Abstract
Conventional CT, MR, and digital subtraction angiography rely on the presence of luminal narrowing for the identification of vascular pathology offering limited insight into the offending pathophysiologic mechanism affecting the vessel. High-resolution MRI vessel wall imaging (VWI) has the potential to directly depict and characterize vessel wall pathology affecting the intracranial circulation increasing diagnostic accuracy for vasculopathies with similar angiographic findings.
Similar content being viewed by others
Background
Blood flow and CSF suppression are essential for optimal vessel wall visualization in the intracranial circulation [1]. Intracranial VWI is especially challenging due to the small caliber and tortuosity of the intracranial vessels necessitating submilimeter spatial resolution and high field strength magnets [1–3]. Black blood MRI (BBMRI) sequences are designed to achieve blood flow suppression and have historically utilized 2-dimensional (2D) pre- and postcontrast T1- or proton-weighted sequences to characterize the vessel wall [1, 3]. The 2D sequences are prone to partial volume artifacts, which are accentuated by the tortuosity and small size of intracranial vessels [2]. Three-dimensional (3D) sequences have more recently become achievable and have the advantage of a large field of view, which allows substantial coverage in a single acquisition in a clinically acceptable scan time obviating the need for prospective slice placement, and isotropic resolution, which allows post hoc reconstruction along the short and long axis of vessels minimizing overestimation of wall thickness (Fig. 1) [2].
Utility of 3D BBMRI. Postcontrast 3D BBMRI images (acquired resolution, 0.52 mm isotropic) of the left middle cerebral artery (MCA) M1 segment in a 48 year old man with hypertension, diabetes, and coronary artery disease who presented with acute on subacute left MCA territory infarctions. Long axis (a) and short axis (b) reconstructions demonstrate eccentric thickening and intense enhancement of the M1 segment vessel wall (arrows)
Specific vessel wall characteristics that are sought on VWI include vessel wall thickening (smooth, irregular, circumferential, concentric, eccentric), signal, and enhancement [4]. A short axis view perpendicular to an intracranial vessel is best for evaluation of vessel wall thickening and pattern of enhancement (Fig. 1b). In this text we review the major current applications of BBMRI in the intracranial circulation.
Atherosclerosis
The hallmark of atherosclerosis on BBMRI is the heterogeneity of the thickened vessel wall due to various plaque components, which may include lipid core, fibrous cap, intraplaque hemorrhage [5], calcifications, and enhancement (Figs. 1, 2, 3 and 4) [1, 6]. These components are better demonstrated in the extracranial circulation due to the larger vessel size, and current MRI techniques cannot characterize consistently individual intracranial atherosclerotic disease (ICAD) components [3, 6]. Nevertheless, plaque features detected by BBMRI, such as enhancement and hemorrhage, have been shown to relate to downstream strokes (Figs. 1, 2, 3 and 4) [5–7]. Furthermore, BBMRI can detect small atherosclerotic plaques in vessels that are not yet stenosed even in advanced atherosclerosis due to remodeling [1, 8]. Subtle atherosclerotic changes, including wall thickening and positive remodeling, have been observed in non-stenotic arteries in stroke patients when compared with controls [9, 10]. Atherosclerotic changes identified on BBMRI in non-stenotic intracranial arteries appear to be the most significant risk factor for white matter hyperintensities [11].
Maximum intensity reconstruction (MIP) from a contrast-enhanced MRA (a) and postcontrast 2D BBMRI (b) images through a right vertebral artery plaque in a 49 year old woman with multifocal intracranial arterial narrowing presenting with an acute distal anterior cerebral artery infarction., The 2D BBMRI slice (b), positioned through focal narrowing of the proximal V4 segment of the right vertebral artery (a, line), demonstrates eccentric wall thickening and enhancement (arrowhead) with central hypointesity (arrow) representing a partially calcified core. BBMRI was achieved using double inversion recovery with the inversion time set to the null point of blood
Imaging through the cavernous carotid arteries of a 56 year old HIV positive man with type 1 diabetes who presented with acute left MCA territory infarcts. Postcontrast 3D BBMRI through the short axes of the juxtasellar cavernous ICA segments demonstrates eccentric wall enhancement and thickening on the left compatible with atherosclerotic plaque with a hypointense rim (arrowhead) that is highlighted by surrounding venous enhancement and compatible with calcifcation. A hypoenhancing focus (white arrow) within the enhancing, thickened wall suggests a lipid core. A small atherosclerotic plaque with minimal enhancement is seen on the right (black arrow)
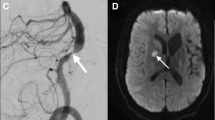
Imaging of the basilar artery terminal branches in a 55 year old man with acute medial bithalamic infarctions. 3D time-of-flight MRA MIP image through the distal basilar artery shows a focal high grade stenosis of the left posterior cerebral artery (PCA) P1 segment. 2D postcontrast BBMRI image (b) oriented through the short axis of the stenosed vessel (a, line) demonstrates circumferential eccentric wall thickening and enhancement (arrow) compatible with an atherosclerotic plaque
Atherosclerotic plaques in the intracranial circulation tend to present as eccentric and usually irregular wall thickening with or without luminal stenosis and variable enhancement (Figs. 1, 2, 3 and 4) [3, 6, 7, 12]. When compared with reversible cerebral vasoconstriction syndrome (RCVS) and vasculitic lesions, ICAD lesions are significantly more likely to have eccentric wall involvement [3], and can demonstrate lesional T2 hyperintensity presumably corresponding to the fibrous cap (80 % sensitivity), which presents as a T2 juxtaluminal hyperintense band occasionally overlying a T2 hypointense component, the lipid core [12]. T2 hyperintensities and heterogeneous signal are reportedly absent in vasculitis and RCVS [12]. Luminal narrowing can be identified with conventional angiographic studies, and the goal of VWI is the definite characterization of a focal stenotic lesion as athrosclerosis, the discrimination of active and stable plaques, and the demonstration of nonstenotic atherosclerotic burden. In a recent study, atherosclerotic plaques involving the basilar artery were more frequently identified on BBMRI than on time-of-flight MRA [13].
Plaque enhancement can be used to identify lesions responsible for cerebrovascular ischemic events (Fig. 1). Plaque enhancement could represent inflammation and/or neovascularization and a more strongly enhancing intracranial plaque more likely represents the culprit lesion for an ischemic event [6, 7, 14]. Enhancement in an ICAD lesion that causes more than 50 % stenosis has been shown to be associated with its likelihood to have caused a recent ischemic event, and this is independent of plaque thickness [6]. Strong plaque enhancement has been observed in the vessel supplying the stroke territory within 4 weeks of the ischemic insult, and the enhancement decreased following the ictus [6, 15]. Plaque enhancement might serve as a more precise marker of stroke risk than luminal stenosis, enabling risk stratification in low-grade or even angiographically occult lesions [6]. Qiao et al. observed a lack of contrast enhancement only in nonculprit plaques [6]. This suggests that BBMRI can identify stable plaques with lack of enhancement, which might not need aggressive treatment [6], though a prospective study is needed to validate plaque enhancement as a predictor of future events.
The effects of intracranial thromboembolism and recanalization have been described in a small number of patients, and include smooth concentric wall thickening and enhancement at the site of recent arterial occlusion, which is more common in patients who received mechanical thrombectomy than in patients treated with medical therapy alone [16]. This pattern of enhancement following thrombectomy is reminiscent of and could be potentially confused with central nervous system (CNS) vasculitis if the patient’s history is unknown. BBMRI also has been used for the identification of eccentric atherosclerotic plaques in the basilar artery and their relationship to the ostia of the major side branches before basilar artery stenting to minimize procedural complications [17].
Vasculitis
CNS vasculitis represents inflammation of intracranial blood vessels [18]. CNS vasculitis is rare and represents a group of diseases with various underlying mechanisms that affect vessels of different sizes and are characterized by non-atheromatous inflammation and necrosis of the arterial wall [14]. Conventional angiographic findings of vasculitis when present include multifocal luminal irregularities and stenosis, which are nonspecific and cannot reliably differentiate vasculitis from atherosclerosis and RCVS. In contrast to intracranial atherosclerosis, wall thickening and enhancement in vasculitis tends to be homogeneous, circumferential and concentric. However, there is overlap with ICAD, and ICAD lesions can present with circumferential vessel wall involvement, and vasculitic lesions can demonstrate eccentric enhancement [3, 7, 12, 14, 19]. Hyperintense T2 signal has been shown to be absent in vasculitic lesions, and a concentric enhancing lesion with hyperintense or heterogeneous T2 signal is more likely ICAD [12]. In our clinical experience, vasculitic enhancement and inflammation can occasionally extend beyond the vessel wall to involve the adjacent perivascular space and/or brain parenchyma (Fig. 5). We hypothesize that this periadventitial enhancement might represent a specific pattern of involvement in vasculitis. Finally, 3D black blood sequences can be used for intraoperative navigation to target individual vascular branches to increase diagnostic yield when biopsy is contemplated in suspected cases of vasculitis.
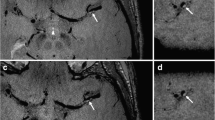
Brain imaging in a 79 year old man with biopsy confirmed amyloid-β-related angiitis. Extensive confluent T2 FLAIR hyperintense vasogenic edema (a) and multiple punctate cortical foci of susceptibility (b) are seen in the right frontal and parietal lobes. Axial postcontrast 3D BBMRI (c) demonstrates 3 small cortical arteries with patent central hypointense lumen (arrows), and extensive circumferential wall enhancement, which appears to extend into the adjacent perivascular brain parenchyma compatible with vasculitis
Reversible cerebral vasoconstriction syndrome
RCVS is a noninflammatory disorder of arterial tone regulation, which results in multifocal segmental narrowing of cerebral arteries that resolves spontaneously within 3 months [14, 20]. The main differential consideration is vasculitis especially when there are overlapping clinical features; and discrimination between the two conditions is important because vasculitis is treated with steroids, which can be harmful in RCVS [4, 20, 21]. Angiographic imaging fails to distinguish the two conditions due to nonspecific luminal narrowing in both [19]. A pilot VWI study that assessed consecutive cases with multifocal segmental narrowing on angiographic imaging demonstrated vessel wall thickening and enhancement in CNS vasculitis and cocaine vasculopathy, and minimal to no enhancement in RCVS [20]. Cocaine vasculopathy creates vasospasm, but unlike RCVS it results in arterial wall inflammation on histopathologic evaluation [22]. A larger study recruited 13 vasculitis and 13 RCVS patients [19]. Twelve out of 13 vasculitis patients had wall thickening and enhancement. The enhancement involved a short segment and was concentric in 9 patients and eccentric in 3 [19]. In RCVS, 10 patients had diffuse uniform wall thickening continuous throughout the entire wall of the diseased vessel likely due to smooth muscle contraction [20], and only 4 patients had mild vessel wall enhancement [19]. Wall enhancement in RCVS was less intense compared to CNS vascultis with early resolution within 3 months when present [19]. These results suggest that BBMRI is helpful in the differentiation of RCVS from CNS vasculitis.
Aneurysm
A pilot study in 5 patients with aneurysmal subarachnoid hemorrhage demonstrated thick vessel wall enhancement in all ruptured aneurysms, and absent enhancement in the unruptured aneurysms [23]. In a larger study that included 117 patients, there was strong aneurysmal wall enhancement in 73.8 % of ruptured versus 4.8 % of unruptured aneurysms [24]. The authors concluded that in patients with multiple aneurysms and subarachnoid hemorrhage the presence of aneurysmal wall enhancement will likely identify the ruptured lesion [23, 24]. However, it is important to note that these are retrospective investigations and the enhancement could be a consequence of the rupture. In surgically treated aneurysms with partial wall enhancement, the enhancement corresponded to the point of rupture during surgery, which might be helpful in treatment planning [24].
In another study that included 87 patients with 108 aneurysms, circumferential aneurysmal wall enhancement was more frequently seen in unstable than in stable aneurysms (87 % versus 28.5 %, respectively) [25]. The unstable aneurysm group included ruptured aneurysms, aneurysms with change in morphology, and symptomatic aneurysms [25]. Identification of aneurysm wall enhancement could correspond to vasa vasorum formation and inflammatory activity, and it may relate to the aneurysm’s risk of rupture [14, 24, 25]. A prospective study could confirm the role of VWI in the non-invasive follow-up of unruptured aneurysms [25].
Moyamoya disease
Moyamoya disease (MMD) is an idiopathic disorder causing progressive narrowing of the distal intracranial internal carotid arteries (ICAs) and the proximal circle of Willis vessels, and is characterized by the development of hypertrophied lenticulostriate branches. MMD and ICAD are both more prevalent in Asians, and differentiation between the two conditions is important because of different treatment strategies (revascularization surgery in MMD versus aggressive medical treatment in ICAD) [26]. Prior reports have suggested that MMD is characterized by little to no wall enhancement, and that this could differentiate it from radiation-induced arteritis, which presents with concentric enhancement, or an ICAD lesion that presents with eccentric enhancement [3, 27, 28]. More recent studies have described concentric enhancement in symptomatic and asymptomatic MMD patients affecting the distal ICAs, which could correspond to intimal hyperplasia pathologically [1, 26, 29]. Ryoo et al. suggested that concentric wall enhancement in bilateral distal ICAs and shrinkage of the middle cerebral arteries in MMD can distinguish it from ICAD, which presents with focal eccentric enhancement on BBMRI [26]. Additional studies are needed to further investigate the appearance of MMD using optimized BBMRI imaging and clarify whether a noninvasive diagnosis can be made.
Dissection
Dissection represents blood tracking into the vessel wall through an intimal tear. BBMRI findings suggestive of dissection include eccentric wall thickening with T1 hyperintense signal representing intramural hematoma, the identification of a false lumen, and eccentric wall enhancement, which might imply involvement by vasa vasorum [3, 14, 30]. However, the hyperintense T1 vessel wall signal is not specific for dissection since it could represent intraplaque hemorrhage in an ICAD lesion [3, 5], and studies have yet to validate these imaging features given the general lack of intracranial vessel specimens for comparison [3].
Conclusions
VWI is a rapidly growing and evolving area of clinical and research interest that holds promise to improve diagnostic accuracy for intracranial vasculopathies. VWI enables direct visualization of offending vessel wall pathology, which wouldn’t be detectable on conventional imaging unless it resulted in luminal narrowing. Specific vessel wall thickening and enhancement patterns have been described in several conditions with similar angiographic findings enabling discrimination of these diseases that was not previously possible. VWI appears to allow for assessment of atherosclerotic burden and for risk stratification of individual ICAD lesions with potential to influence treatment decisions. Further prospective studies are needed to better define the role of VWI in predicting risk from intracranial vasculopathies and determining the best management approach.
Abbreviations
- 2D:
-
2-dimensional
- 3D:
-
3-dimensional
- BBMRI:
-
Black blood MRI
- CNS:
-
central nervous system
- ICA:
-
internal carotid artery
- ICAD:
-
intracranial atherosclerotic disease
- MCA:
-
middle cerebral artery
- MMD:
-
Moyamoya disease
- PCA:
-
posterior cerebral artery
- RCVS:
-
reversible cerebral vasoconstriction syndrome
- VWI:
-
MRI vessel wall imaging
References
Dieleman N, van der Kolk AG, Zwanenburg JJ, Harteveld AA, Biessels GJ, Luijten PR, et al. Imaging intracranial vessel wall pathology with magnetic resonance imaging: current prospects and future directions. Circulation. 2014;130:192–201.
Qiao Y, Steinman DA, Qin Q, Etesami M, Schar M, Astor BC, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. J Magn Reson Imaging. 2011;34:22–30.
Swartz RH, Bhuta SS, Farb RI, Agid R, Willinsky RA, Terbrugge KG, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology. 2009;72:627–34.
Miller TR, Shivashankar R, Mossa-Basha M, Gandhi D. Reversible Cerebral Vasoconstriction Syndrome, Part 2: Diagnostic Work-Up, Imaging Evaluation, and Differential Diagnosis. AJNR Am J Neuroradiol. 2015;36:1580–8.
Turan TN, Bonilha L, Morgan PS, Adams RJ, Chimowitz MI. Intraplaque hemorrhage in symptomatic intracranial atherosclerotic disease. J Neuroimaging. 2011;21:e159–61.
Qiao Y, Zeiler SR, Mirbagheri S, Leigh R, Urrutia V, Wityk R, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology. 2014;271:534–42.
Vergouwen MD, Silver FL, Mandell DM, Mikulis DJ, Swartz RH. Eccentric narrowing and enhancement of symptomatic middle cerebral artery stenoses in patients with recent ischemic stroke. Arch Neurol. 2011;68:338–42.
van der Kolk AG, Hendrikse J, Brundel M, Biessels GJ, Smit EJ, Visser F, et al. Multi-sequence whole-brain intracranial vessel wall imaging at 7.0 tesla. Eur Radiol. 2013;23:2996–3004.
Lee WJ, Choi HS, Jang J, Sung J, Kim TW, Koo J, et al. Non-stenotic intracranial arteries have atherosclerotic changes in acute ischemic stroke patients: a 3 T MRI study. Neuroradiology. 2015;57:1007–13.
de Havenon A, Yuan C, Tirschwell D, Hatsukami T, Anzai Y, Becker K, et al. Nonstenotic Culprit Plaque: The Utility of High-Resolution Vessel Wall MRI of Intracranial Vessels after Ischemic Stroke. Case Rep Radiol. 2015;2015:356582.
Kim TH, Choi JW, Roh HG, Moon WJ, Moon SG, Chun YI, et al. Atherosclerotic arterial wall change of non-stenotic intracracranial arteries on high-resolution MRI at 3.0 T: Correlation with cerebrovascular risk factors and white matter hyperintensity. Clin Neurol Neurosurg. 2014;126:1–6.
Mossa-Basha M, Hwang WD, De Havenon A, Hippe D, Balu N, Becker KJ, et al. Multicontrast high-resolution vessel wall magnetic resonance imaging and its value in differentiating intracranial vasculopathic processes. Stroke. 2015;46:1567–73.
Kim YS, Lim SH, Oh KW, Kim JY, Koh SH, Kim J, et al. The advantage of high-resolution MRI in evaluating basilar plaques: a comparison study with MRA. Atherosclerosis. 2012;224:411–6.
Portanova A, Hakakian N, Mikulis DJ, Virmani R, Abdalla WM, Wasserman BA. Intracranial vasa vasorum: insights and implications for imaging. Radiology. 2013;267:667–79.
Skarpathiotakis M, Mandell DM, Swartz RH, Tomlinson G, Mikulis DJ. Intracranial atherosclerotic plaque enhancement in patients with ischemic stroke. AJNR Am J Neuroradiol. 2013;34:299–304.
Power S, Matouk C, Casaubon LK, Silver FL, Krings T, Mikulis DJ, et al. Vessel wall magnetic resonance imaging in acute ischemic stroke: effects of embolism and mechanical thrombectomy on the arterial wall. Stroke. 2014;45:2330–4.
Jiang WJ, Yu W, Ma N, Du B, Lou X, Rasmussen PA. High resolution MRI guided endovascular intervention of basilar artery disease. J Neurointerv Surg. 2011;3:375–8.
Kuker W, Gaertner S, Nagele T, Dopfer C, Schoning M, Fiehler J, et al. Vessel wall contrast enhancement: a diagnostic sign of cerebral vasculitis. Cerebrovasc Dis. 2008;26:23–9.
Obusez EC, Hui F, Hajj-Ali RA, Cerejo R, Calabrese LH, Hammad T, et al. High-resolution MRI vessel wall imaging: spatial and temporal patterns of reversible cerebral vasoconstriction syndrome and central nervous system vasculitis. AJNR Am J Neuroradio. 2014;35:1527–32.
Mandell DM, Matouk CC, Farb RI, Krings T, Agid R, ter Brugge K, et al. Vessel wall MRI to differentiate between reversible cerebral vasoconstriction syndrome and central nervous system vasculitis: preliminary results. Stroke. 2012;43:860–2.
Miller TR, Shivashankar R, Mossa-Basha M, Gandhi D. Reversible Cerebral Vasoconstriction Syndrome, Part 1: Epidemiology, Pathogenesis, and Clinical Course. AJNR Am J Neuroradiol. 2015;36:1392–9.
Han JS, Mandell DM, Poublanc J, Mardimae A, Slessarev M, Jaigobin C, et al. BOLD-MRI cerebrovascular reactivity findings in cocaine-induced cerebral vasculitis. Nat Clin Pract Neurol. 2008;4:628–32.
Matouk CC, Mandell DM, Gunel M, Bulsara KR, Malhotra A, Hebert R, et al. Vessel wall magnetic resonance imaging identifies the site of rupture in patients with multiple intracranial aneurysms: proof of principle. Neurosurgery. 2013;72:492–6. discussion 496.
Nagahata S, Nagahata M, Obara M, Kondo R, Minagawa N, Sato S, et al. Wall Enhancement of the Intracranial Aneurysms Revealed by Magnetic Resonance Vessel Wall Imaging Using Three-Dimensional Turbo Spin-Echo Sequence with Motion-Sensitized Driven-Equilibrium: A Sign of Ruptured Aneurysm? Clinical neuroradiology 2014.
Edjlali M, Gentric JC, Regent-Rodriguez C, Trystram D, Hassen WB, Lion S, et al. Does aneurysmal wall enhancement on vessel wall MRI help to distinguish stable from unstable intracranial aneurysms? Stroke. 2014;45:3704–6.
Ryoo S, Cha J, Kim SJ, Choi JW, Ki CS, Kim KH, et al. High-resolution magnetic resonance wall imaging findings of Moyamoya disease. Stroke. 2014;45:2457–60.
Aoki S, Hayashi N, Abe O, Shirouzu I, Ishigame K, Okubo T, et al. Radiation-induced arteritis: thickened wall with prominent enhancement on cranial MR images report of five cases and comparison with 18 cases of Moyamoya disease. Radiology. 2002;223:683–8.
Kim JM, Jung KH, Sohn CH, Park J, Moon J, Han MH, et al. High-resolution MR technique can distinguish moyamoya disease from atherosclerotic occlusion. Neurology. 2013;80:775–6.
Kim YJ, Lee DH, Kwon JY, Kang DW, Suh DC, Kim JS, et al. High resolution MRI difference between moyamoya disease and intracranial atherosclerosis. Eur J Neurol. 2013;20:1311–8.
Mauermann ML, Phillips CD, Worrall BB. Intraplaque dissection of the basilar artery. Neurology. 2006;66:1544.
Acknowledgements
BAW has received grant support from the National Heart, Lung, and Blood Institute (RO1HL105930-01A1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kontzialis, M., Wasserman, B.A. Intracranial vessel wall imaging: current applications and clinical implications. Neurovasc Imaging 2, 4 (2016). https://doi.org/10.1186/s40809-016-0014-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40809-016-0014-5