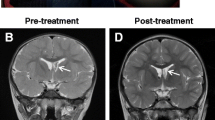
Abstract
Neuropsychiatric symptoms are very common in tuberous sclerosis complex (TSC). Autism is present in up to 60% of these patients, and TSC accounts for 1-4% of all cases of autism. In this study, we illustrate a 27 year-old female patient with TSC, autism, and renal angiomyolipomas, in whom everolimus treatment was associated with improvement in behavioral deficits. She took part in an everolimus clinical trial (EXIST-2: ClinicalTrials.gov number NCT00790400) to assess the efficacy of this drug in TSC. It was a randomized, double-blind, placebo-controlled study of everolimus (RAD001) (10 mg/day during 18 months) in the treatment of TSC-related angiomyolipoma. The Japanese version of the Aberrant Behavior Checklist (ABC) and the Pervasive Developmental Disorders - Autism Society Japan Rating Scale (PARS) were used to assess the severity of behavioral deficits. Clinical improvement after everolimus treatment was more remarkable for irritability, stereotypic behavior and inappropriate speech scores on the ABC scale. In addition, stereotypic behavior and lethargy/social withdrawal subscale scores showed an overall reduction of 10 and 8 points, respectively. The severity of autistic symptoms measured with the PARS also showed a marked reduction after treatment. There were no abnormal EEG findings before the treatment and no changes after the treatment. Our findings are consistent with those of animal models proposing that treatment of TSC1 and TSC2 mutant mice with the mTOR inhibitor rapamycin, reversed impaired social interaction. This makes everolimus a promising drug for the treatment of TSC patients with autism. Our findings warrant further investigation in future clinical trials.
Similar content being viewed by others
Background
Tuberous sclerosis complex (TSC) is a neurocutaneous disorder caused by mutations in either of the two tumor suppressor genes TSC1 or TSC2, encoding hamartin and tuberin, respectively (van Slegtenhorst et al, 1997; European Chromosome 16 Tuberous Sclerosis Consortium, 1993). Typical TSC lesions include hypomelanic macules and facial angiofibromas, as well as brain cortical tubers, subependymal nodules, and subependymal giant cell astrocytomas (SEGAs) (Holmes et al, 2007; Curatolo et al, 2008; Sahin, 2012). In addition to manifestations in the skin and nervous system, TSC is associated with hallmark tumors in the kidney, lung, heart and liver such as angiomyolipomas, lymphangioleiomyomatosis, and rhabdomyomas (Crino et al, 2006; Curatolo et al, 2008; Orlova and Crino, 2010; Ehninger, 2013).
Neuropsychiatric symptoms are very common in TSC, including epilepsy and a broad range of cognitive and behavioral problems (Kopp et al, 2008). It is estimated that approximately 70 to 90% of all TSC patients have seizures at some point during their life (Holmes et al, 2007; Ess, 2010; Sahin, 2012). West syndrome (infantile spasms) is the commonest epileptic disorder, which is associated with more intellectual disability and a less favorable neurological outcome (Joinson et al, 2003). This syndrome is seen in up to 50% of children with TSC (Ess, 2010).
Autism is a heterogeneous neurodevelopmental disorder with onset in early childhood, characterized by impairments in communication, reciprocal social interaction, and restricted and stereotyped patterns of interests and activities (Curatolo et al, 2004). Autism spectrum disorders (ASD) are frequently diagnosed in patients with TSC. Autism may be present in up to 60% of these patients, and TSC accounts for 1 to 4% of all cases of autism (Fombonne, 2003; Curatolo et al, 2004; Ehninger, 2013). In fact, the incidence of autism in patients with TSC may be higher than that of cardiac and renal abnormalities, for which screening is routinely conducted (Curatolo et al, 2004). Although the majority of TSC individuals with autism have a history of infantile spasms, there are also subjects who develop autism but with no history of seizures (Numis et al., 2011), suggesting that additional factors also play a role in this association.
To date, the treatment of TSC is entirely symptomatic, targeting. A recent review by Ehninger (2013) highlights that currently available therapies do not treat the cause of TSC symptoms but rather use general pharmacological and behavioral approaches to manage specific sets of symptoms, such as seizures, attention deficit hyperactivity disorder, (ADHD), anxiety, and depression, etc. However, no treatments are currently available to target neurocognitive dysfunction associated with this disorder. It has been demonstrated that hamartin and tuberin bind together to form a biochemical complex that inhibits the mammalian target of rapamycin (mTOR) that controls translation, proliferation, and cell growth (Curatolo et al, 2010). The discovery that the products of the TSC genes regulate mTOR signaling (Tee et al., 2002) has paved the way to current experimental mTORC inhibitor-based treatment approaches. Although the dysregulation of intracellular signaling through the activation of mTOR pathway is thought to contribute to the pathogenesis of epilepsy, cognitive dysfunction and behavioral abnormalities in TSC, most studies on mTOR inhibition therapy in humans have focused on seizure control and tumor growth reduction.
Clinical trials in TSC populations using everolimus (RAD001, Novartis), as mTOR inhibitor, have shown promising results regarding epilepsy, skin manifestations, subependymal giant cell astrocytomas (SEGAs), and renal and lung manifestations (Bissler et al., 2008) and also improved subependymal giant cell astrocytomas (SEGAs), specific brain tumors associated with TSC (Krueger et al, 2010; Józwiak et al 2012; Curran et al 2012; Franz et al, 2013). In fact, everolimus has already been approved to treat this kind of TSC lesions (Kohrman, 2012).
Preclinical studies in animal models of TSC, however, have assessed the potential efficacy of mTOR-based treatment on TSC-related cognitive impairments. In adult mice with a heterozygous, inactivating mutation in the TSC2 gene, mTOR inhibition reversed TSC-related learning and memory deficits. These cognitive abnormalities emerged in the absence of neuropathology and seizures (Ehninger et al. 2008, 2009). A recent study by Sato et al. (2012) in TSC mutant adult mice found that impaired social behavior also reversed by mTOR inhibitor treatment, which associated with mTOR inhibition at the molecular level. Others have also provided evidence that autistic-like behavior can be prevented with mTOR treatment in mouse models of TSC (Tsai et al, 2012; Talos et al, 2012; Reith et al. 2013). Altogether, these results propose that considerable therapeutic opportunities may exist for neuropsychiatric impairments associated with TSC, even if treatment is initiated in adulthood.
In humans, the effects of mTOR inhibitors on neurocognitive function and autistic phenotypes have rarely been explored. Thus, it is unknown whether mTOR inhibition can improve autistic features in individuals with TSC. An open label trial of rapamycin for angiomyolipomas and lymphangiomyomatosis reported certain improvement in neurocognitive function, particularly in recall memory in patients with TSC (Davies et al 2011). A placebo-controlled double blind trial of everolimus in patients with TSC and neurocognitive deficits is currently underway (ClinicalTrials.gov; NCT01289912). This study is enrolling individuals with TSC between the ages of 6 and 21 years, with IQ greater than or equal to 60, who are stable on an anti-seizure medication regimen and have no evidence of SEGAs. The primary endpoint is improvement in neurocognitive tests while autism, seizure frequency, and sleep habits are evaluated as secondary endpoints (Sahin, 2012). Furthermore, another clinical trial entitled “Efficacy of RAD001/Everolimus in autism and neuropsychological deficits in children with TSC” is currently recruiting patients to target cognitive functions and autistic behavior (http://clinicaltrials.gov/show/NCT01730209). In the present study, we illustrate a patient with TSC, autism, and renal angiomyolipomas, in whom everolimus treatment was associated with improvement in behavioral deficits in addition to a reduction in the volume of the kidney tumor.
Case presentation
In this study, we illustrate a 27 year-old female patient with TSC, autism, and renal angiomyolipomas, in whom everolimus treatment was associated with improvement in behavioral deficits. The patient had a history of cardiac rhabdomyoma since the fetal period. Eventually, she developed epilepsy (i.e., West syndrome), and multiple skin lesions, particularly hypomelanic macules, facial angiofibromas, Shagreen patch, scalp and forehead plaques, and periungual fibromas. Developmental problems were noticed early during the course of the disease (7-8 months of age). There was maladaptive behavior in the kindergarten, impaired social communication, and mental retardation. At age 15, she showed aggressive behavior, mainly toward her mother. Consequently, she was admitted in the department of Psychiatry at Osaka University Hospital and diagnosed as autism. The patient also had a renal cell carcinoma, which was operated, as well as bilateral renal angiomyolipomas (n = 8). Genetic analysis revealed TSC1 mutation: TSC1 intron 3 (c.328 + 15A > G).
Two years ago, she took part in an everolimus clinical trial (EXIST-2: ClinicalTrials.gov number NCT00790400) to assess the efficacy of this drug in TSC. It was a randomized, double-blind, placebo-controlled study of everolimus (RAD001) (10 mg/day during 18 months) in the treatment of TSC-related angiomyolipoma [3]. Out of 6 patients from the department of Dermatology and Psychiatry at Osaka University enrolled in the study, this was the only patient diagnosed with TSC-related autism. Informed consent was obtained from the patient and her family. Before enrollment, all patients or parents (if patients had developmental delays) agreed to write informed consent according to local guidelines. The ethics committee of Osaka University Hospital approved the protocol for the clinical trial (EXIST-2: ClinicalTrials.gov number NCT00790400). The safety reviews every 6 months were done by an independent data monitoring committee. This research was carried out in compliance with the Helsinki Declaration.
The Japanese version of the Aberrant Behavior Checklist (ABC) and the Pervasive Developmental Disorders - Autism Society Japan Rating Scale (PARS) were used to assess the severity of behavioral deficits. Scores on these scales are shown in Table 1. Clinical improvement after everolimus treatment was more remarkable for irritability, stereotypic behavior and inappropriate speech scores on the ABC scale. For instance, irritability had a 24-point reduction at 6 months of treatment, and completely disappeared at endpoint. Inappropriate speech symptoms also disappeared at endpoint. In addition, stereotypic behavior and lethargy/social withdrawal subscale scores showed an overall reduction of 10 and 8 points, respectively. The severity of autistic symptoms measured with the PARS also showed a marked reduction after treatment. The PARS-present score was reduced in 18 points, which represented a 46.2% improvement in the severity of behavioral problems at the end of the study. Other TSC signs, particularly facial angiofibromas and the volume of renal angiomyolipomas also showed a sustained reduction (Table 1). The EEG was measured during wakefulness, whereas sleep data were unavailable. There were no abnormal EEG findings before the treatment, showing 8 ~ 9 Hz alpha rhythm at the bilateral parietal and occipital area with no spikes and slow waves, and no changes after the treatment.
Conclusions
This case study shows that everolimus treatment reversed behavioral deficits in a patient with autism associated with TSC without EEG abnormalities. The improvement was more remarkable for irritability, stereotypic behavior and inappropriate speech, as indicated by changes in the respective ABC subscale scores. The severity of autistic symptoms in this patient, as measured with the PARS, also showed a marked reduction after everolimus treatment. Although the improvement of autistic features by everolimus is the most striking finding of our study, other manifestations of TSC, including facial angiofibromas had a notable improvement, as well. Similarly, the volume of renal angiomyolipomas showed a sustained reduction after everolimus was administered.
In line with a sustained improvement of angiomyolipomas in this study, recent everolimus studies have demonstrated the efficacy of this drug in the treatment of some tumors associated with TSC. An open-label study of five patients with sporadic abdominopelvic and lung lymphangioleiomyomatosis reported a significant shrinkage or complete resolution of the tumors during a 6-month treatment, although cessation of everolimus resulted in recurrence of symptoms (Mohammadieh et al, 2013). Larger open-label studies of everolimus for SEGAs in TSC showed that the treatment was associated with a marked and sustained tumor reduction (Krueger et al 2010). This was confirmed by a phase III, randomized, placebo-controlled trial, demonstrating that everolimus was associated with a SEGA response rate of 35% compared with 0% in the placebo group. The double-blind, placebo-controlled, phase 3 trial everolimus for angiomyolipoma in TSC where this patient took part, found an angiomyolipoma response rate of 42% for everolimus treatment, which contrasted with a 0% response for placebo (Bissler et al, 2013). Taken together, these findings demonstrate that some TSC-related tumors volume can significantly be reduced by everolimus therapy with an acceptable safety profile.
It is well documented that autism has devastating effects on the patients, their parents and the society, and a symptomatic or etiological treatment is still lacking. Although this study reports treatment effects on a single patient, to our knowledge, this is the first study providing evidence that in addition to tumor growth suppression properties, mTOR inhibition by everolimus in humans might be an effective pharmacological treatment of ASD associated with TSC. Our findings are consistent with those of Tsai et al (2012) in animal models, proposing that treatment of TSC1 and TSC2 mutant mice with the mTOR inhibitor rapamycin, prevented both TSC-related cerebellar pathology and autistic-like behavior. Further support to our results is provided by other preclinical studies demonstrating that rapamycin reversed deficient social interaction in mouse models (Sato et al, 2012; Reith et al, 2013). This makes everolimus a promising drug for the treatment behavior of patients with autism. Our findings warrant further investigation in future clinical trials (Wang and Doering, 2013).
Cognitive abnormalities in adult mouse models of TSC have also been reported to reverse with mTOR inhibitors, even in the absence of neuropathology and seizures (Ehninger et al. 2008, 2009). This suggests that at least some of the TSC-related cognitive impairments are caused by disinhibited mTOR signaling in adults, and are the consequence of functional changes rather than irreversible structural defects during development (Ehninger and Silva, 2011).The possibility that mTOR inhibitors may have benefit in the treatment of TSC-brain disease was also highlighted by Meikle et al (2008) study of a neuronal model of TSC. They found improvement in biochemical and signaling profiles, reduction in neurofilament expression and phosphorylation, and markedly improved myelination during rapamycin and everolimus treatment. All together, these studies support the notion that mTOR inhibition can be the key for an effective treatment of ASD associated with TSC.
Overall, the results of our study, along with animal evidence for a role of mTOR inhibition in improving behavior and social interaction deficits (Sato et al, 2012; Tsai et al, 2012; Reith et al, 2013), makes everolimus a promising drug for the treatment of TSC patients with ASD without EEG abnormalities. These findings warrant further investigation with physiological assessments in future clinical trials of mTOR inhibitors in autism.
Consent
Informed consent was obtained from the patient and her family. Before enrollment, all patients or parents (if patients had developmental delays) agreed to write informed consent according to local guidelines.
References
Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, Schmithorst VJ, Laor T, Brody AS, Bean J, Salisbury S, Franz DN (2008) Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med 358:140–151
Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, Sauter M, Nonomura N, Brakemeier S, de Vries PJ, Whittemore VH, Chen D, Sahmoud T, Shah G, Lincy J, Lebwohl D, Budde K (2013) Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 381:817–824
Crino PB, Nathanson KL, Henske EP (2006) The tuberous sclerosis complex. N Engl J Med 355:1345–1356
Curatolo P, Porfirio MC, Manzi B, Seri S (2004) Autism in tuberous sclerosis. Eur J Paediatr Neurol 8:327–332
Curatolo P, Bombardieri R, Jozwiak S (2008) Tuberous sclerosis. Lancet 372:657–668
Curatolo P, Napolioni V, Moavero R (2010) Autism spectrum disorders in tuberous sclerosis: pathogenetic pathways and implications for treatment. J Child Neurol 25:873–880
Curran MP (2012) Everolimus: in patients with subependymal giant cell astrocytoma associated with tuberous sclerosis complex. Paediatr Drugs 14:51–60
Davies DM, de Vries PJ, Johnson SR, McCartney DL, Cox JA, Serra AL, Watson PC, Howe CJ, Doyle T, Pointon K, Cross JJ, Tattersfield AE, Kingswood JC, Sampson JR (2011) Sirolimus therapy for angiomyolipoma in tuberous sclerosis and sporadic lymphangioleiomyomatosis: a phase 2 trial. Clin Cancer Res 17:4071–4081
Ehninger D (2013) From genes to cognition in tuberous sclerosis: Implications for mTOR inhibitor-based treatment approaches. Neuropharmacology 68:97–105
Ehninger D, Silva AJ (2011) Rapamycin for treating Tuberous sclerosis and Autism spectrum disorders. Trends Mol Med 17:78–87
Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ (2008) Reversal of learning deficits in a Tsc2+/- mouse model of tuberous sclerosis. Nat Med 14:843–848
Ehninger D, de Vries PJ, Silva AJ (2009) From mTOR to cognition: molecular and cellular mechanisms of cognitive impairments in tuberous sclerosis. J Intellect Disabil Res 53:838–851
Ess KC (2010) Tuberous sclerosis complex: a brave new world? Curr Opin Neurol 23:189–193
European Chromosome 16 Tuberous Sclerosis Consortium (1993) Identification and characterization of the tuberous sclerosis gene on chromosome 16. Cell 75:1305–1315
Fombonne E (2003) Epidemiological surveys of autism and other pervasive developmental disorders: an update. J Autism Dev Disord 33:365–382
Franz DN, Belousova E, Sparagana S, Bebin EM, Frost M, Kuperman R, Witt O, Kohrman MH, Flamini JR, Wu JY, Curatolo P, de Vries PJ, Whittemore VH, Thiele EA, Ford JP, Shah G, Cauwel H, Lebwohl D, Sahmoud T, Jozwiak S (2013) Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 381:125–132
Holmes GL, Stafstrom CE, Tuberous Sclerosis Study Group (2007) Tuberous sclerosis complex and epilepsy: recent developments and future challenges. Epilepsia 48:617–630
Joinson C, O'Callaghan FJ, Osborne JP, Martyn C, Harris T, Bolton PF (2003) Learning disability and epilepsy in an epidemiological sample of individuals with tuberous sclerosis complex. Psychol Med 33:335–344
Józwiak S, Stein K, Kotulska K (2012) Everolimus (RAD001): first systemic treatment for subependymal giant cell astrocytoma associated with tuberous sclerosis complex. Future Oncol 8:1515–1523
Kohrman MH (2012) Emerging treatments in the management of tuberous sclerosis complex. Pediatr Neurol 46:267–275
Kopp CM, Muzykewicz DA, Staley BA, Thiele EA, Pulsifer MB (2008) Behavior problems in children with tuberous sclerosis complex and parental stress. Epilepsy Behav 13:505–510
Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, Wilson KA, Byars A, Sahmoud T, Franz DN (2010) Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med 363:1801–1811
Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, Kwiatkowski DJ (2008) Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci 28:5422–532
Mohammadieh AM, Bowler SD, Silverstone EJ, Glanville AR, Yates DH (2013) Everolimus treatment of abdominal lymphangioleiomyoma in five women with sporadic lymphangioleiomyomatosis. Med J Aust 199:121–123
Numis AL, Major P, Montenegro MA, Muzykewicz DA, Pulsifer MB, Thiele EA (2011) Identification of risk factors for autism spectrum disorders in tuberous sclerosis complex. Neurology 76:981–987
Orlova KA, Crino PB (2010) The tuberous sclerosis complex. Ann N Y Acad Sci 1184:87–105
Reith RM, McKenna J, Wu H, Hashmi SS, Cho SH, Dash PK, Gambello MJ (2013) Loss of Tsc2 in Purkinje cells is associated with autistic-like behavior in a mouse model of tuberous sclerosis complex. Neurobiol Dis 51:93–103
Sahin M (2012) Targeted treatment trials for tuberous sclerosis and autism: no longer a dream. Curr Opin Neurobiol 22:1–7
Sato A, Kasai S, Kobayashi T, Takamatsu Y, Hino O, Ikeda K, Mizuguchi M (2012) Rapamycin reverses impaired social interaction in mouse models of tuberous sclerosis complex. Nat Commun 3:1292–1300
Talos DM, Sun H, Zhou X, Fitzgerald EC, Jackson MC, Klein PM, Lan VJ, Joseph A, Jensen FE (2012) The interaction between early life epilepsy and autistic-like behavioral consequences: a role for the mammalian target of rapamycin (mTOR) pathway. PLoS One 7:e35885
Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J (2002) Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci U S A 99:13571–13576
Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, Steinberg J, Crawley JN, Regehr WG, Sahin M (2012) Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature 488:647–651
van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S, Lindhout D, van den Ouweland A, Halley D, Young J, Burley M, Jeremiah S, Woodward K, Nahmias J, Fox M, Ekong R, Osborne J, Wolfe J, Povey S, Snell RG, Cheadle JP, Jones AC, Tachataki M, Ravine D, Sampson JR, Reeve MP, Richardson P, Wilmer F, Munro C, Hawkins TL et al (1997) Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 277:805–808
Wang H, Doering LC (2013) Reversing autism by targeting downstream mTOR signaling. Front Cell Neurosci 7:28
Acknowledgments
We thank our patients and their families for their participation and contribution to the trial and Novartis for supporting this trial.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RI, MWK, NN and YN participated in the study design and discussions, did the research, recruited and enrolled patients, oversaw data collection and collected the data. RI, MWK and LC participated in the data analysis and discussions and interpreted the data, did the literature review and wrote the manuscript. RI, MWK, LC and MT edited and reviewed the manuscript. All authors approved the final draft of the manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Ishii, R., Wataya-Kaneda, M., Canuet, L. et al. Everolimus improves behavioral deficits in a patient with autism associated with tuberous sclerosis: a case report. Neuropsychiatr Electrophysiol 1, 6 (2015). https://doi.org/10.1186/s40810-015-0004-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40810-015-0004-x