Abstract
Background
Contracting skeletal muscle produces reactive oxygen species (ROS) originating from both mitochondrial and cytosolic sources. The use of non-specific antioxidants, such as vitamins C and E, during exercise has produced inconsistent results in terms of exercise performance. Consequently, the effects of the mitochondrial-targeted coenzyme Q10, named Mitoquinone (MitoQ) on exercise responses are currently under investigation.
Methods
In this study, we conducted a meta-analysis to quantitatively synthesize research assessing the impact of MitoQ on aerobic endurance performance and exercise-induced oxidative damage. PubMed, Web of Science, and SCOPUS databases were used to select articles from inception to January 16th of 2024. Inclusion criteria were MitoQ supplementation must be compared with a placebo group, showing acute exercise responses in both; for crossover designs, at least 14 d of washout was needed, and exercise training can be concomitant to MitoQ or placebo supplementation if the study meets the other inclusion criteria points. The risk of bias was evaluated through the Critical Appraisal Checklist (JBI).
Results
We identified eight studies encompassing a total sample size of 188 subjects. Our findings indicate that MitoQ supplementation effectively reduces exercise-induced oxidative damage (SMD: -1.33; 95% CI: -2.24 to -0.43). Furthermore, our findings indicate that acute and/or chronic MitoQ supplementation does not improve endurance exercise performance (SMD: -0.50; 95% CI: -1.39 to 0.40) despite reducing exercise-induced oxidative stress. Notably, our sensitivity analysis reveals that MitoQ may benefit subjects with peripheral artery disease (PAD) in improving exercise tolerance.
Conclusion
While MitoQ effectively reduces exercise-induced oxidative damage, no evidence suggests that aerobic exercise performance is enhanced by either acute or chronic MitoQ supplementation. However, acute MitoQ supplementation may improve exercise tolerance in subjects with PAD. Future research should investigate whether MitoQ supplementation concurrent with exercise training (e.g., 4–16 weeks) alters adaptations induced by exercise alone and using different doses.
Key points
Contracting skeletal muscle produces ROS from both mitochondrial and cytosolic sources, potentially impairing exercise performance.
MitoQ supplementation reduces exercise-induced oxidative damage but does not improve endurance exercise performance.
Similar content being viewed by others
Background
Oxidative metabolism efficiently generates significant energy by completely oxidizing glucose and fatty acids within mitochondria [1]. This process involves an enzymatic cascade that produces reactive oxygen species (ROS), such as singlet oxygen, hydrogen peroxide, and the hydroxyl radical. Excess ROS production is associated with pathophysiological conditions, including insulin resistance and cardiovascular diseases [2,3,4]. Additionally, the overproduction of ROS can induce muscle fatigue during intense contractions [5, 6] and lead to oxidative damage in cellular compartments, proteins, and lipids [7]. Exercise adaptations include increased ROS-detoxifying enzymes, enhancing antioxidant capacity [8], and modifications within the mitochondrial electron transport chain to minimize ROS production [9]. These adaptations decrease ROS bursts, maintaining the redox state within physiological levels [10]. Importantly, ROS serve as critical signaling factors, mediating metabolic and physical adaptations to exercise [11, 12]. Thus, balancing ROS signaling and preventing oxidative damage is essential. However, non-specific antioxidants like vitamins C and E have not demonstrated benefits superior to placebo regarding exercise adaptations or responses [13,14,15,16].
Studies on the supplementation with antioxidant vitamins C and E have shown that they specifically prevent the mitochondrial adaptations induced by exercise [11, 12]. However, the mechanism remains unclear, as these antioxidants appear to block the entire pathway, from nuclear transcription factors to the expression of the electron transport chain machinery and mitochondrial DNA [11, 12]. Contracting skeletal muscle generates both mitochondrial and cytosolic ROS. The primary sources of mitochondrial ROS are complexes I and III of the electron transport chain [17], whereas the main cytosolic source is NADPH oxidase isoform 2 (NOX2) [18]. It should be noted that during exercise, ROS production from Complex I and Complex III is reduced to below basal levels [17]. Which may suggest that reducing mitochondrial ROS during exercise could be a desirable target to maintain muscle homeostasis. Notably, NOXs-related ROS production is crucial in key exercise events such as glucose uptake and calcium kinetics [18, 19]. In addition, ROS production by NOX enzymes appears to mediate exercise adaptations, including improvements in mitochondrial function [20] and insulin sensitivity [21]. Consequently, a novel research topic in exercise sciences is supplementing with a mitochondrial-targeted antioxidant during exercise to reduce excessive mitochondrial ROS while preserving cytosolic ROS production.
Coenzyme Q supplementation as an antioxidant has seen limited success due to its lipophilic nature which limits its bioavailability [22]. In contrast, mitoquinone (MitoQ) is an orally available mitochondrial-targeted coenzyme Q variant [23]. Mitoquinone, a conjugate of coenzyme Q, effectively positions the quinone moiety within the hydrophobic core of the polarized IMM, making MitoQ an efficient superoxide scavenger that attenuates lipid peroxidation [24]. Moreover, as training is known to increase Coenzyme Q10 levels [25, 26] which may enhance mitochondrial function [27]. There is growing interest in the effects of MitoQ supplementation on exercise performance. However, studies have yielded mixed results; some report improvements in performance [28], while others find no significant effects [29, 30]. Nevertheless, no study has already synthetized the availably data to reach a solid conclusion. Therefore, our study aims to quantitatively summarize, through a meta-analysis of randomized trials, the effects of MitoQ supplementation on aerobic exercise performance and exercise-induced oxidative damage.
Methods
Data Search and Study Selection
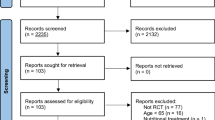
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement was used to report the items of research studies used [31]. The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42023477400). The authors systematically searched for eligible articles in PubMed, Web of Science, and SCOPUS databases from inception to January 16th of 2024. To be considered, the studies must be published in English or Spanish and performed on adult humans (≥ 18 year.). Inclusion criteria: (1) MitoQ supplementation must be compared with a placebo group; for crossover designs, at least 14 d of washout was needed; (2) acute exercise-induced oxidative damage and/or exercise performance must be reported for both placebo and MitoQ groups; (3) exercise training can be concomitant to mitoQ or placebo supplementation if the study meets the other inclusion criteria points. Exclusion criteria: concomitant supplementation with other(s) antioxidants and studies not performed in humans. As the present topic is relatively recent, we performed a broad search strategy as follows: (“exercise” or “training” or “sport”) and (“mitoquinone” or “mitoquinol” or “mitoQ” or “mitochondrial-targeted antioxidant” or “mitochondrial targeted antioxidant” or “mitochondria-targeted coenzyme Q10” or “mitochondria-targeted coenzyme Q10”). To identify missing studies, each selected study was individually scrutinized by clicking on the “cited” and “similar” tabs of the databases. The two authors independently selected the studies. Studies were screened based on their titles and abstracts. Discrepancies that arose during the study selection were resolved by consensus after discussion.
Data Extraction, Synthesis, and Analysis
The two researchers independently extracted the following data: number of subjects in each group, sex, age, body mass index (BMI), weight, height, health status, plasma oxidative stress markers, and aerobic endurance performance markers. If data were not presented in the text or tables, data were extracted using WebPlotDigitalizer [32]. The mean and standard deviation (ΔSD) change was recorded for each treatment (i.e., MitoQ and Placebo) and outcome. One study [29] did not report preliminary data, so changes were not reported. As this was a crossover trial, we collected only the post-Placebo and post-MitoQ time to exhaustion data, and the effect size was calculated using the post-Placebo mean (SD) and the post-MitoQ mean (SD). We used a similar approach previously when both sets of data came from the same subjects [33, 34]. Additionally, a sensitivity analysis was used to confirm that this approach did not affect the overall results. When ΔSD was not reported we calculated it assuming a correlation coefficient of 0.7 as previously suggested [35, 36]:
Regarding aerobic endurance performance, two studies performed time trials [37, 38] and two performed time to exhaustion trials [29, 39]. In this regard, we included the study by Shill et al. [30] in the analysis of aerobic exercise performance as it reported VO2max during a graded exercise test.
All analyses were performed using the meta for package of R software [40]. The meta-analyses were performed using random-effects models with DerSimonian‒Laird methods to assess the effects of MitoQ on exercise-induced oxidative stress and aerobic exercise performance. Effect sizes are presented as standardized mean differences (SMDs) and 95% CIs, as the outcomes have noncomparable scales.
Risk of Bias and Heterogeneity
The Critical Appraisal Checklist for Randomized Controlled Trials of the Faculty of Health and Medical Sciences at the University of Adelaide, South Australia [41] was used to evaluate study bias. The checklist comprises 13 items relating to the article’s title, abstract, introduction, methods, results, and discussion sections. Quality scores were calculated as the total points and percentage of the applicable items. The overall quality was considered medium when the score ranged from 50 to 75% and high for it was > 75% [33]. Heterogeneity was reported as the Tau (τ)2 value which is an estimate of between-studies variance, and the confidence intervals was derived from τ.
Publication bias was assessed using visual inspection of Funnel plots and accompanying Egger’s Tests. Publication bias pertains to the tendency of significant results to be more likely published than null results. A p-value less than 0.05 in the Egger test indicates publication bias. As a sensitivity analysis, we employed the leave-one-out method to assess whether any of the included studies significantly influenced the overall effect. If the leave-one-out test yielded positive results, we reported the effect size of the model with that particular study excluded from the analysis.
Results
After deduplication, a total of 81 studies were screened (Fig. 1). Eight studies (involving 188 subjects) met the inclusion criteria and were included in the meta-analysis (Fig. 1). Two studies [42, 43] evaluated the effects of MitoQ supplementation and MitoQ in conjunction with training on basal blood redox state. However, since they did not analyze the response to an acute exercise, they were excluded from the analysis. Six of the included studies assessed healthy recreational subjects [28,29,30, 37, 38, 44], one study involved a population with peripheral artery disease (PAD) [39] and another study involved subjects with chronic kidney disease [45]. Six of these studies examined the effects of chronic supplementation (with an average duration of 28 days, ranging from 10 to 42 days), and three studies investigated the effects of acute supplementation [29, 39, 44] (Table 1). The risk of bias analysis of the studies indicated medium to high quality (Suppl Table 1).
Effect of MitoQ Supplementation on Aerobic Performance
The effects of MitoQ supplementation on aerobic performance assessed in five studies (Fig. 2A). We found that MitoQ did not improve aerobic exercise performance (SMD: -0.50; 95% CI: -1.39 to 0.40; τ2 = 1.03; p = 0.398). Regarding publication bias, the Egger test yielded a non-significant result (p = 0.239, Fig. 2B). The sensitivity analysis revealed that the study by Park et al. [39] influenced the overall effects. When excluding this study from the analysis, the result yielded a similar result (SMD: -0.03; 95% CI: -0.46 to 0.41; τ2 = 0.06; p = 0.909).
Effect of MitoQ Supplementation on Exercise-induced Oxidative Damage
Exercise-induced oxidative stress was evaluated in six studies (Fig. 2C). We found that MitoQ supplementation decreased blood markers of oxidative damage (SMD: -1.33; 95% CI: -2.24 to -0.43; τ2 = 1.01; p = 0.004). Regarding publication bias, the Egger test yielded a significant result (p = 0.002, Fig. 2D). At the same time, the sensitivity analysis revealed that the study by Park et al. [39] was influencing the overall effects. When excluding this study from the analysis, the result yielded a more robust result (SMD: -0.7; 95% CI: -1.07 to -0.39; τ2 = 0.00; p < 0.001).
Discussion
Muscle contractions, such as those induced by exercise, increase ROS production. In this response, NOX enzymes are known to be a primary source of ROS, which likely facilitates performance by increasing glucose uptake [18]. On the other hand, although mitochondrial ROS production is limited during high-intensity exercise compared to NOX sources, it may still be sufficient to induce oxidative damage [44]. Therefore, our meta-analysis aimed to synthesize quantitatively the studies assessing the effects of the mitochondrial-targeted antioxidant MitoQ on aerobic endurance performance and exercise-induced oxidative damage.
The main finding of our work is that MitoQ supplementation does not enhance aerobic exercise performance. The main potential explanation for this phenomenon is that mitochondrial ROS production can decrease during exercise, even falling below resting levels [17]. Thus, targeting mitochondrial ROS production may have limited effects during exercise. Additionally, it is now known that several CoQ pools exist in mammalian mitochondria [46]. When glycolysis is the main source of electrons, they primarily pass from NADH to Complex I (CI) to Complex III (CIII) through a CI-linked Q-pool [47]. However, when β-oxidation predominates, the relative amount of FADH2 increases, and electrons are mainly taken up by a free Q-pool linked to CIII [47]. Failure to modulate the available CoQ pools according to physiological needs decreases maximal exercise capacity in rodents [48]. It has been suggested that fatty acid-linked respiration increases more than CI and Complex II (CII)-linked respiration after an exercise period [49]. Therefore, muscle mitochondria adapt strongly to take electrons from FADH2 to a Q-pool dedicated to CIII. Thus, it would be logical that higher CoQ levels, achieved by intake of Q analogs such as MitoQ, may help channel electrons to CIII. However, when MitoQ is supplemented during exercise, there are no improvements in muscular oxidative capacity [30]. Moreover, 20 days of MitoQ supplementation does not increase mitochondrial content [38], which can have significant implications if CIII (and/or related dehydrogenases) does not increase correspondingly with CoQ levels, as reductive stress can arise [50]. Therefore, the accumulation of reducing equivalents can explain the unchanged exercise performance while preventing oxidative stress. In addition, this situation can also trigger long-term CI degradation via reverse electron transport [47], which would be counterproductive for maximal exercise performance. In the future, studies addressing MitoQ in muscle physiology should consider assessing electron transport chain complexes as well as mitochondrial respiration using different substrates.
Our sensitivity analyses identified the study by Park et al. [39] as a source of heterogeneity. This study was conducted in subjects with PAD and found that an acute 80 mg dose of MitoQ, administered one hour before an exercise test, improved walking time by 17% compared to placebo. It also enhanced flow-mediated dilation in the legs, contrasting with another recent study where an acute 80 mg dose of MitoQ reduced VO2max and maximal ventilation in sedentary healthy subjects [29]. The latter study suggested that this effect might be due to an inhibition of exercise-induced pulmonary vasoconstriction. Another study examined the effects of 28 days of MitoQ supplementation in patients with chronic kidney disease [45]. Although aerobic exercise capacity was not improved, MitoQ supplementation enhanced microvascular vasodilation [45]. Taken together, these data suggest that MitoQ could potentially impact blood flow by increasing vasodilation. While this can be beneficial for patients with some degree of vascular dysfunction, in the context of training, it could elicit a negative response because local vasoconstriction is necessary during intense exercise. However, it should be highlighted that although patients with some degree of vascular dysfunction could benefit in terms of aerobic performance, this was only observed in patients with PAD [39], not in those with chronic kidney disease [45]. One possible explanation could be that the study by Kirkman et al. [45] assessed fatigue during progressive exercise on a cycle ergometer, where exercise limitation can be due to local rather than central fatigue. Additionally, these studies differed in the duration of supplementation, which was acute (1 h before exercise) in the study by Park et al. [39], and chronic (over 28 consecutive days) in the study by Kirkman et al. [45], which can likely exert different physiological effects.
The study by Park et al. [39] also contributed significantly to the heterogeneity among studies analyzing exercise-induced oxidative damage. ROS are known to act as crucial signaling molecules, regulating gene expression, enzyme activity, and membrane transport during exercise [51]. However, the ROS burst induced by exercise can surpass physiological limits, leading to significant oxidative damage in conditions with enhanced mitochondrial ROS production, such as in patients with PAD. This may also explain the observed improvement in walking time after MitoQ supplementation in PAD patients. Indeed, high-intensity muscle contractions can produce excessive ROS, potentially reducing muscle force generation [6]. In such scenarios, limiting ROS production or redirecting it to cytosolic sources might enhance endurance performance. However, it should be noted that the Park et al. [39] study employed the thiobarbituric Acid Reactive Substances (TBARS) assay to indirectly assess lipid peroxidation, a method reported to be a less reliable marker for detecting lipid peroxidation in response to exercise [52]. Therefore, the precise impact of MitoQ supplementation on excessive ROS production during exercise in subjects with altered mitochondrial function, like those with PAD, remains unclear. Additionally, the dose-response relationship of MitoQ merits further investigation, as varying dosages, such as the 20 mg used by Williamson et al. [44] versus the 80 mg used by Park et al. [39], may account for the significant differences observed in exercise-induced oxidative damage between these two studies employing an acute supplementation design. Nevertheless, it appears likely that similar doses, for instance, 80 mg, may have differing effects on exercise time to exhaustion depending on the health status of the subjects [29, 39].
Limitations and Strengths
The main limitation of our study is the relative novelty of MitoQ supplementation in sports science, resulting in limited published research. Other limitations are the relatively small participant number, as well as the relatively short duration (i.e., acute and 3 weeks) of supplementation in published studies. In addition, there were only 17 females out of 188 participants and, thus, it should be considered as a limitation. Finally, we investigated oxidative stress biomarkers in plasma, which limits our ability to draw mechanistic conclusions about the effects of MitoQ supplementation on exercise-induced muscle oxidative stress. However, the novelty of this research topic also serves as a strength, as significant findings can guide the development of future studies. There is a clear need to investigate whether MitoQ supplementation concurrent with exercise training (e.g., 4–16 weeks) alters adaptations induced by exercise alone. Additionally, studies examining various doses, ranging from 10 to 80 mg, are warranted, as well as comparisons between non-specific antioxidants and MitoQ to discern the impact of ROS sources on exercise adaptations.
Conclusions
We conducted the first meta-analysis of randomized trials exploring the effects of MitoQ on aerobic exercise performance and exercise-induced oxidative damage. Our findings indicate that while MitoQ effectively reduces exercise-induced oxidative damage, no evidence suggests that aerobic exercise performance is enhanced by either acute or chronic MitoQ supplementation. However, acute MitoQ supplementation may improve exercise tolerance in subjects with PAD. Investigating whether similar benefits extend to other diseases characterized by significant alterations in mitochondrial ROS production could be highly valuable.
Data Availability
Data used for the present study is available from the corresponding author.
Abbreviations
- MitoQ:
-
Mitoquinone
- NOX:
-
NADPH oxidase
- PAD:
-
Peripheral artery disease
- ROS:
-
Reactive oxygen species
- SMDs:
-
Standardized mean differences
References
Huertas JR, Casuso RA, Agustín PH, Cogliati S. Stay fit, stay young: Mitochondria in Movement: the role of Exercise in the new mitochondrial paradigm. Oxid Med Cell Longev. 2019;2019:1–18.
Sugamura K, Keaney JF. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–92.
Panth N, Paudel KR, Parajuli K. Reactive oxygen species: a key Hallmark of Cardiovascular Disease. Adv Med. 2016;2016:1–12.
Hurrle S, Hsu WH. The etiology of oxidative stress in insulin resistance. Biomed J. 2017;40:257–62.
Lamb GD, Westerblad H. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J Physiol. 2011;589:2119–27.
Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–76.
Powers SK, Nelson WB, Hudson MB. Exercise-induced oxidative stress in humans: cause and consequences. Free Radic Biol Med. 2011;51:942–50.
Powers SK, Deminice R, Ozdemir M, Yoshihara T, Bomkamp MP, Hyatt H. Exercise-induced oxidative stress: friend or foe? J Sport Health Sci. 2020;9:415–25.
Huertas JR, Al Fazazi S, Hidalgo-Gutierrez A, López LC, Casuso RA. Antioxidant effect of exercise: exploring the role of the mitochondrial complex I superassembly. Redox Biol. 2017;13:477–81.
Brooks SV, Vasilaki A, Larkin LM, McArdle A, Jackson MJ. Repeated bouts of aerobic exercise lead to reductions in skeletal muscle free radical generation and nuclear factor κB activation. J Physiol. 2008;586:3979–90.
Gomez-Cabrera M-C, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, et al. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–9.
Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–70.
Stepanyan V, Crowe M, Haleagrahara N, Bowden B. Effects of vitamin E supplementation on exercise-induced oxidative stress: a meta-analysis. Appl Physiol Nutr Metab. 2014;39:1029–37.
Righi NC, Schuch FB, De Nardi AT, Pippi CM, de Almeida Righi G, Puntel GO, et al. Effects of vitamin C on oxidative stress, inflammation, muscle soreness, and strength following acute exercise: meta-analyses of randomized clinical trials. Eur J Nutr. 2020;59:2827–39.
Clifford T, Jeffries O, Stevenson EJ, Davies KAB. The effects of vitamin C and E on exercise-induced physiological adaptations: a systematic review and Meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2020;60:3669–79.
Casuso RA, Gallego JG, Huertas JR, Antioxidants. Intervention studies. Encyclopedia of Human Nutrition. Elsevier; 2023. pp. 48–60.
Goncalves RLS, Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Brand MD. Sites of Superoxide and Hydrogen Peroxide production by Muscle Mitochondria Assessed Ex Vivo under conditions mimicking Rest and Exercise. J Biol Chem. 2015;290:209–27.
Henríquez-Olguin C, Knudsen JR, Raun SH, Li Z, Dalbram E, Treebak JT, et al. Cytosolic ROS production by NADPH oxidase 2 regulates muscle glucose uptake during exercise. Nat Commun. 2019;10:4623.
Hidalgo C, Sánchez G, Barrientos G, Aracena-Parks P. A transverse tubule NADPH oxidase activity stimulates calcium release from isolated triads via ryanodine receptor type 1 S -glutathionylation. J Biol Chem. 2006;281:26473–82.
Henríquez-Olguín C, Renani LB, Arab-Ceschia L, Raun SH, Bhatia A, Li Z, et al. Adaptations to high-intensity interval training in skeletal muscle require NADPH oxidase 2. Redox Biol. 2019;24:101188.
Xirouchaki CE, Jia Y, McGrath MJ, Greatorex S, Tran M, Merry TL, et al. Skeletal muscle NOX4 is required for adaptive responses that prevent insulin resistance. Sci Adv. 2021;7:eabl4988.
López-Lluch G. Coenzyme Q homeostasis in aging: response to non-genetic interventions. Free Radic Biol Med. 2021;164:285–302.
Smith RAJ, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci. 2010;1201:96–103.
Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem. 2001;276:4588–96.
Gohil K, Rothfuss L, Lang J, Packer L. Effect of exercise training on tissue vitamin E and ubiquinone content. J Appl Physiol (1985). 1987;63:1638–41.
Bailey DM, McEneny J, Mathieu-Costello O, Henry RR, James PE, McCord JM, et al. Sedentary aging increases resting and exercise-induced intramuscular free radical formation. J Appl Physiol (1985). 2010;109:449–56.
Zoladz JA, Koziel A, Woyda-Ploszczyca A, Celichowski J, Jarmuszkiewicz W. Endurance training increases the efficiency of rat skeletal muscle mitochondria. Pflugers Arch. 2016;468:1709–24.
Broome SC, Braakhuis AJ, Mitchell CJ, Merry TL. Mitochondria-targeted antioxidant supplementation improves 8 km time trial performance in middle-aged trained male cyclists. J Int Soc Sports Nutr. 2021;18:58.
Hughes RP, Carlini NA, Fleenor BS, Harber MP. Mitochondrial-targeted antioxidant ingestion acutely blunts VO2max in physically inactive females. Physiol Rep. 2023;11:e15871.
Shill DD, Southern WM, Willingham TB, Lansford KA, McCully KK, Jenkins NT. Mitochondria-specific antioxidant supplementation does not influence endurance exercise training-induced adaptations in circulating angiogenic cells, skeletal muscle oxidative capacity or maximal oxygen uptake. J Physiol. 2016;594:7005–14.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;n71.
Jelicic Kadic A, Vucic K, Dosenovic S, Sapunar D, Puljak L. Extracting data from figures with software was faster, with higher interrater reliability than manual extraction. J Clin Epidemiol. 2016;74:119–23.
Pérez-Rodríguez M, Huertas JR, Villalba JM, Casuso RA. Mitochondrial adaptations to calorie restriction and bariatric surgery in human skeletal muscle: a systematic review with meta-analysis. Metabolism. 2023;138:155336.
Casuso RA, Huertas JR, Aragón-Vela J. The role of muscle disuse in muscular and cardiovascular fitness: a systematic review and meta‐regression. Eur J Sport Sci. 2024.
Mason SA, Keske MA, Wadley GD. Effects of vitamin C supplementation on Glycemic Control and Cardiovascular Risk factors in people with type 2 diabetes: a GRADE-Assessed systematic review and Meta-analysis of Randomized controlled trials. Diabetes Care. 2021;44:618–30.
Galan-Lopez P, Casuso RA. Metabolic adaptations to Morning Versus Afternoon training: a systematic review and Meta-analysis. Sports Med. 2023;53:1951–61.
Broome SC, Atiola RD, Braakhuis AJ, Mitchell CJ, Merry TL. Mitochondria-targeted antioxidant supplementation does not affect muscle soreness or recovery of maximal voluntary isometric contraction force following muscle-damaging exercise in untrained men: a randomized clinical trial. Appl Physiol Nutr Metab. 2022;47:762–74.
Broome SC, Pham T, Braakhuis AJ, Narang R, Wang HW, Hickey AJR, et al. MitoQ supplementation augments acute exercise-induced increases in muscle PGC1α mRNA and improves training-induced increases in peak power independent of mitochondrial content and function in untrained middle-aged men. Redox Biol. 2022;53:102341.
Park S-Y, Pekas EJ, Headid RJ, Son W-M, Wooden TK, Song J, et al. Acute mitochondrial antioxidant intake improves endothelial function, antioxidant enzyme activity, and exercise tolerance in patients with peripheral artery disease. Am J Physiol Heart Circ Physiol. 2020;319:H456–67.
Viechtbauer W. Conducting Meta-analyses in R with the metafor Package. J Stat Softw. 2010;36.
Barker TH, Stone JC, Sears K, Klugar M, Tufanaru C, Leonardi-Bee J, et al. The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid Synth. 2023;21:494–506.
Masoumi-Ardakani Y, Najafipour H, Nasri HR, Aminizadeh S, Jafari S, Moflehi D. Effect of combined endurance training and MitoQ on cardiac function and serum level of antioxidants, NO, miR-126, and miR-27a in hypertensive individuals. Biomed Res Int. 2022;2022:8720661.
Masoumi-Ardakani Y, Najafipour H, Nasri HR, Aminizadeh S, Jafari S, Safi Z. Moderate endurance training and MitoQ improve Cardiovascular function, oxidative stress, and inflammation in Hypertensive individuals: the role of miR-21 and miR-222: a Randomized, Double-Blind, clinical trial. Cell J. 2022;24:577–85.
Williamson J, Hughes CM, Cobley JN, Davison GW. The mitochondria-targeted antioxidant MitoQ, attenuates exercise-induced mitochondrial DNA damage. Redox Biol. 2020;36:101673.
Kirkman DL, Stock JM, Shenouda N, Bohmke NJ, Kim Y, Kidd J, et al. Effects of a mitochondrial-targeted ubiquinol on vascular function and exercise capacity in chronic kidney disease: a randomized controlled pilot study. Am J Physiol Ren Physiol. 2023;325:F448–56.
Enriquez JA, Lenaz G. Coenzyme q and the respiratory chain: coenzyme q pool and mitochondrial supercomplexes. Mol Syndromol. 2014;5:119–40.
Guarás A, Perales-Clemente E, Calvo E, Acín-Pérez R, Loureiro-Lopez M, Pujol C, et al. The CoQH2/CoQ ratio serves as a Sensor of Respiratory Chain Efficiency. Cell Rep. 2016;15:197–209.
Calvo E, Cogliati S, Hernansanz-Agustín P, Loureiro-López M, Guarás A, Casuso RA, et al. Functional role of respiratory supercomplexes in mice: SCAF1 relevance and segmentation of the Qpool. Sci Adv. 2020;6:eaba7509.
Pesta D, Hoppel F, Macek C, Messner H, Faulhaber M, Kobel C, et al. Similar qualitative and quantitative changes of mitochondrial respiration following strength and endurance training in normoxia and hypoxia in sedentary humans. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1078–87.
Herrero Martín JC, Salegi Ansa B, Álvarez-Rivera G, Domínguez-Zorita S, Rodríguez-Pombo P, Pérez B, et al. An ETFDH-driven metabolon supports OXPHOS efficiency in skeletal muscle by regulating coenzyme Q homeostasis. Nat Metab. 2024;6:209–25.
Powers SK, Schrager M. Redox signaling regulates skeletal muscle remodeling in response to exercise and prolonged inactivity. Redox Biol. 2022;54:102374.
Groussard C, Rannou-Bekono F, Machefer G, Chevanne M, Vincent S, Sergent O, et al. Changes in blood lipid peroxidation markers and antioxidants after a single sprint anaerobic exercise. Eur J Appl Physiol. 2003;89:14–20.
Acknowledgements
The authors would like to acknowledge Universidad Loyola Andalucía for supporting their research activities.
Funding
Rafael A. Casuso is supported by a grant from the Spanish Ministry of Science and Innovation (PID2022-140453OB-I00 financed by MICIU/AEI/https://doi.org/10.13039/501100011033 and the FEDER, UE).
Author information
Authors and Affiliations
Contributions
Following the CRediT author statement each of the authors contributed as follows: OGS; Conceptualization, Investigation, Data curation, Writing-Review & Editing and Visualization. RAC; Conceptualization, Investigation, Methodology, Software, Formal analysis, Writing- Original Draft, Visualization and Supervision. Both authors read and approved the final version.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gonzalo-Skok, O., Casuso, R.A. Effects of Mitoquinone (MitoQ) Supplementation on Aerobic Exercise Performance and Oxidative Damage: A Systematic Review and Meta-analysis. Sports Med - Open 10, 77 (2024). https://doi.org/10.1186/s40798-024-00741-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40798-024-00741-5