determine the feasibility and acceptability of utilising an accelerometer as the primary outcome in a definitive randomised controlled trial
-
To determine the acceptability of the patient-reported and performance-based outcome measures to participants
Methods
Ethics
The trial protocol has received a favourable ethical approval from the Health Research Authority via the Integrated Research Application System (IRAS number: 303710). This will ensure that the trial is conducted following best practice ethical guidelines. It will follow guidance from the Research Governance Framework for Health and Social Care and comply with the Data Protection Act 2018. Although clarification will be sought from the research team if a participant withdraws, they may do so without offering one, and their normal care will not be affected in anyway. If a person withdraws, data collected up to the point of withdrawal will be used to inform the analysis unless the participants specifically withdraw consent for this.
Trial design
The trial will utilise a single-centre mixed-methods feasibility design including a quantitative prospective, single-arm feasibility trial with a 6-month follow-up [18, 26] and a qualitative component to provide an in-depth examination of the intervention’s feasibility and acceptability [20] in the view of participants, physiotherapists and research staff. Six months are the planned primary end point of the anticipated future definitive RCT. This is based on the generally accepted definition of behavioural maintenance in the literature [27] and large drop off in reported clinical effectiveness on PA behaviours [12] around this time point. This trial is registered with the International Standard Randomised Controlled Trial Number (ISRCTN) database (Trial ID: ISRCTN12002764) and follows Standard Protocol Items Recommendations for Interventional Trials (SPIRIT) guidelines [28]. The trial is reported in accordance with the CONSORT Statement for Pilot and Feasibility studies [29] and the COnsolidated criteria for REporting Qualitative research (COREQ) guidelines for quantitative and qualitative components respectively [30]. Figures 1 and 2 outline the flow of patients with lower-limb OA through the trial and the schedule of enrolment, intervention and assessments, respectively.
Trial setting and participant eligibility criteria
Patients referred for physiotherapy treatment of their hip or knee OA symptoms at the Royal Orthopaedic Hospital (ROH), Birmingham, will be invited to participate (please see Table 1 for eligibility criteria).
All intervention procedures will take place in the ROH physiotherapy clinics or virtually via phone or ‘Attend Anywhere’, the secure NHS video call service. Ongoing PA will be undertaken by people with lower-limb OA in their own home or the community.
Participant identification and consent
Feasibility trial
Potential participants will be identified from the physiotherapy waiting lists at the ROH by a member of the research team. If the patient appears to meet the eligibility criteria, they will be sent a participant information sheet (PIS) and trial consent form via post. Each potential participant will be phoned by a research nurse a minimum of 1 week later. Potential participants will be asked if they have reviewed the PIS and if they are interested in trial participation. If they report being interested, the research nurse will confirm eligibility, outline the trial and answer any outstanding questions. Consent will be taken by the research nurse during the phone call and witnessed by another ROH staff member. During the meeting, the research nurse will reiterate that participants are free to withdraw from the trial at any time, and that this will not impact on their current or future healthcare [31].
Semi-structured interviews with participants
A purposive sample of patients who undergo the physiotherapy intervention (n = 8–10) will be invited to attend individual semi-structured interviews. Participation in the semi-structured interviews will be discussed at the 3-month post-baseline assessment with the research nurse, and consent will be gained in the same manner as for the main trial. If the participant consents to having an interview, the lead researcher (MW) will contact the participant approximately 1 week later. If participants choose not to engage with the interviews, the research nurse will gently probe for any underlying reasons. If the participant chooses to divulge their reasons, these will be anonymously fed back to the lead researcher for data recording and further discussion in study steering meetings. Interviews will be offered either by telephone or skype/zoom depending on the participant’s preference and will be scheduled as close to the end of their treatment sessions as convenient for the participant.
Focus groups with Royal Orthopaedic Hospital physiotherapists and research staff
All physiotherapists who deliver intervention (n = 6–7) and the ROH trial research staff team will be invited to attend focus groups. The participating ROH physiotherapists and research staff will be approached by the site lead researcher (GS), an advanced physiotherapy practitioner within the ROH. Prior to the focus groups, participants will be given the opportunity to ask any questions, and the PIS will be reviewed in real time. MW will conduct all semi-structured interviews and focus groups and establish informed consent prior to focus groups (intervention physiotherapists and ROH research staff, respectively). MW is experienced in clinical practice, establishing consent (has in-date GCP training), and conducting and analysing qualitative data obtained from semi-structured interviews and focus groups.
Multicomponent behaviour change intervention
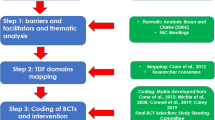
The complex, multicomponent theoretical behaviour change intervention was developed iteratively through several interconnecting projects including the following:
-
A systematic review that identified the most effective BCTs used in physiotherapy interventions to promote PA adherence in patients with lower-limb OA [12, 32]
-
Semi-structured interviews with patients with lower-limb OA [17, 33]
-
Focus groups with outpatient physiotherapists
-
A scoping review and mapping exercise to identify appropriate theories of behaviour change to underpin the intervention delivery and physiotherapy training
The resulting intervention, which is underpinned by self-determination theory (SDT), has the overarching aim of creating a more motivationally empowering treatment climate and to implement particular BCTs to support patients with lower-limb OA to adopt and maintain appropriate individual levels of PA. Briefly, SDT outlines three universal psychological needs (competence, relatedness and autonomy) which influence the quality of an individuals’ motivation (i.e. how self-determined that motivation is) [34]. An individual’s competence relates to their ability to effectively do the behaviour; autonomy refers to whether the engagement is voluntary and the degree of agency that an individual has with regard to the behaviour; relatedness involves feelings of being associated and optimally supported by others in regard to the behaviour [25]. SDT postulates that fulfilment of the 3 psychological needs leads to more autonomous forms of motivation and facilitates behaviour adoption and maintenance. A primarily SDT-based training programme Empowering Coaching, [35, 36], which has been adapted for delivery to physiotherapists (i.e. the bespoke Empowering Physio training), was selected to guide the training of the trial physiotherapists in regard to the motivational aspects of the intervention delivery. A brief overview of the sequential development of the intervention and associated training programme is outlined in the additonal file 2, Appendix 1 and further detail will be provided in a subsequent paper.
Intervention materials and associated behaviour change techniques
Support material has been developed and will be utilised to assist delivery of intervention BCTs. Participants will be given two paper-based workbooks. The first workbook targets facilitating PA adoption by encouraging the patient to reflect on their PA preferences to identify appropriate patient-centred PA goals (‘goal setting’; ‘review behaviour goals’) and includes a weekly activity planner (‘action planner’). Each participant will be given a Yamax pedometer (Tokyo, Japan) to keep that will act as a self-monitoring device (‘self-monitoring of behaviour’; ‘feedback of behaviour’) and as a thanks for partaking in the trial.
Participants will be given the second workbook in session 4. This workbook aids participants to identify necessary modifications to their physical and/or social environment (‘problem-solving’; ‘restructuring of the social environment’) to help develop a PA routine and then maintain these behaviours (‘habit formation’; ‘generalisation of the target behaviour’). A detailed list of local PA services and supports is also included [‘social support (practical)’]. Each workbook is written using needs supportive language to aid participants’ feelings of competence, autonomy and relatedness towards establishing PA as a lifestyle behaviour.
The trial-specific workbooks will be supported by the Versus Arthritis information booklets on knee or hip OA which outline general information on OA epidemiology, pathophysiology, management strategies (exercise and medication) and appropriate support services (‘information on health consequences of performing the behaviour’; ‘information on social and environmental consequences’). An outline of the intervention materials, their intended sequencing and associated BCTs is outlined in Table 2.
The use of manual therapy techniques and supportive braces/walking devices will be incorporated into the intervention at the discretion of the treating physiotherapist [1]. Participants will be asked to not seek any additional treatments for their OA symptoms for the duration of the trial and to maintain their standard medications. Any co-interventions will be recorded in the assessment appointments with the research nurses.
Intervention delivery
Participants will attend individual intervention sessions (approximately six based on patient preference reflected in previous work related to the intervention development [33] and the standard number of physiotherapy sessions offered by the ROH) with the trained physiotherapists. Physiotherapy sessions will be conducted in person, via phone or ‘Attend Anywhere’ (based on participant preferences). Appointment frequency will depend on the participants’ availability and related PA goals and the physiotherapists’ clinical schedules. The ROH’s standard physiotherapy sessions are 45 min for an initial appointments and 30 min for a follow-up session.
Physiotherapist training
The bespoke Empowering Physio™ training programme aims to enhance physiotherapists’ understanding of differences in the quality of patient motivation (and implications) and awareness of the treatment climate they create and how it can influence patients’ psychological needs of competence, autonomy, relatedness and their subsequent autonomous motivation to adopt and maintain their PA goals. The training will involve presentations, and interactive exercises to convey why, when and how physiotherapists interact and provide feedback with patients during their treatment sessions may influence participants’ levels of controlled and autonomous motivation to engage in PA. Delivery of the BCTs across PA adoption, routine formation and/or maintenance will be addressed including their theoretical background. The physiotherapists will receive printed and electronic versions of the Empowering Physio™ training workbook, a handbook with BCT definitions and clinical examples and key research articles relevant the intervention.
Intervention physiotherapists (n = 6–7) will be trained by two members of the research team, Professor Joan Duda (JD), the founder of the Empowering Coaching programme who possesses a PhD in psychology and is an internationally renowned expert in motivational processes, and MW, whose PhD has focused on the development of the intervention. Training will include two 3-h, in-person group training sessions conducted on consecutive weeks and a follow-up 2-h ‘top-up’ session approximately 1 month later.
Trial outcomes and success criteria
Once data analyses are complete, the overall trial’s success (i.e. trial-related procedures and intervention feasibility and acceptability) will be determined by evaluating the results against a priori established criteria for success (Table 3). The criteria were established by the Trial Management Group, which includes experts in musculoskeletal physiotherapy, ROH physiotherapists and researchers with expertise in trial methodology and behaviour change supported by contemporary literature in contemporary feasibility trial methodology [37]. Each criterion will be evaluated against guidance outlined by Thabane et al. (2010) [38] to determine if progression to a definitive trial is recommended. Possible outcomes include the following:
-
Stop: A definitive trial is not feasible.
-
Continue with modification: Modification of the protocol is required to make definitive trial feasible.
-
Continue without modification: Modification of the protocol is not required to make definitive trial feasible but requires close monitoring.
-
Continue without modifications: A definitive trial appears feasible.
Once it has been established if each criterion has been met, further consultation with patient and public involvement (PPI) at Trial Steering Group meetings will be utilised to determine if and what modifications the intervention requires to progress to the full-scale RCT (e.g. adaptions to intervention to enable delivery in alternative NHS physiotherapy departments).
Methods of data collection
Patient-reported and performance-based outcomes during trial
Participants will complete outcome assessment at baseline, 3-month post-baseline and 6-month post-baseline. Baseline outcome assessment appointments will be scheduled immediately prior to participants’ initial physiotherapy session. Outcome assessments are anticipated to be conducted virtually in the participants’ own homes via phone or ‘Attend Anywhere’. However, if participants do not feel confident about completing their performance-based outcomes at home, they will be offered a choice of having their baseline assessment done in person. Outcomes to be assessed include the use of an accelerometer to measure daily PA, patient-reported outcome measures (PROMs) and performance-based outcomes. Accelerometers and PROMs will be posted to participants’ home addresses 1 week prior to outcome assessment appointments. A 150-cm tape measure will be included for standardisation of one of the performance-based tests (figure-of-8 walking test: Table 4) [47]. Participants will be asked to complete PROMs prior to assessment appointments so the session can be used to collect performance-based outcomes and answer any questions. A stamped, addressed envelope will be included for return of accelerometers (including simple fitting instructions and a belt to secure the accelerometer) and PROMs to the ROH. If accelerometers and PROMs are not returned within 2 weeks of their assessment appointment, participants will be contacted via text/telephone and asked to send them back at their earliest convenience.
The accelerometer will be fitted over the right anterior superior iliac spine [45, 46, 67]. Following a standardised and widely used protocol [68], participants will be asked to wear accelerometers for 7 consecutive days [43, 69,70,71] with removal for sleep or when in water (e.g. swimming or shower) [45, 46, 71, 72]. The patient-reported and performance-based outcomes to be assessed, including their rationale, are detailed in Table 4.
Collection of qualitative data
As the main aim of the qualitative component of the trial is to gain in-depth perspectives on the feasibility and acceptability of the trial intervention and associated processes, the epistemological stance is based on phenomenology [73]. Questions will initially be asked in an open manner with clarification sought if key points are identified. Participants, physiotherapists.and research staff will be invited to introduce new topics and/or issues as the discussions progress. The respective topic guides have been developed a priori with questions determined through collaboration of the trial management team which includes clinical physiotherapists, researchers with expertise in qualitative research methods and behaviour change and PPI [74].
For individuals attending semi-structured interviews and focus groups, demographic detail will be recorded to enable description of participants, intervention physiotherapists and ROH research staff, respectively.
Patient and public involvement
All patient-facing documents were designed in collaboration with a lay person (ED) with extensive experience in public involvement and a lifetime’s work in effective communication. ED is a central member of the Trial Management and Steering Groups. They have reviewed and offered extensive feedback on all patient-facing documents including the patient information sheets, consent forms, semi-structured interview forms, intervention content, trial protocol, ethics application and PROMs.
Sample size
Although a formal calculation was not completed, total sample sizes of between n = 24 to 50 are recommended in feasibility trials and a sample of 30 [18] to 35 [75] considered sufficient to ensure normal distribution of participants. An audit of the ROH physiotherapy waiting lists suggested that approximately 65 patients with musculoskeletal pain are referred to the ROH each week, and that approximately 5% would be eligible to participate in the trial (i.e. three per week). Based on previous trials conducted at the ROH, approximately one in three of those approached consents to participate. Therefore, based on trial resources and timelines, recruitment of a target sample of up to 35 participants over a 9-month period is estimated.
Data analysis
Quantitative
The primary quantitative data analysis will be descriptive [19, 31]. A CONSORT diagram will outline the number of participants who were identified, recruited, commenced and finished treatment, and the recruitment rate (number/month and number approached/number recruited) will be reported as a percentage [29]. Where possible, reasons for refusal and dropout during the intervention will be recorded and reported. A Shapiro–Wilk test will test the normality of continuous data (e.g. PROM and accelerometer data), and the mean and 95% confidence intervals will be reported for each. The number of non-completed/partially completed PROMs, performance measures and accelerometer data at each timepoint will be expressed as a percentage. Partially completed PROM and accelerometer data will not be included in the data analysis, but potential reasons, including practicality, will be discussed in the interviews.
Intervention fidelity
Fidelity assessment of delivery of the intervention will include evaluation of two aspects: what was delivered (e.g. the BCTs) and how these BCTs were delivered/overall exchanges between the physiotherapist and patient (i.e. the physiotherapy treatment motivational climate) in regard to promoting the patient’s feelings of competence, autonomy and relatedness. Physiotherapists will be asked to audio record one session from each of the adoption, routine formation and maintenance phases from their first and third participants. The specific sessions to be recorded in each intervention phase will be randomised. The audio recording will be transcribed verbatim.
What was delivered: evaluation of behaviour change techniques delivered
-
Two members of the research team will code all transcripts to identify the BCTs delivered. A coding manual will be provided to coders to optimise consistency. It will include intervention BCTs definition as per the V1 taxonomy [76] and examples of how the technique may be delivered or described in the intervention. BCTs will only be coded once per session and will be written as either (1) fully delivered or (2) partially delivered [77]. BCTs that were present but not part of the specific protocol will also be noted [78]. Coders will have completed online training for BCT identification using the V1 taxonomy and have experience coding BCTs from intervention manuscripts [12] and transcribed interviews with high levels of reliability. The median and interquartile range of BCTs per session in the adoption, routine formation and maintenance phases will be reported respectively. The number of times each BCT was fully delivered compared to when it was outlined in the treatment manual will be averaged across physiotherapists and calculated as a percentage. Overall fidelity will be categorised as high if ≥ 80% of essential BCTs are present, moderate if 50–79% are present, and lowif 0–49% are present [40, 78].
How was it delivered: evaluation of the treatment motivational climate
Two researchers will use the Interpersonal Support in Physical Activity Consultations Observational Tool (ISPACOT) [39], a 21-item tool that is grounded in SDT, which will be used to evaluate the treatment climate of the physiotherapy sessions. The tool assesses four aspects of the motivational climate created as follows: autonomy support, involvement, structure and interpersonal control and has demonstrated moderate to high levels of inter-rater reliability when assessments are carried out by trained users [39]. A 7-point Likert scale, ranging from 1 ‘not at all true’ to 7 ‘very true’, will be used to determine the degree of need support. The mean Likert scores will be calculated for all physiotherapists and reported for the adoption, routine formation and maintenance phases, respectively. In line with previous research, scores of ≥ 4/7 will be considered the minimum required to deliver a need supportive treatment climate [41, 79].
Qualitative data analysis
The interviews and focus groups will be audio recorded and transcribed verbatim. Interviews and focus groups will not be repeated. However, transcripts will be returned to participants/physiotherapists/research staff for member checking and any supplementary comments which are highlighted will be presented to the Trial Steering Group, noted and integrated with the main analysis [80]. MW will take supplementary field notes during the interview process to enable triangulation during data analysis.
All transcripts will be analysed following a six-step deductive method as outlined in Braun and Clarke (2006) [81]. To analyse the semi-structured interviews, two researchers will code the first transcript together to establish a preliminary framework and identify initial themes using NVivo 11 software (QSR International, Melbourne, Australia). A second transcript will be coded independently by the same two researchers with in-depth meetings planned to test the initial framework. The remaining transcripts will be coded by both researchers, with codes and emerging themes clarified through researcher meeting (approximately every two–three transcripts). Focus groups will be undergo the same analysis by two researchers. Iterative results from semi-structured interviews and focus groups, supported by direct quotations, will be presented to the research team and at Trial Steering Group meetings for feedback and challenge.
Additional information
Further trial information, including data storage, monitoring of adverse events and intended dissemination of findings, can be viewed in the additional file 2, Appendix 2.
Discussion
Lower-limb OA is a prevalent and painful condition that impacts the quality of life in adults ≥ 45 years old. International guidelines support optimising PA to help manage clinical symptoms associated with OA. However, people with lower-limb OA are generally less active than other people of a similar age. Optimising PA levels requires people to alter their behaviours, and this is thought to be most easily accomplished if the treatment incorporates behaviour change theory. Theoretical interventions have demonstrated significant improvements in PA levels in several populations with secondary noncommunicable diseases. Therefore, the main aim of this trial is to determine the acceptability and feasibility of delivery of a complex, multicomponent theoretical physiotherapy behaviour change intervention that aims to optimise adoption and maintenance of prescribed PA in patients with lower-limb OA.
The behaviour change intervention has been developed targeting the specific behavioural determinants of PA adoption and maintenance. It has been developed sequentially over a series of projects including a systematic review and a qualitative project incorporating the views of patients with lower-limb OA and heavily influenced from feedback from PPI.
Key methodological considerations were discussed extensively within the SSC at the trial planning stage. These included the primary data collection end point and choice of a single-arm or randomised parallel two-arm trial design.
Within the behaviour change literature, the concept of behavioural ‘maintenance’ suffers from considerable heterogeneity [16]. However, the most utilised definition in empirical studies is that proposed in the transtheoretical model, which suggests behavioural maintenance occurs after approximately 6 months of behaviour change practice (i.e. when a behaviour change intervention commences) [82]. Although this definition is based on addictive behaviours, it has also been utilised in previous systematic reviews examining PA in healthy young and middle-aged [83] and inactive adult populations [84].
Interestingly, the effectiveness of physiotherapy delivered BCTs on PA behaviours (as measured by effectiveness ratios) was lowest at 6-month post-baseline compared to other time points in our systematic review [12]. Furthermore, there appeared to be a drop off in the amount of available data for analysis at this timepoint. Therefore, a 6-month post-baseline was chosen as the primary end point for our feasibility study. However, if a definitive RCT is indicated once analysis is complete, it is anticipated that the interventions effectiveness will be measured at further time points post-baseline (e.g. 9 and/or 12 months) to capture this data for further analysis.
There have been an extensive number of RCTs conducted on patients with lower-limb OA, and methodological aspects such as randomisation have already been established. As information on these features exists, and STAPLO feasibility trial does not aim to test the interventions effectiveness, adding a parallel arm was considered unresourceful and potentially unethical. To this end, a single-arm study with a sample size great enough to provide a normal distribution of patients was planned [85].
This trial design should enable purposive sampling to gain a wide range of opinions on the feasibility and acceptability of the intervention from participants, intervention physiotherapists and research staff. Furthermore, study research nurses were not informed of the trials’ overall design (single arm versus parallel arms) or primary aims during training. Therefore, they are considered blind assessors in the STAPLO trial. The results of several large scales, low risk-of-bias RCTs, from those identified in our systematic review, [12, 86] and conducted locally, [87] will be used to inform methodological decisions, such as a sample size calculation, if a full-scale definitive trial is indicated.
The proposed intervention protocol has been concept tested against physiotherapists working clinically and further refined to encompass theories of behaviour change. Feasibility examination is required prior to its effectiveness being determined.
Availability of data and materials
The datasets used and/or analysed during the current trial will be available from the corresponding author on reasonable request.
Abbreviations
- OA:
-
Osteoarthritis
- PA:
-
Physical activity
- BCT:
-
Behaviour change technique
- UK:
-
United Kingdom
- NHS:
-
National Health Service
- MRC:
-
Medical Research Council
- SDT:
-
Self-determination theory
- CONSORT: ISRCTN:
-
International Standard Randomised Controlled Trial Number
- SPRIT:
-
Standard Protocol Items Recommendations for Interventional Trials
- COREQ:
-
COnsolidated criteria for REporting Qualitative research
- ROH:
-
Royal Orthopaedic Hospital Birmingham
- PIS:
-
Participant information sheet
- PROM:
-
Patient-reported outcome measure
- EC:
-
Empowerment coaching
- V1:
-
Version 1
- ISPACOT:
-
Interpersonal Support in Physical Activity Consultations Observational Tool
References
NICE. National Institute for for Health and Care Excellence 2022. Osteoarthritis in over 16s: diagnosis and management. clinical guideline NG266. 2022. Available from: https://www.nice.org.uk/guidance/ng226/resources/osteoarthritis-in-over-16s-diagnosis-and-management-pdf-66143839026373. Accessed 28 Mar 2023.
Wilkie R, Hay EM, Croft P, et al. Exploring how pain leads to productivity loss in primary care consulters for osteoarthritis: a prospective cohort study. PLoS One. 2015;10(4). https://doi.org/10.1371/journal.pone.0120042 [published Online First: 2015/04/08].
Speerin R, Slater H, Li L, et al. Moving from evidence to practice: models of care for the prevention and management of musculoskeletal conditions. Best Pract Res Clin Rheumatol. 2014;28(3):479–515. https://doi.org/10.1016/j.berh.2014.07.001. [publishedOnlineFirst:2014/12/08]
Eyles JP, Hunter DJ, Bennell KL, et al. Priorities for the effective implementation of osteoarthritis management programs: an OARSI international consensus exercise. Osteoarthritis Cartilage. 2019;27(9):1270–9. https://doi.org/10.1016/j.joca.2019.05.015. [publishedOnlineFirst:2019/06/05]
Safiri S, Kolahi AA, Smith E, et al. Global, regional and national burden of osteoarthritis 1990–2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79(6):819-828. https://doi.org/10.1136/annrheumdis-2019-216515.
Casperson CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health related research. Public Health Rep. 1985;100(2):126–31.
Fernandes L, Hagen KB, Bijlsma JW, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis. 2013;72(7):1125–35. https://doi.org/10.1136/annrheumdis-2012-202745. [publishedOnlineFirst:2013/04/19]
Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2020;72(2):149–62. https://doi.org/10.1002/acr.24131/.
Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–89. https://doi.org/10.1016/j.joca.2019.06.011. [publishedOnlineFirst:2019/07/07]
Foster NE, Healey EL, Holden MA, et al. A multicentre, pragmatic, parallel group, randomised controlled trial to compare the clinical and cost-effectiveness of three physiotherapy-led exercise interventions for knee osteoarthritis in older adults: the BEEP trial protocol. BMC Musculoskelet Disord. 2014;15:254.
Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee: a Cochrane systematic review. Br J Sports Med. 2015;49(24):1554–7. https://doi.org/10.1136/bjsports-2015-095424. [publishedOnlineFirst:2015/09/26]
Willett M, Duda J, Fenton S, et al. Effectiveness of behaviour change techniques in physiotherapy interventions to promote physical activity adherence in lower limb osteoarthritis patients: a systematic review. PLoS One. 2019;14(7). https://doi.org/10.1371/journal.pone.0219482 [published Online First: 2019/07/11].
Pisters MF, Veenhof C, Schellevis FG, et al. Long-term effectiveness of exercise therapy in patients with osteoarthritis of the hip or knee: a randomized controlled trial comparing two different physical therapy interventions. Osteoarthritis Cartilage. 2010;18(8):1019–26. https://doi.org/10.1016/j.joca.2010.05.008.
Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ. 2008;337. https://doi.org/10.1136/bmj.a1655 [published Online First: 2008/10/01].
Michie S, Prestwich A. Are interventions theory-based? Development of a theory coding scheme. Health Psychol. 2010;29(1):1–8. https://doi.org/10.1037/a0016939. [publishedOnlineFirst:2010/01/13]
Kwasnicka D, Dombrowski SU, White M, et al. Theoretical explanations for maintenance of behaviour change: a systematic review of behaviour theories. Health Psychol Rev. 2016;10(3):277–96. https://doi.org/10.1080/17437199.2016.1151372. [publishedOnlineFirst:2016/02/09]
Willett M, Greig C, Fenton S, et al. Utilising the perspectives of patients with lower-limb osteoarthritis on prescribed physical activity to develop a theoretically informed physiotherapy intervention. BMC Musculoskelet Disord. 2021;22:155. https://doi.org/10.1186/s12891-021-04036-8.
Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10(2):307–12.
Tickle-Degnen L. Nuts and bolts of conducting feasibility studies. Am J Occup Ther. 2013;67(2):171–6. https://doi.org/10.5014/ajot.2013.006270. [publishedOnlineFirst:2013/02/26]
Bowen DJ, Kreuter M, Spring B, et al. How we design feasibility studies. Am J Prev Med. 2009;36(5):452–7. https://doi.org/10.1016/j.amepre.2009.02.002. [publishedOnlineFirst:2009/04/14]
Hurley DA, Jeffares I, Hall AM, et al. Feasibility cluster randomised controlled trial evaluating a theory-driven group-based complex intervention versus usual physiotherapy to support self-management of osteoarthritis and low back pain (SOLAS). Trials. 2020;21(1):807. https://doi.org/10.1186/s13063-020-04671-x. [publishedOnlineFirst:2020/09/25]
Jordan KP, Kadam UT, Hayward R, et al. Annual consultation prevalence of regional musculoskeletal problems in primary care: an observational study. BMC Musculoskelet Disord. 2010;11:144. https://doi.org/10.1186/1471-2474-11-144.
Tiffreau V, Mulleman D, Coudeyre E, et al. The value of individual or collective group exercise programs for knee or hip osteoarthritis. Clinical practice recommendations. Ann Readapt Med Phys. 2007;50(9):741–6. https://doi.org/10.1016/j.annrmp.2007.10.001. 34–40. [published Online First: 2007/10/30].
Fransen M, McConnell S, Harmer AR, et al. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2015;1:CD004376. https://doi.org/10.1002/14651858.CD004376.pub3.
Deci EL, Ryan RM. Intrinsic motivation and self-determination in human behavior. New York: Plenum Press; 1985.
Arain M, Campbell MJ, Cooper CL, et al. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med Res Methodol. 2010;10:67. https://doi.org/10.1186/1471-2288-10-67.
Prochaska JO, Di Clemente CC, Norcross JC. In search of how people change: applications to addictive behaviors. Am Psychol. 1992;47(9):1102–11.
Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–7.
Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot and feasibility studies. 2016;2:64. https://doi.org/10.1186/s40814-016-0105-8.
Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–57.
Bowling A. Research methods in health: investigating health and health services. UK: McGraw-Hill Education; 2014.
Willett M, Duda J, Gautrey C, et al. Effectiveness of behavioural change techniques in physiotherapy interventions to promote physical activity adherence in patients with hip and knee osteoarthritis: a systematic review protocol. BMJ Open. 2017;7(6). https://doi.org/10.1136/bmjopen-2017-015833.
Willett MJ, Greig C, Rogers D, et al. Barriers and facilitators to recommended physical activity in lower-limb osteoarthritis: protocol for a qualitative study exploring patients and physiotherapist perspectives using the theoretical domains framework and behaviour change taxonomy. BMJ Open. 2019;9(10). https://doi.org/10.1136/bmjopen-2019-029199.
Deci EL, Ryan RM. The “what” and “why” of goal pursuits: human needs and the self-determination of behaviour. Psychological Inquir. 2000;11(4):227–68.
Duda JL. The conceptual and empirical foundations of Empowering Coaching™: setting the stage for the PAPA project. International Journal of Sport and Exercise Psychology. 2013;11(4):311–8. https://doi.org/10.1080/1612197x.2013.839414.
Duda JL, Appleton PR. Empowering and disempowering coaching climates: conceptualization, measurement considerations, and intervention implications. 2016:373–88. doi: https://doi.org/10.1016/b978-0-12-803634-1.00017-0
O’Cathain A, Hoddinott P, Lewin S, et al. Maximising the impact of qualitative research in feasibility studies for randomised controlled trials: guidance for researchers. Pilot Feasibility Stud. 2015;1:32. https://doi.org/10.1186/s40814-015-0026-y. [publishedOnlineFirst:2015/09/07]
Thabane L, Ma J, Chu R, et al. A tutorial on pilot studies: the what, why and how. BMC medical research methodology. 2010;10(1). https://doi.org/10.1186/1471-2288-10-1.
Rouse PC, Duda JL, Ntoumanis N, et al. The development and validation of the interpersonal support in physical activity consultations observational tool. Eur J Sport Sci. 2016;16(1):106–14. https://doi.org/10.1080/17461391.2014.987320. [publishedOnlineFirst:2014/12/10]
Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent. 2011;71 Suppl 1:S52-63. https://doi.org/10.1111/j.1752-7325.2011.00233.x. [publishedOnlineFirst:2011/04/19]
Keogh A, Matthews J, Segurado R, et al. Feasibility of training physical therapists to deliver the theory-based self-management of osteoarthritis and low back pain through activity and skills (SOLAS) intervention within a trial. Phys Ther. 2018;98(2):95–107.
Knoll N, Hohl DH, Motter S, et al. Facilitating physical activity and reducing symptoms in patients with knee osteoarthritis: study protocol of a randomized controlled trial to test a theory-based PrevOP-psychological adherence program (PrevOP-PAP). BMC Musculoskelet Disord. 2018;19(1):221. https://doi.org/10.1186/s12891-018-2158-8. [publishedOnlineFirst:2018/07/20]
Svege I, Kolle E, Risberg MA. Reliability and validity of the physical activity scale for the elderly (PASE) in patients with hip osteoarthritis. BMC Musculoskelet Disord. 2012;13:26. https://doi.org/10.1186/1471-2474-13-26. [publishedOnlineFirst:2012/02/23]
Freedson PS, Melanson E, Sirard J. Calibration of the computer science and applications. Inc accelerometer> Med Sci Sports Exerc. 1998;30(5):777–81.
Myers ND, Lee S, Bateman AG, et al. Accelerometer-based assessment of physical activity within the Fun for Wellness online behavioral intervention: protocol for a feasibility study. Pilot Feasibility Stud. 2019;5:73. https://doi.org/10.1186/s40814-019-0455-0. [publishedOnlineFirst:2019/06/06]
Adlard KN, Jenkins DG, Salisbury CE, et al. Peer support for the maintenance of physical activity and health in cancer survivors: the PEER trial - a study protocol of a randomised controlled trial. BMC Cancer. 2019;19(1):656. https://doi.org/10.1186/s12885-019-5853-4. [publishedOnlineFirst:2019/07/05]
Barker KL, Batting M, Schlussel M, et al. The reliability and validity of the figure of 8 walk test in older people with knee replacement: does the setting have an impact? Physiotherapy. 2019;105(1):76–83. https://doi.org/10.1016/j.physio.2018.07.003. [publishedOnlineFirst:2018/09/23]
Craig CL, Marshall AL, Sjostrom M, et al. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. https://doi.org/10.1249/01.MSS.0000078924.61453.FB. [publishedOnlineFirst:2003/08/06]
Newman-Beinart NA, Norton S, Dowling D, et al. The development and initial psychometric evaluation of a measure assessing adherence to prescribed exercise: the Exercise Adherence Rating Scale (EARS). Physiotherapy. 2017;103(2):180–5. https://doi.org/10.1016/j.physio.2016.11.001. [publishedOnlineFirst:2016/12/04]
Meade LB, Bearne LM, Godfrey EL. Comprehension and face validity of the exercise adherence rating scale in patients with persistent musculoskeletal pain. Musculoskeletal Care. 2018;16(3):409–12.
Rolfson O, Wissig S, van Maasakkers L, et al. Defining an International Standard Set of Outcome Measures for Patients With Hip or Knee Osteoarthritis: Consensus of the International Consortium for Health Outcomes Measurement Hip and Knee Osteoarthritis Working Group. Arthritis Care Res (Hoboken). 2016;68(11):1631–9. https://doi.org/10.1002/acr.22868. [publishedOnlineFirst:2016/10/27]
Alghadir AH, Anwer S, Iqbal A, et al. Test-retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J Pain Res. 2018;11:851–6. https://doi.org/10.2147/JPR.S158847. [publishedOnlineFirst:2018/05/08]
Ferraz MB, Quaresma MR, Aquino LR, et al. Reliability of pain scales in the assessment of literate and illiterate patients with rheumatoid arthritis. J Rheumatol. 1990;17(8):1022–4 (8):1022–4.
Perruccio AV, Stefan Lohmander L, Canizares M, et al. The development of a short measure of physical function for knee OA KOOS-Physical Function Shortform (KOOS-PS) - an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16(5):542–50. https://doi.org/10.1016/j.joca.2007.12.014. [publishedOnlineFirst:2008/02/26]
Davis AM, Perruccio AV, Canizares M, et al. The development of a short measure of physical function for hip OA HOOS-Physical Function Shortform (HOOS-PS): an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16(5):551–9. https://doi.org/10.1016/j.joca.2007.12.016. [publishedOnlineFirst:2008/02/26]
Ozden F, Coskun G, Bakirhan S. The test-retest reliability and concurrent validity of the five times sit to stand test and step test in older adults with total hip arthroplasty. Experimental gerontology. 2020;142. https://doi.org/10.1016/j.exger.2020.111143.
Bilbao A, Garcia-Perez L, Arenaza JC, et al. Psychometric properties of the EQ-5D-5L in patients with hip or knee osteoarthritis: reliability, validity and responsiveness. Qual Life Res. 2018;27(11):2897–908. https://doi.org/10.1007/s11136-018-1929-x. [publishedOnlineFirst:2018/07/07]
Resnick B, Jenkins LS. Testing the reliability and validity of the Self-Efficacy for Exercise Scale. Nurs Res. 2000;49(3):154–9.
McAuley E. The role of efficacy cognitions in the prediction of exercise behavior in middle-aged adults. J Behav Med. 1992;15(1):65–88.
Markland D, Tobin V. A modification to the behavioural regulation in exercise questionnaire to include an assessment of amotivation. J Sport Exerc Psychol. 2004;26(2):191–6.
Wilson PM, Rodgers WM, Loitz CC, et al. “It’s who I am…really!” The importance of integrated regulation in exercise contexts. Journal of Biobehavioral Research. 2006;11(2):79–104.
Brooks JM, Kaya C, Chan F, et al. Validation of the Behavioural Regulation in Exercise Questionnaire-2 for adults with chronic musculoskeletal pain. Int J Ther Rehabil. 2018;25(8):395–404. https://doi.org/10.12968/ijtr.2018.25.8.395. [publishedOnlineFirst:2018/08/02]
Liu L, Xiang M, Guo H, et al. Reliability and Validity of the Behavioral Regulation in Exercise Questionnaire-2 for Nursing Home Residents in China. Asian Nurs Res (Korean Soc Nurs Sci). 2020;14(1):11–6. https://doi.org/10.1016/j.anr.2019.12.002.
Wilson PM, Rogers WT, Rodgers WM, et al. The Psychological Need Satisfaction in Exercise Scale. J Sport Exerc Psychol. 2006;28(3):231–51.
Gunnell KE, Wilson PM, Zumbo BD, et al. Assessing psychological need satisfaction in exercise contexts: issues of score invariance, item modification, and context. Meas Phys Educ Exerc Sci. 2012;16(3):219–36. https://doi.org/10.1080/1091367x.2012.693340.
Williams GC, Freedman ZR, Deci EL. Supporting autonomy to motivate glucose control in patients with diabetes. Diabetes Care 1998;21(1644–1651).
Baird JF, Sasaki JE, Sandroff BM, et al. An intervention for changing sedentary behavior among African Americans with multiple sclerosis: protocol. JMIR research protocols. 2019;8(5). https://doi.org/10.2196/12973.
Song J, Semanik P, Sharma L, et al. Assessing physical activity in persons with knee osteoarthritis using accelerometers: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken). 2010;62(12):1724–32. https://doi.org/10.1002/acr.20305. [publishedOnlineFirst:2010/09/02]
Crombie KM, Leitzelar BN, Almassi NE, et al. Translating a “stand up and move more” intervention by state aging units to older adults in underserved communities: protocol for a randomized controlled trial. Medicine (Baltimore). 2019;98(27). https://doi.org/10.1097/MD.0000000000016272 [published Online First: 2019/07/07].
Missud DC, Parot-Schinkel E, Connan L, et al. Physical activity prescription for general practice patients with cardiovascular risk factors-the PEPPER randomised controlled trial protocol. BMC Public Health. 2019;19(1):688. https://doi.org/10.1186/s12889-019-7048-y. [publishedOnlineFirst:2019/06/05]
Ryan JM, Fortune J, Stennett A, et al. Changing physical activity behaviour for people with multiple sclerosis: protocol of a randomised controlled feasibility trial (iStep-MS). BMJ Open. 2017;7. https://doi.org/10.1136/bmjopen-2017-018875.
Jones AV, Evans RA, Esliger DW, et al. Protocol for a feasibility trial to inform the development of a breathlessness rehabilitation programme for chronic obstructive pulmonary disease and chronic heart failure (the COHERE trial). BMJ Open. 2019;9. https://doi.org/10.1136/bmjopen-2019-029387.
McLeod J. Qualitative research in counselling and psychotherapy. SAGE Publications Ltd; 2011.
Brett J, Staniszewska S, Mockford C, et al. Mapping the impact of patient and public involvement on health and social care research: a systematic review. Health Expect. 2014;17(5):637–50. https://doi.org/10.1111/j.1369-7625.2012.00795.x. [publishedOnlineFirst:2012/07/20]
Teare MD, Dimairo M, Shephard N, et al. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study. Trials. 2014;15(1):264.
Michie S, Richardson M, Johnston M, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95. https://doi.org/10.1007/s12160-013-9486-6. [publishedOnlineFirst:2013/03/21]
Wood CE, Richardson M, Johnston M, et al. Applying the behaviour change technique (BCT) taxonomy v1: a study of coder training. Transl Behav Med. 2015;5(2):134–48. https://doi.org/10.1007/s13142-014-0290-z. [publishedOnlineFirst:2015/06/02]
Keogh A, Matthews J, Hurley DA. An assessment of physiotherapist’s delivery of behaviour change techniques within the SOLAS feasibility trial. Br J Health Psychol. 2018;23(4):908–32. https://doi.org/10.1111/bjhp.12323.
Tessier D, Sarrazin P, Ntoumanis N. The effect of an intervention to improve newly qualified teachers’ interpersonal style, students motivation and psychological need satisfaction in sport-based physical education. Contemp Educ Psychol. 2010;35(4):242–53. https://doi.org/10.1016/j.cedpsych.2010.05.005.
Thomas DR. Feedback from research participants: are member checks useful in qualitative research? Qual Res Psychol. 2016;14(1):23–41. https://doi.org/10.1080/14780887.2016.1219435.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101. https://doi.org/10.1191/1478088706qp063oa.
Prochaska JO, DiClemente CC. Transtheoretical therapy: toward a more integrative model of change. Psychotherapy. 1982;19(3):276–88. https://doi.org/10.1037/h0088437.
Murray JM, Brennan SF, French DP, et al. Mediators of behavior change maintenance in physical activity interventions for young and middle-aged adults: a systematic review. Ann Behav Med. 2018;52(6):513–29. https://doi.org/10.1093/abm/kay012. [publishedOnlineFirst:2018/04/20]
Howlett N, Trivedi D, Troop NA, et al. Are physical activity interventions for healthy inactive adults effective in promoting behavior change and maintenance, and which behavior change techniques are effective? A systematic review and meta-analysis. Transl Behav Med. 2019;9(1):147–57. https://doi.org/10.1093/tbm/iby010. [publishedOnlineFirst:2018/03/06]
Teare MD, Dimairo M, Shephard N, et al. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study. Trials 2014;15(264)
Bennell KL, Ahamed Y, Jull G, et al. Physical therapist-delivered pain coping skills training and exercise for knee osteoarthritis: randomized controlled trial. Arthritis Care Res (Hoboken). 2016;68(5):590–602. https://doi.org/10.1002/acr.22744. [publishedOnlineFirst:2015/09/30]
Hay E, Dziedzic K, Foster N, et al. Optimal primary care management of clinical osteoarthritis and joint pain in older people: a mixed-methods programme of systematic reviews, observational and qualitative studies, and randomised controlled trials. Southampton (UK)2018.
Acknowledgements
The authors would like to thank Dr. Eric Deeson for representing patient and public involvement throughout the intervention and trial development.
Funding
This trial is primarily funded by the Private Physiotherapy Educational Foundation (A1 scheme) and the Musculoskeletal Association of Chartered Physiotherapists research awards. The funding bodies had no role in the design and writing of the trial protocol and does not have a role in data collection, management, analysis and interpretation of data, writing of the report and the decision to submit the report for publication.
Private Physiotherapy Educational Foundation, A1 Scheme App 364,Matthew Willett, Musculoskeletal Association of Chartered Physiotherapists, Level 1 award, Matthew Willett
Author information
Authors and Affiliations
Contributions
MW is a PhD student. JD, AR and CG are the academic supervisors of MW. JD is the CI, and MW is the PI of the trial; MW, JD, AR and CG conceived the trial. MW and JD contributed to the unpinning theory of the intervention. MW applied for funding and ethical approval. GS is the site lead, and SR is the trial co-ordinator and will assist in data collection. GS and SR advised on trial design and site-specific feasibility parameters. SF advised and will assist on accelerometry collection and data analysis. MW drafted the protocol. Critical feedback was provided by JD, AR, CG, GS and SF. MW, AR, JD, ED, GS and SF will contribute to Trial Steering and Management Groups respectively. MW, GS and SR will be responsible for data monitoring. MW will be responsible for data analysis. The authors read and approved the final manuscript.
Ethics declarations
Ethics approval and consent to participate
Ethics approval was granted on the 10/11/2021 from the Health Research Authority reference number (21/PR/1490). Written informed consent will be obtained from all participants.
Consent for publication
Not applicable. No identifiable details (including images and videos) relating to an individual person will be published.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
The STaying Active with Physiotherapy in patients with Lower-limb Osteoarthritis (STAPLO): Feasibility Trial – Consent Form. The STaying Active with Physiotherapy in patients with Lower-limb Osteoarthritis (STAPLO): Feasibility Trial – Interview Consent Form. Physiotherapist focus group consent form. Research staff focus group consent form.
Additional file 2:
Appendix 1. Overview of intervention development. Appendix 2. Additional Trial Information. Table 1: Adverse Event Definitions.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Willett, M., Rushton, A., Stephens, G. et al. Feasibility of a theoretically grounded, multicomponent, physiotherapy intervention aiming to promote autonomous motivation to adopt and maintain physical activity in patients with lower-limb osteoarthritis: protocol for a single-arm trial. Pilot Feasibility Stud 9, 54 (2023). https://doi.org/10.1186/s40814-023-01274-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40814-023-01274-6