Abstract
Background
Flexibility is an important component of physical fitness for competitive and recreational athletes. It is generally suggested that flexibility training should start from childhood (6–11 years of age) to optimize joint range of motion (ROM) increases; however, evidence is limited and inconsistent.
Objective
To examine whether there is a difference in the effect of stretching training on flexibility during childhood (6–11 years of age) and adolescence (12–18 years of age).
Design
Systematic review and meta-analysis.
Methods
We searched PubMed Central, Web of Science, Scopus, Embase, and SPORTDiscus, to conduct this systematic review. Randomized controlled trials and non-randomized controlled trials were eligible. No language and date of publication restrictions were applied. Risk of bias was assessed using Cochrane RoB2 and ROBINS-I tools. Meta-analyses were conducted via an inverse variance random-effects model. GRADE analysis was used to assess the methodological quality of the studies.
Results
From the 2713 records retrieved 28 studies were included in the meta-analysis (n = 1936 participants). Risk of bias was low in 56.9% of all criteria. Confidence in cumulative evidence was moderate. We found that stretching was effective in increasing ROM in both children (SMD = 1.09; 95% CI = 0.77–1.41; Z = 6.65; p < 0.001; I2 = 79%) and adolescents (SMD = 0.90; 95% CI = 0.70–1.10; Z = 8.88; p < 0.001; I2 = 81%), with no differences between children and adolescents in ROM improvements (p = 0.32; I2 = 0%). However, when stretching volume load was considered, children exhibited greater increases in ROM with higher than lower stretching volumes (SMD = 1.21; 95% CI = 0.82–1.60; Z = 6.09; p < 0.001; I2 = 82% and SMD = 0.62; 95% CI = 0.29–0.95; Z = 3.65; p < 0.001; I2 = 0%, respectively; subgroup difference: p = 0.02; I2 = 80.5%), while adolescents responded equally to higher and lower stretching volume loads (SMD = 0.90; 95% CI = 0.47–1.33; Z = 4.08; p < 0.001; I2 = 83%, and SMD = 0.90; 95% CI = 0.69–1.12; Z = 8.18; p < 0.001; I2 = 79%, respectively; subgroup difference: p = 0.98; I2 = 0%).
Conclusions
Systematic stretching training increases ROM during both childhood and adolescence. However, larger ROM gains may be induced in childhood than in adolescence when higher stretching volume loads are applied, while adolescents respond equally to high and low stretching volume loads.
Registration: INPLASY, registration number: INPLASY202190032; https://inplasy.com/inplasy-2021-9-0032/
Similar content being viewed by others
Key Points
-
Systematic stretching training increases ROM during both childhood and adolescence.
-
Larger ROM gains may be induced in childhood than in adolescence when higher stretching volume loads are applied
-
Adolescents respond equally to high and low stretching volume loads
Background
Long-term athlete development models provide general frameworks to prepare children and adolescents for sports and a physically active lifestyle [1]. These models aim to align sport practice with growth, maturation, and early sport specialization and consider factors such as injury risk [2, 3] and the limitations of the existing training practice schedules [4]. Muscular strength and power, speed, agility, mobility, and flexibility are central fitness components in all the long-term athlete development models [3, 5, 6]. Most long-term athlete development models encourage participation in mobility and flexibility training from a very young age (45 years), with an underlying assumption that flexibility can be enhanced more with early training [7, 8].
Flexibility is an important component of physical fitness for competitive and recreational athletes [9] and a performance determinant in sports requiring the ability to move comfortably through a large range of motion (ROM) [10]. Flexibility is defined as the ROM in a joint or series of joints [9] and from a functional perspective represents the ability to move comfortably without constraints or pain through a full ROM [11]. The importance of flexibility in children and adolescents is task and sport specific [10]. For example, in gymnastics the athlete executes skills assuming extreme body positions [12, 13], while in other sports, a large ROM is utilized to enhance the mechanical effectiveness of a task [14, 15]. For example, in throwing activities an enhanced joint ROM can increase the distance over which muscle force is applied or absorbed thus allowing the athlete to generate a higher power output [15, 16]. In sports such as gymnastics [12, 13] and throwing [16], increased hip and shoulder ROM are typically associated with higher performance level. There is also evidence suggesting that decreased joint ROM is a risk factor for injury in young athletes [17, 18]. For example, adolescent swimmers with limited ROM were found to have a 3.6 times higher risk of developing shoulder pain than swimmers with normal ROM [17].
Despite its importance, flexibility is a largely under-researched area of study within the pediatric populations [15]. It has been suggested that childhood is a key time period for flexibility development, with the age range of 6–11 years proposed as being a “window of opportunity” for flexibility development [8]. One possible mechanism for this is the increased pliability and reduced musculotendinous stiffness associated with childhood [19], which may enable greater ROM to be attained, and this may, in turn, render flexibility training more effective. For example, Kubo et al. [19] reported that the tendon structures in younger boys (10–11 years old) are more compliant than those in older boys (14–15 years old) and young men, although the association between muscle and tendon mechanical properties and joint ROM in children and adolescents has not been investigated. Furthermore, children and adolescents are generally more flexible than adults [20, 21], while joint ROM gradually diminishes with age [22]. However, research on flexibility training in youth is limited, and evidence regarding the existence of “windows of opportunity” for different motor skills development is controversial [23]. Previous long-term athletic development models did not suggest an appropriate period for flexibility development [7]. More recently, the Youth Physical Development Model [8] suggested that middle childhood (ages 6–11) may be an optimal time frame for flexibility and mobility training. According to the authors of this model, the rationale for this selection is that it incorporates a period that has previously been termed a “critical period” of flexibility development, which is supported mainly by empirical evidence [10, 24]. For example, in sports such as gymnastics and dance, children are submitted to extensive daily flexibility training schedules on the assumption that ROM gains may be maximized with early training [10]. On the other hand, the levels of flexibility tend to plateau or even decrease at the time of the adolescent growth spurt and into adulthood, especially in boys, thus lending support to the notion of a “window of opportunity” earlier in childhood, at least in boys [25].
Short-term stretching training improvements in joint ROM are usually attributed to increased stretch tolerance and/or are related to a decreased tissue resistance to stretch [9]. The loading characteristics of the stretching protocol are key elements for chronic joint ROM increases [26]. Past research in adults has reported that total stretch duration is more important for ROM enhancement than the duration of each stretching bout [27]. Cross-sectional studies in adults also reported that higher stretching volume load (i.e., the total duration of stretching applied over the intervention period) is a crucial factor for improvement in ROM [28]. However, evidence for the effects of stretching training on ROM improvement in children and adolescents is limited and, in many cases, contradictory [29]. Thus, although a “window of opportunity” for flexibility development has been widely suggested, there is only sparse evidence to verify its existence. Moreover, the effect of confounding variables such as the loading characteristics of the stretching protocols has not yet been collectively assessed. Therefore, the aim of this systematic review and meta-analysis was to examine whether there is a difference in the effect of stretching training on flexibility during childhood (6–11 years of age) and adolescence (12–18 years of age).
Methods
Study Design
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [30] (see Additional file 1: PRISMA checklist). The review was preregistered in the International Platform of Registered Systematic Review and Meta-analysis Protocols (INPLASY, registration number: INPLASY202190032; https://inplasy.com/inplasy-2021-9-0032/).
Search and Selection Strategy
Five electronic databases were searched through, until March 2022 by two independent investigators (OD, IP): PubMed Central, Scopus, Web of Science, Embase and SPORTDiscus. No language and date restrictions were applied. The search was carried out in the field type “Title and abstract.” The topic was systematically searched using a Boolean search strategy with the operator “AND” and “OR.” The keywords with more than one word were enclosed in quotes. The keyword algorithm used in the selected databases can be found in Additional file 1. Additional records that were not picked up in systematic searches were identified through: (1) searching the reference lists of original studies and some related study reviews, (2) examining the reference citations and the researchers’ publications, (3) contacting by email the corresponding authors (if they were not defined, the first author was used), and (4) screening the researchers’ personal lists in ResearchGate and Google Scholar (first authors) [31, 32]. Based on our knowledge of the area, we also contributed additional studies which we had knowledge of but were not picked up in systematic searches. Two investigators (IP, AK) selected the eligible studies based on the eligibility criteria. In the case of a disagreement between the investigators, GCB and OD made the ultimate decision for the searching and selection procedures by majority consensus.
Inclusion and Exclusion Criteria
We followed PICOS (Population, Intervention, Comparison, Outcome, Study Design) for selecting studies for inclusion. We included randomized controlled trials and non-randomized controlled trials (not randomized trials that include a comparison or control group). The included studies investigated the chronic effects (> 2 weeks) of static stretching in healthy (i.e., non-clinical) children (5–11 years old), and adolescents (12–18 years old). We included pupils, recreationally active, and trained participants. Studies also had to include an implementation of a static stretching intervention because evidence for other types of stretching (e.g., dynamic, ballistic, proprioceptive neuromuscular facilitation stretching, and nerve-directed stretching) is limited in children and adolescents and these types of stretching are not commonly used in physical education and sport settings in these age groups. Due to the limited evidence, we also decided to include only studies that examined lower limbs. The comparison conditions included pre- and post-stretching interventions in experimental and control conditions. Data regarding ROM maintenance following a detraining period were not included in the study. We excluded single group studies, studies without a control group, studies which had no clearly defined stretching protocol or a protocol also including a different stimulus (e.g., vibration or strength training). In addition, studies which focused on very small joints (e.g., fingers, toes), non-human studies, and in vitro studies were excluded. Retrospective studies, review papers, case reports, special communications, letters to the editor, invited commentaries, and conference papers were excluded. Related articles were included up to March 2022.
Risk of Bias Assessment and Methodological Quality
IP and OD independently assessed the risk of bias of the included studies and any conflict was resolved through discussion with AK and PCD. The updated Cochrane Risk of Bias 2 (RoB2) and ROBINS-I tools were used for the randomized controlled trials and controlled trials without randomization, respectively. The updated Risk of Bias 2 (RoB2) Cochrane Library includes the following sources of bias: bias arising from the randomization process, bias due to deviations from intended interventions (effect of assignment to intervention and effect of adhering to intervention), bias due to missing outcome data, bias in the measurement of the outcome, and bias in selection of the reported result [33]. ROBINS-I includes the following bias domains: bias due to confounding, bias in selection of participants into the study, bias in classification of interventions, bias due to deviations from intended interventions, bias due to missing data, bias in measurement of outcomes, and bias in selection of the reported results [34].
Confidence in the Cumulative Evidence
The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) quality rating analysis was used to assess the quality of the outcomes. GRADE has four levels of evidence quality: very low, low, moderate, and high [35, 36]. For GRADE analysis, five evaluation components were adopted to lower quality (risk of bias, inconsistency of results, indirectness, imprecision, and publication bias) and three evaluation components to higher quality (large effect, dose–response, and confounding). All evaluation components were assessed independently by OD and IP and verified by GCB and PCD. The same authors estimated the overall quality and confidence in the cumulative evidence.
Data Extraction
Three independent investigators (AK, IP, and OD) extracted the data from the included papers in the systematic review. The data extraction was supervised by two other investigators (PCD and GCB). We extracted data regarding: (a) author and year of publication, (b) type of publication (journal paper or grey literature), (c) study design (randomized controlled trial or controlled trial), (d) sample size in total, and for the experimental and control groups, (e) sex (males and females), (f) age (for the experimental and the control groups), (g) anthropometric characteristics (body mass, height), (h) participants’ physical activity level (e.g., recreationally active, athlete, or pupil), (i) the main outcome of the study, and (j) the means and standard deviations for outcome measures for both the experimental and the control groups. The term “Range of motion (ROM)” was used to indicate the linear or angular distance and direction a joint can move between the flexed position and the extended position [10]. The characteristics of the included studies can be found in Table 1. In addition, we extracted the characteristics of the stretching interventions, the joint, and muscle examined and the test used to assess ROM. Additional details regarding the stretching intervention characteristics (i.e., the duration of every stretching bout, the number of exercises, the number of sets, and the frequency of stretching training per week) were extracted and from these data, and we calculated the daily stretching duration (s) (the duration of each stretching bout × number of sets × number of exercises), the stretching duration per week (s) (the duration of the daily stretching × the number of stretching trainings per week), and the total duration of the stretching intervention (s) (the stretching duration per week × the number of weeks). These characteristics can be found in an open repository file (https://doi.org/10.6084/m9.figshare.17104640).
Data Synthesis and Meta-analysis Methods
All the included studies in the systematic review provided data for the meta-analysis. We extracted pre- and post-intervention means and standard deviations. In the case of data being given in the form of a graph and in the case of missing data, the corresponding or first authors of the included studies were contacted via email, to retrieve these data. We have calculated the Δ scores of the means by subtracting the baseline values from the post-intervention values. The standard deviations for the Δ scores were calculated according to the following equation: \(\sqrt {\left( {{\text{SD}}^{2} {\text{pre}} + {\text{SD}}^{2} {\text{post}}} \right){-}\left( {2 \times 0.70 \times {\text{SDpre}} \times {\text{SD post}}} \right) }\) [33]. This approach removed the bias acquired from the significant differences in baseline values that might have played a role in the post-intervention differences between the experimental and control groups. We conducted an inverse-variance, continuous, random-effects model meta-analysis using RevMan 5.3 software [34]. We tested the differences in ROM between an experimental (stretching group) and a control group (i.e., no stretching). Heterogeneity was tested using the I2 statistic [35]. I2 values indicate the degree of heterogeneity in the effects: 0–40% were not important, 30–60% moderate heterogeneity, 50–90% substantial heterogeneity, and 75–100% considerable heterogeneity [36]. A cutoff value of 75% was adopted as an index of considerable heterogeneity. In all the meta-analyses, we used the standardized mean differences due to the different scale measurements that the variables displayed [33]. We performed between group analyses, which included comparisons of age (children 5–11 years of age vs. adolescents 12–18 years of age) irrespective of the stretching protocol, and between group analyses which included comparisons between high and low stretching volume loads (< 3600 s vs. ≥ 3600 s) irrespective of age. Subgroups analyses were performed according to age groups, as follows: child participants (≤ 11 years of age) following either a lower (< 3600 s) or a higher stretching volume load protocol (≥ 3600 s), and adolescents (≥ 12 years of age) following either a lower (< 3600 s) or a higher stretching volume load protocol (≥ 3600 s). The age groups were selected based on evidence of age-related differences in growth [37, 38], motor skill competence, and health-related physical fitness [39, 40]. The cutoff value for the stretching volume load was determined by calculating the total stretching duration (in s) of 10 weeks of training, including three sessions per week, and performing in each session two sets of two exercises lasting 30 s each (< 3600 s). This duration was selected to reflect typical stretching training protocols in sports and school practice [41]. No comparisons between the athletic and non-athletic populations were performed because, in the studies involving primary or secondary school students, extracurricular activities (e.g., sport participation) were either not controlled for or not reported. In addition, no subgroup comparisons between male and female participants were conducted because the studies including both males and females reported collective values for both sexes. According to Hopkins et al. [42], we defined the effects for a standardized mean difference (SMD) of < 0.2, 0.2–0.6, 0.6–1.2, 1.2–2.0, 2.0–4.0, and > 4.0 as trivial, small, moderate, large, very large, and extremely large, respectively. An alpha level of 0.05 was defined for the statistical significance of all the tests, apart from heterogeneity (p < 0.10). Moreover, visual inspection of the funnel plot was applied to detect possible publication bias.
Results
Results of the Searching Procedure
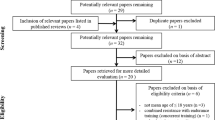
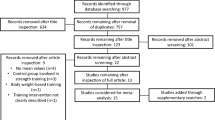
The initial search procedure retrieved 2713 papers. After duplicates were removed (n = 523), 2190 papers remained for eligibility evaluation. From these 2190 papers, 163 were conference papers, one was a letter to the editor, 162 papers were reviews, 25 were published proceedings and 1791 were considered irrelevant because they examined adult or clinical populations, acute interventions, or interventions not relevant to the study purpose. Finally, 48 papers were found to be eligible for this study. We then checked the reference lists and citations of the eligible studies to determine whether additional studies were relevant. Following this additional search, 8 more relevant papers were identified, of which 6 papers were eligible. Also, two more papers were added from our own library. After the screening of the full texts of the 56 eligible papers, 28 papers were excluded for different reasons (i.e., the study had no control group, or the study included some other type of stretching or stretching was combined with other interventions such as vibration or strength training). Therefore, in total, 28 papers (54 entries) were included in this systematic review and were used in the meta-analysis. A flowchart of the search process is presented in Fig. 1.
PRISMA flowchart illustrating different phases of the search and study selection [30]
Characteristics of the Included Studies
The 28 eligible studies in this systematic review and meta-analysis were published between 2004 and 2021 and involved 1936 participants (975 males). In total, 652 participants were between 5 and 11 years of age and 1284 participants were between 12 and 18 years (mean age: 9.3 ± 1.4 years vs. 14.0 ± 2.7 years, respectively). The characteristics of the participants can be found in Table 1. Out of the 28 eligible studies, six were controlled trials (CTs) [29, 43,44,45,46,47], and 22 were randomized controlled trials (RCTs) [48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. All the eligible studies used static stretching, and all the protocols targeted the lower limbs.
Risk of Bias Within Studies
A summary of the risk of bias assessment is illustrated in Figs. 2 and 3 for the RCTs and CTs, respectively. A detailed description of the risk of bias assessment for all the included studies in the current systematic review can be found in Additional file 1.
Meta-analysis Outcomes
Primary outcomes were any assessments related to ROM changes, both short-term (> 2 weeks) and long-term (~ 9 months) in children and adolescents (≤ 11 vs. ≥ 12 years of age, respectively). These outcomes were used only if there were pre- and post-intervention assessments. Secondary outcomes included differences in ROM according to the stretching volume load (< 3600 s vs. ≥ 3600 s of total stretching duration). Subgroups analyses were performed according to age groups, as follows: child participants (≤ 11 years of age) following either a lower (< 3600 s) or a higher stretching volume load protocol (≥ 3600 s), and adolescents (≥ 12 years of age) following either a lower (< 3600 s) or a higher stretching volume load protocol (≥ 3600 s).
Primary Outcomes
After all the participants had been analyzed together, it was found that stretching interventions were moderately effective in increasing ROM in the experimental groups compared with age-matched controls (SMD = 0.96; 95% CI = 0.79–1.13; Z = 11.23; p < 0.001; I2 = 80%; Fig. 4). In particular, the results showed that stretching was moderately effective in increasing ROM in children (SMD = 1.09; 95% CI = 0.77–1.41; Z = 6.65; p < 0.001; I2 = 79%; Fig. 4) and adolescents (SMD = 0.90; 95% CI = 0.70–1.10; Z = 8.88; p < 0.001; I2 = 81%; Fig. 4). However, no differences were found in ROM improvements between age groups (≤ 11 years of age vs. ≥ 12 years of age; SMD: 1.09 vs. 0.90, p = 0.32; I2 = 0%; Fig. 4).
Effect of static stretching training on joint range of motion in children and adolescents. SD: standard deviation, 95% CI: confidence interval. Note: CON: continuous stretching; INT: intermittent stretching; LL: left leg; RL: right leg; DKE: dorsiflexion with knee extension; DKF: dorsiflexion with knee flexion; HR: hip rotation; ER: external rotation; IR: internal rotation; HBD: heel-to-buttocks distance; SLR: straight leg raise; SAR: sit and reach; TT: toe-touch; and PKE: passive knee extension
Secondary Outcomes
Out of the 54 entries analyzed, 27 had “low” total volume (i.e., < 3600 s) and 27 had “high” total volume (≥ 3600 s). The characteristics of stretching interventions in the two subgroups (“high” and “low” volume) differed only in the number of exercises per session (two exercises vs. six exercises, p ˂ 0.001), and in the duration of the intervention (8.2 ± 2.7 weeks vs. 18.4 ± 9.5 weeks, p < 0.001), while the number of sets, the duration of each stretching bout, and the frequency of training per week were similar (p ˃ 0.08) (see, published file: https://doi.org/10.6084/m9.figshare.17104640).
After all the participants had been analyzed together, lower stretching volume loads (< 3600 s) increased ROM in the experimental groups compared with the age-matched controls (SMD = 0.87; 95% CI = 0.67–1.06; Z = 8.74; p < 0.001; I2 = 76%; Fig. 5) and the same was found for higher stretching volume loads (≥ 3600 s) (SMD = 1.08; 95% CI = 0.78–1.37; Z = 7.16; p < 0.001; I2 = 83%; Fig. 5). No differences were observed in ROM increases between higher and lower stretching volume loads when children and adolescents were analyzed together (SMD: 0.87 vs. 1.08; p = 0.25; I2 = 23.3%; Fig. 5).
Effect of high and low stretching volume load on joint range of motion. SD: standard deviation, 95% CI: confidence interval. Note: CON: continuous stretching; INT: intermittent stretching; LL: left leg; RL: right leg; DKE: dorsiflexion with knee extension; DKF: dorsiflexion with knee flexion; HR: hip rotation; ER: external rotation; IR: internal rotation; HBD: heel-to-buttocks distance; SLR: straight leg raise; SAR: sit and reach; TT: toe-touch; and PKE: passive knee extension
Subgroup Analyses: Age and Stretching Volume Interaction
Subgroup analyses in children (≤ 11 years of age) showed that both lower (< 3600 s) and higher (≥ 3600 s) stretching volume loads were effective in increasing ROM in the experimental groups compared with the controls (SMD = 1.09; 95% CI = 0.77–1.41; Z = 6.65; p < 0.001; I2 = 79%; Fig. 6). However, higher stretching volume loads were more effective in increasing ROM during childhood (SMD = 1.21; 95% CI = 0.82–1.60; Z = 6.09; p < 0.001; I2 = 82%; Fig. 6) compared with lower stretching volume loads (SMD = 0.62; 95% CI = 0.29–0.95; Z = 3.65; p = 0.0003; I2 = 0%; Fig. 6; SMD: 0.62 vs. 1.21, subgroup difference: p = 0.02; I2 = 80.5%; Fig. 6).
Subgroup analyses in adolescents (≥ 12 years of age) showed that both stretching volume loads, i.e., lower (< 3600 s) and higher (≥ 3600 s), were effective in increasing ROM in the experimental groups compared with the controls (SMD = 0.90; 95% CI = 0.70–1.10; Z = 8.88; p < 0.001; I2 = 81%; Fig. 7). Higher stretching volume loads increased ROM during adolescence (SMD = 0.90; 95% CI = 0.47–1.33; Z = 4.08; p < 0.001; I2 = 83%; Fig. 7), and the same was found for lower stretching volume loads (SMD = 0.90; 95% CI = 0.69–1.12; Z = 8.18; p < 0.001; I2 = 79%; Fig. 7). No differences were found in ROM increases in adolescents between the two stretching volume loads (SMD = 0.90 vs. 0.90; subgroup difference: p = 0.98; I2 = 0%; Fig. 7).
Effect of high and low stretching volume load on joint range of motion in adolescents. SD: standard deviation, 95% CI: Confidence Interval. Note: LL: left leg; RL: right leg; DKE: dorsiflexion with knee extension; DKF: dorsiflexion with knee flexion; HR: hip rotation; ER: external rotation; IR: internal rotation; HBD: heel-to-buttocks distance; SLR: straight leg raise; TT: toe-touch; and PKE: passive knee extension
Confidence in Cumulative Evidence
Confidence in the cumulative evidence is equivalent to the quality of the evidence [35]. GRADE assessments are presented in Additional file 1. For randomized controlled trials, GRADE starts by assuming high quality, which can be downgraded according to five dimensions (risk of bias, inconsistency of results, indirectness, imprecision, and publication bias) [35, 36]. In this study, randomized controlled trials and controlled trials were included and GRADE thus started assuming moderate quality. The quality of evidence was not downgraded for risk of bias but was downgraded due to inconsistency of the results (one level) and indirectness (one level). For GRADE analysis, the following evaluation components were adopted to higher quality (large effect, dose–response, and confounding). Overall, the analysis showed that we can be moderately confident in the effect estimates. This implies that the true effect is likely to be close to the estimate of the effect. Visual inspection of the funnel plot implied no publication bias (Additional file 2: Fig. S1).
Discussion
The aim of this systematic review and meta-analysis was to examine whether there is a difference in the effect of stretching training on flexibility during childhood and adolescence. The main meta-analysis, which included 28 studies and 54 effect sizes, indicated an increase in joint ROM after training in both children and adolescents with a medium magnitude of change (SMD = 0.96, p < 0.001), but no difference between children and adolescents when the effect of stretching volume load was not considered. However, the subgroup analyses showed that higher stretching volume loads result in larger ROM gains only during childhood and not in adolescence.
The main meta-analysis showed an equal increase in ROM in children (6–11 years of age) and adolescents (12–18 years of age), following stretching training. This finding appears to contradict the current suggestions in the pediatric literature regarding a “window of opportunity” for flexibility, i.e., an age range where training responses are maximized [3, 70]. Consequently, it has been suggested that if appropriate training is not performed during this “window,” maximum potential may not be reached [7]. The long-term athlete development model and the youth development model have suggested that middle childhood serves as an important time frame for flexibility development because it incorporates a period that has been termed “critical” for ROM enhancement [4, 8]. Although this suggestion may provide coaches and clinicians with a valuable insight into the components of a successful athletic development program, there is still no conclusive evidence to support this suggestion [1]. This is because evidence regarding ROM improvement following stretching training in children and adolescents is limited and inconsistent [71, 72], despite the fact that flexibility in young athletes is often associated with a higher performance, at least in sports such as gymnastics, swimming, and dance. The results of the current meta-analysis show that flexibility can be developed throughout childhood and adolescence, and there does not appear to be an effect of age on ROM development, at least for the training periods examined in the current systematic review (2–9 months). Along this line, Lloyd et al. [3] recently suggested that the concept of a “window of opportunity” is questionable and that most fitness components are trainable throughout childhood and adolescence, while training should not be considered as more effective in certain ages.
However, the subgroup analyses revealed a very interesting finding, i.e., that higher stretching volume loads result in larger ROM gains only in children and not in adolescents (Fig. 6). In contrast with the lack of difference in ROM improvements between children and adolescents, the interaction of age and stretching volume load seems to suggest that there may be indeed a “window of opportunity” during childhood for flexibility development, provided that the stretching volume load is more than 3600 s. It should be noted that the importance of flexibility is sport specific, and in sports such as gymnastics and dance, athletes are required to perform technical elements requiring large ROM from a very young age (7–9 years old) [72]. Therefore, if it is important to have a large joint ROM, then higher stretching volume loads could be successfully implemented during childhood. This finding warrants further investigation, because of the small number of studies implementing low-volume stretching protocols (i.e., lower than 3600 s) in children. Nevertheless, it was shown that, in childhood, higher training volumes can induce larger ROM gains, a finding possibly associated with the increased pliability and reduced musculotendinous stiffness observed during this period of development which may enable greater ROM to be attained [19]. A recent study found that the greater ankle dorsiflexion in the stretched compared with the control leg after 12 weeks of high-volume stretching training was accompanied by a concomitant increase in resting fascicle length of gastrocnemius medialis, greater fascicle elongation of gastrocnemius medialis and lateralis, and larger increases in gastrocnemius cross-sectional area in female adolescent athletes [64]. There is, however, a paucity of studies that have examined the association between joint ROM and muscle morphology, as well as other factors (i.e., growth, age, sex, training status, and type of joint/muscle examined) affecting flexibility at different developmental ages.
On the other hand, the subgroup analyses showed that in adolescence, higher and lower stretching volume loads both induce similar increases in ROM. The mechanisms associated with the response of children and adolescents to high-volume stretching have not yet been studied. Growth, maturation, muscle and tendon morphology, and neurophysiological differences between children and adolescents may underpin this response [19, 73,74,75]. During puberty, the growth of bones is faster than that of muscles, which can result in reduced muscle–tendon extensibility in postural and biarticular muscles, and substantial limitations on ROM [76,77,78]. In addition, the rise of hormone levels associated with puberty (e.g., testosterone) [79] may affect tendon stiffness and consequently ROM, at least in boys [80]. Since levels of flexibility tend to temporarily plateau or even decrease at the time of the adolescent growth spurt [81], the results of this meta-analysis suggest that higher stretching volume loads may not result in larger ROM gains at this age range. This finding is important because it suggests that the maintenance of the previously acquired levels of flexibility should be the training focus in adolescents for future athletic development [15].
The cutoff value for the stretching volume load in this systematic review (i.e., 3600 s), was determined by calculating the total stretching duration of 10 weeks of training, including three sessions per week, and two sets of two exercises performed for 30 s each. These stretching characteristics are commonly used in sports practice [41]. It should be noted that the two subgroups (“high” and “low” volume load) differed only in the number of exercises per session (two exercises vs. six exercises, p < 0.001), and in the duration of the intervention (8.2 ± 2.7 weeks vs. 18.4 ± 9.5 weeks, p < 0.001), while the number of sets and the frequency of training per week were similar. Thus, the sixfold difference in the mean stretching volume between the two subgroups (2062 vs. 12436 s) (see dataset file/https://doi.org/10.6084/m9.figshare.17104640) was mainly due to the number of exercises per session and the training duration in weeks. The more than twofold training duration of the studies in the “high” subgroup (8.2 weeks vs. 18.4 weeks) may indicate that flexibility is a fitness component that is improved slowly, possibly due to the morphological adaptations that require more time to develop [64]. Although some flexibility gains may be noticed following only a few weeks of training, the large ROM adaptations observed in certain sports such as gymnastics and dance may need several months or even years to occur [43]. In this respect, more evidence is needed regarding the effects of long-term stretching protocols applied throughout childhood and adolescence, which could be a suggestion for future studies. Furthermore, it would be interesting to compare the effects of other types of training, such as strength and eccentric exercises, on ROM at developmental ages [82, 83].
To the best of the authors’ knowledge, this is the first systematic review and meta-analysis to have examined flexibility development during childhood and adolescence despite the importance of flexibility for young athletes. In this systematic review, a robust methodology was implemented [84,85,86], together with well-established tools to assess the quality of the included studies [87]. As indicated by the GRADE analysis, the findings of this meta-analysis are based on studies with a moderate quality of evidence, and thus, we are confident that the true effect is likely to be close to the estimate of the effect. In terms of population, a large sample of children (n = 652) and adolescents (n = 1284) was included in this meta-analysis, and thus, generalization of the findings to the respective populations is possible.
Limitations
One limitation is that in this systematic review no comparisons were made between male and female participants because the studies including both males and females reported collective values for both sexes, with the exception of three studies [29, 52, 53]. Furthermore, no comparisons between athletic and non-athletic populations were performed because in the studies involving primary or secondary school students, extracurricular activities (e.g., sport participation) were not controlled for or were not reported. Finally, most of the included studies examined the hip joint (22 out of 28 studies, see https://doi.org/10.6084/m9.figshare.17104640), while there is a sparsity of information regarding upper limb flexibility.
Conclusions
In conclusion, this meta-analysis indicated that systematic stretching training increases ROM during both childhood and adolescence. This may initially suggest that a “window of opportunity” for flexibility development during childhood which has been widely suggested in the literature is not evident, and flexibility can be developed throughout childhood and adolescence. However, the subgroup analyses showed that higher stretching volume loads result in larger ROM gains only in children and not in adolescents, thus suggesting that the interaction of age and stretching volume load may create a “window of opportunity” during childhood for flexibility development, provided that the stretching volume load is more than 3600 s. In contrast, the lack of a stretching volume load effect in adolescents may be due to the faster linear growth of bones compared with muscles, which may reduce muscle–tendon extensibility in postural and biarticular muscles and induce substantial limitations on ROM [76,77,78]. Thus, during adolescence, flexibility training seems to be independent of stretching volume load. It should be noted that these findings are based on limited evidence from the subgroup analyses, so that future randomized studies examining the effect of different stretching protocols on flexibility enhancement at different stages of development as well as on the factors associated with flexibility in young athletic and non-athletic populations are needed.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the Figshare data repository file (https://doi.org/10.6084/m9.figshare.17104640).
Abbreviations
- ROM:
-
Range of motion
- GRADE:
-
Grading of recommendations: assessment: development and evaluations
- RoB:
-
Risk of bias
- CTs:
-
Controlled trials
- RCTs:
-
Randomized controlled trials
- SMD:
-
Standardized mean difference
- CI:
-
Confidence interval
- I2 :
-
I2 statistic describes the percentage of variation across studies that is due to heterogeneity rather than chance
References
Pichardo AW, Oliver JL, Harrison CB, et al. Integrating models of long-term athletic development to maximize the physical development of youth. Int J Sports Sci Coach. 2018;13(6):1189–99. https://doi.org/10.1177/1747954118785503.
Smith JJ, Eather N, Morgan PJ, et al. The health benefits of muscular fitness for children and adolescents: a systematic review and meta-analysis. Sports Med. 2014;44(9):209–1223. https://doi.org/10.1007/s40279-014-0196-4.
Lloyd RS, Oliver JL, Faigenbaum AD, et al. Long-term athletic development, part 2: barriers to success and potential solutions. J Strength Cond Res. 2015;29(5):1451–64. https://doi.org/10.1519/01.JSC.0000465424.75389.56.
Lloyd RS, Oliver JL, Faigenbaum AD, et al. Long-term athletic development-part 1: a pathway for all youth. J Strength Cond Res. 2015;29(5):1439–50. https://doi.org/10.1519/JSC.0000000000000756.
Meyers RW, Oliver JL, Hughes MG, et al. New insights into the development of maximal sprint speed in male youth. J Strength Cond. 2017;39(2):2–10. https://doi.org/10.1519/SSC.0000000000000290.
Lesinski M, Prieske O, Granacher U. Effects and dose–response relationships of resistance training on physical performance in youth athletes: a systematic review and meta-analysis. Br J Sports Med. 2016;50(13):781–95. https://doi.org/10.1136/bjsports-2015-095497.
Balyi I, Hamilton A. Long-term athlete development: trainability in childhood and adolescence. Olymp Coach. 2004;16(1):4–9.
Lloyd RS, Oliver JL. The youth physical development model: a new approach to long-term athletic development. J Strength Cond. 2012;34(3):61–72. https://doi.org/10.1519/SSC.0b013e31825760ea.
Magnusson SP, Renström P. The European college of sports sciences position statement: the role of stretching exercises in sports. Eur J Sport Sci. 2006;6:87–91. https://doi.org/10.1080/17461390600617865.
Sands W, McNeal J. Mobility and flexibility training for young athletes. In: Strength and conditioning for young athletes. Routledge, 2019, p. 265–275.
Heyward VH. Designs for fitness: a guide to physical fitness appraisal and exercise prescription. Minneapolis: Burgess Publishing Company; 1984.
Donti O, Bogdanis GC, Kritikou M, et al. The relative contribution of physical fitness to the technical execution score in youth rhythmic gymnastics. J Hum Kinet. 2016;51(1):143–52. https://doi.org/10.1515/hukin-2015-0183.
Douda HT, Toubekis AG, Avloniti AA, et al. Physiological and anthropometric determinants of rhythmic gymnastics performance. Int J Sports Physiol Perform. 2008;3(1):41–54. https://doi.org/10.1123/ijspp.3.1.41.
Drabik J. Children and sports training. Island Pond, VT: Stadion Publishing Company; 1996.
Falsone S. Optimising flexibility. UD Joyce, D Lewindon (ur.) High-performance training for sports, 2014, p. 61–70.
Wilk KE, Meister K, Fleisig G, et al. Biomechanics of the overhead throwing motion. Sports Med Arthrosc Rev. 2000;8(2):124–34.
Cejudo A, Sánchez-Castillo S, de Baranda PS, et al. Low range of shoulders horizontal abduction predisposes for shoulder pain in competitive young swimmers. Front Psychol. 2019;10:478. https://doi.org/10.3389/fpsyg.2019.00478.
Møller M, Nielsen RO, Attermann J, et al. Handball load and shoulder injury rate: a 31-week cohort study of 679 elite youth handball players. Br J Sports Med. 2017;51(4):231–7. https://doi.org/10.1136/bjsports-2016-096927.
Kubo K, Kanehisa H, Kawakami Y, et al. Growth changes in the elastic properties of human tendon structures. Int J Sports Med. 2001;22(02):138–43. https://doi.org/10.1055/s-2001-11337.
Corbin CB, Noble L. Flexibility: a major component of physical fitness. J Phys Educ Recreat. 1980;51(6):23–60. https://doi.org/10.1080/00971170.1980.10622349.
Feldman D, Shrier I, Rossignol M, et al. Adolescent growth is not associated with changes in flexibility. Clin J Sport Med. 1999;9:24–9. https://doi.org/10.1097/00042752-199901000-00005.
Malina RM, Bouchard C, Bar-Or O. Growth, maturation, and physical activity. Champaign: Human Kinetics; 2004.
Van Hooren B, Croix MDS. Sensitive periods to train general motor abilities in children and adolescents: Do they exist? A critical appraisal. Strength Cond J. 2020;42(6):7–14. https://doi.org/10.1519/SSC.0000000000000545.
Malina RM, Geithner CA, O’Brien R, Tan SK. Sex differences in the motor performances of elite young divers. Ital J Sport Sci. 2005;2005(12):18–23.
Beunen GP, Malina RM, Van’t Hof MA, et al. Adolescent growth and motor performance: A longitudinal study of Belgian boys. Champaign: Human Kinetics Publishers; 1988.
Kay AD, Blazevich AJ. A systematic review of the effects of acute static stretch on maximal muscular performance. In: 16th Annual congress of the European college of sport science (ECSS), 2011, July.
Cipriani D, Abel B, Pirrwitz D. A comparison of two stretching protocols on hip range of motion: implications for total daily stretch duration. J Strength Cond Res. 2003;17(2):274–8. https://doi.org/10.1519/1533-4287(2003)017%3c0274:acotsp%3e2.0.co;2.
Freitas SR, Vilarinho D, Vaz JR, et al. Responses to static stretching are dependent on stretch intensity and duration. Clin Physiol Funct Imaging. 2015;35(6):478–84. https://doi.org/10.1111/cpf.12186.
Coledam DHC, Arruda GAD, Oliveira ARD. Chronic effect of static stretching performed during warm-up on flexibility in children. Rev Bras Cineantropometria Desempenho Hum. 2012;14(3):296–304. https://doi.org/10.5007//1980-0037.2012v14n3p296.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. 2021;372: n71. https://doi.org/10.1136/bmj.n71.
Mayorga-Vega D, Bocanegra-Parrilla R, Ornelas M, et al. Criterion-related validity of the distance-and time-based walk/run field tests for estimating cardiorespiratory fitness: a systematic review and meta-analysis. PLOS ONE. 2016;11(3): e0151671. https://doi.org/10.1371/journal.pone.0151671.
Greenhalgh T, Peacock R. Effectiveness and efficiency of search methods in systematic reviews of complex evidence: audit of primary sources. Br Med J. 2005;331(7524):1064–5.
Higgins JPT, Green S (eds.) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration (2011), http://handbook.cochrane.org, Accessed 28th Sep 2018
Centre TNC. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen. The Cochrane Collaboration. 2014.
Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Review of Interventions version 6.2 (updated February 2021) In: Cochrane, ed.
Schünemann H, Brożek J, Guyatt G, et al. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach, 2013.
Logan SW, Webster EK, Getchell N, et al. Relationship between fundamental motor skill competence and physical activity during childhood and adolescence: a systematic review. Kinesiol Rev. 2015;4(4):416–26. https://doi.org/10.1123/kr.2013-0012.
Cattuzzo MT, dos Santos HR, Ré AHN, et al. Motor competence and health related physical fitness in youth: a systematic review. J Sci Med Sport. 2006;19:123. https://doi.org/10.1016/j.jsams.2014.12.004.
Berk LE, Petersen A. Development through the lifespan. Boston: Allyn and Bacon; 2004. p. 667.
Gu X, Thomas KT, Chen YL. The role of perceived and actual motor competency on children’s physical activity and cardiorespiratory fitness during middle childhood. J Teach Phys Educ. 2017;36(4):388–97. https://doi.org/10.1123/jtpe.2016-0192.
Simenz CJ, Dugan CA, Ebben WP. Strength and conditioning practices of National Basketball Association strength and conditioning coaches. J Strength Cond Res. 2005;19(3):495–504.
Hopkins WG, Marshall SW, Batterham AM, et al. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009;41(1):3–13. https://doi.org/10.1249/MSS.0b013e31818cb278.
Donti Ο, Papia K, Toubekis A, et al. Flexibility training in preadolescent female athletes: acute and long-term effects of intermittent and continuous static stretching. J Sports Sci. 2018;36(13):1453–60. https://doi.org/10.1080/02640414.2017.1397309.
Donti O, Papia K, Toubekis A, et al. Αcute and long-term effects of two different static stretching training protocols on range of motion and vertical jump in preadolescent athletes. Biol Sport. 2021;38(4):579–86. https://doi.org/10.5114/biolsport.2021.101127.
Hill GM, Najera W. Effect of a two-sessions-per-week stretching program on hamstring extensibility in Latino High School students. J Phys Educ. 2020;77(3):455–63. https://doi.org/10.18666/TPE-2020-V77-I3-10042.
Sermaxhaj S, Arifi F, Bahtiri A, et al. The impact of recuperation with static stretching in flexibility and agility with and without ball of young soccer players. Acta Kinesiol. 2017;11(1):33–8.
Sermaxhaj S, Arifi F, Havolli J, et al. The effect of physical exercise according to a programme for the development of flexibility in the motor abilities of young football players. Sport Mont. 2021;19(1):25–9.
Azuma N, Someya F. Injury prevention effects of stretching exercise intervention by physical therapists in male high school soccer players. Scand J Med Sci Sports. 2020;30(11):2178–92. https://doi.org/10.1111/sms.13777.
Becerra-Fernández C, Merino-Marban R, Mayorga-Vega D. Effect of a physical education-based dynamic stretching program on hamstring extensibility in female high-school students. Kinesiol. 2016;48(2):258–66.
de Baranda-Andújar P. El trabajo de la flexibilidad en educación física: Programa de intervención. Cult Cienc y Deporte. 2019;4(10).
Hadjicharalambous M. The effects of regular supplementary flexibility training on physical fitness performance of young high-level soccer players. J Sports Med Phys Fitness. 2015;56(6):699–708.
Kamandulis S, Emeljanovas A, Skurvydas A. Stretching exercise volume for flexibility enhancement in secondary school children. J Sports Med Phys Fitness. 2013;53(6):687–92.
Knapik DM, LaTulip S, Salata MJ, et al. Impact of routine gastrocnemius stretching on ankle dorsiflexion flexibility and injury rates in high school basketball athletes. Orthop J Sports Med. 2019;7(4):2325967119836774. https://doi.org/10.1177/2325967119836774.
Mayorga-Vega D, Merino-Marban R, Real J, et al. A physical education-based stretching program performed once a week also improves hamstring extensibility in schoolchildren: a cluster-randomized controlled trial. Nutr Hosp. 2015;32(4):1715–21. https://doi.org/10.3305/nh.2015.32.4.9302.
Mayorga-Vega D, Merino-Marban R, Redondo-Martín FJ, et al. Effect of a one-session-per-week physical education-based stretching program on hamstring extensibility in schoolchildren. Kinesiol. 2017;49(1):101–8.
Mayorga-Vega D, Merino-Marban R, Manzano-Lagunas J, et al. Effects of a stretching development and maintenance program on hamstring extensibility in schoolchildren: a cluster-randomized controlled trial. J Sci Med Sport. 2016;15(1):65.
Mayorga-Vega D, Merino-Marban R, Garrido FJ, et al. Comparison between warm-up and cool-down stretching programs on hamstring extensibility gains in primary schoolchildren. Phys Act Rev. 2014;2:16–24.
Mayorga-Vega D, Merino-Marban R, Sánchez-Rivas E, et al. Effect of a short-term static stretching training program followed by five weeks of detraining on hamstring extensibility in children aged 9–10 years. J Phys Educ Sport. 2014;14(3):355. https://doi.org/10.7752/jpes.2014.03054.
Mayorga Vega D, Merino Marban R, Vera Estrada F, et al. Effect of a short-term physical education-based flexibility program on hamstring and lumbar extensibility and its posterior reduction in primary schoolchildren. Kinesiol. 2014;46(2):227–33.
Santonja-Medina F, De Baranda PS, García Rodriguez P, et al. Effects of frequency of static stretching on straight-leg raise in elementary school children. J Sports Med Phys Fitness. 2007;47(3):304–8.
Merino-Marban R, Mayorga-Vega D, Fernandez-Rodriguez E, et al. Effect of a physical education-based stretching programme on sit-and-reach score and its posterior reduction in elementary schoolchildren. Eur Phy Educ Rev. 2015;21(1):83–92. https://doi.org/10.1177/1356336X14550942.
Moreira RFC, Akagi FH, Wun PYL, et al. Effects of a school based exercise program on children’s resistance and flexibility. Work. 2012;41(Supplement 1):922–8. https://doi.org/10.3233/WOR-2012-0264-922.
Nelson RT, Bandy WD. Eccentric training and static stretching improve hamstring flexibility of high school males. J Athl Train. 2004;39(3):254.
Panidi I, Bogdanis GC, Terzis G, et al. Muscle architectural and functional adaptations following 12-weeks of stretching in adolescent female athletes. Front Physiol. 2021. https://doi.org/10.3389/fphys.2021.701338.
Piqueras-Rodríguez F, Palazón-Bru A, Gil-Guillén VF. Effectiveness analysis of active stretching versus active stretching plus low-frequency electrical stimulation in children who play soccer and who have the short hamstring syndrome. Clin J Sport Med. 2016;26(1):59–68. https://doi.org/10.1097/JSM.0000000000000188.
Rodríguez PL, Santonja FM, López-Miñarro PA, et al. Effect of physical education stretching programme on sit-and-reach score in schoolchildren. Sci Sports. 2008;23(3–4):170–5. https://doi.org/10.1016/j.scispo.2007.12.013.
Sánchez Rivas E, Mayorga-Vega D, Fernández Rodríguez E, et al. Effect of a hamstring stretching programme during physical education lessons in primary education. J Sport Health Res. 2014;6(2):159–68.
Reid DA, McNair PJ. Passive force, angle, and stiffness changes after stretching of hamstring muscles. Med Sci Sports Exerc. 2004;36(11):1944–8. https://doi.org/10.1249/01.mss.0000145462.36207.20.
Van Rensburg LJ, Coetzee FF. Effect of stretching techniques on hamstring flexibility in female adolescents. Afr J Phys Health Edu Recreat Dance. 2014;20:1237–48.
Lloyd RS, Cronin JB, Faigenbaum AD, et al. National strength and conditioning association position statement on long-term athletic development. J Strength Cond Res. 2016;30:1491–509. https://doi.org/10.1519/JSC.0000000000001387.
Jagomägi G, Jürimäe T. The influence of anthropometrical and flexibility parameters on the results of breaststroke swimming. Anthropol Anz. 2005;63:213–9.
Sands WA, McNeal JR, Penitente G, et al. Stretching the spines of gymnasts: a review. Sports Med. 2016;46(3):315–27.
Ratel S, Kluka V, Vicencio SG, et al. Insights into the mechanisms of neuromuscular fatigue in boys and men. Med Sci Sports Exerc. 2015;47:2319–28. https://doi.org/10.1249/MSS.0000000000000697.
Lexell J, Sjostrom M, Nordlund AS, et al. Growth and development of human muscle: a quantitative morphological study of 1484 S. Ratel, A. J. Blazevich 123 whole vastus lateralis from childhood to adult age. Muscle Nerve. 1992;15:404–9. https://doi.org/10.1002/mus.880150323.
Dotan R, Mitchell C, Cohen R, et al. Child—adult differences in muscle activation—a review. Pediatr Exerc Sci. 2012;24(1):2–21. https://doi.org/10.1123/pes.24.1.2.
Tanner J. Issues and advances in adolescent growth and development. J Adolesc Health Care. 1987;8:470–8. https://doi.org/10.1016/0197-0070(87)90048-9.
Philippaerts R, Vaeyens R, Janssens M, et al. The relationship between peak height velocity and physical performance in youth soccer players. J Sports Sci. 2006;24:221–30. https://doi.org/10.1080/02640410500189371.
Robles-Palazón F, Ayala F, Cejudo A, et al. Effects of age and maturation on lower extremity range of motion in male youth soccer players. J Strength Cond Res. 2020. https://doi.org/10.1519/JSC.0000000000003642.
Forbes H, Bullers A, Lovell A, et al. Relative torque profiles of elite male youth footballers: Effects of age and pubertal development. Int J Sports Med. 2009;30:592–7. https://doi.org/10.1055/s-0029-1202817.
Hansen M, Kjaer M. Sex Hormones and Tendon. In: Ackermann P, Hart D, editors. Metabolic influences on risk for tendon disorders: advances in experimental medicine and biology, vol. 920. Cham: Springer; 2016. https://doi.org/10.1007/978-3-319-33943-6_13.
Malina RM. Growth, maturation, and development: applications to young athletes and in particular to divers. In: Malina RM, Gabriel JL, editors. USA diving coach development reference manual. Indianapolis: USA Diving; 2007. p. 3–29.
Morton SK, Whitehead JR, Brinkert RH, et al. Resistance training vs static stretching: effects on flexibility and strength. J Strength Cond Res. 2011;25(12):3391–8. https://doi.org/10.1519/JSC.0b013e31821624aa.
O’Sullivan K, McAuliffe S, DeBurca N. The effects of eccentric training on lower limb flexibility: a systematic review. Br J Sports Med. 2012;46:838–45. https://doi.org/10.1136/bjsports-2012-091530.
Khan KS, Kunz R, Kleijnen J, et al. Five steps to conducting a systematic review. J R Soc Med. 2003;96(3):118–21.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA Statement for reporting reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. https://doi.org/10.1016/j.jclinepi.2009.06.006.
Harris JD, Quatman CE, Manring MM, et al. How to write a systematic review. Am J Sports Med. 2014;42(11):2761–8. https://doi.org/10.1177/0363546513497567.
Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. Br Med J. 2011;343:5928. https://doi.org/10.1136/bmj.d5928.
Acknowledgements
Not applicable.
Funding
Open-access funding was provided by a grant (Project P 32078-B) from the Austrian Science Fund (FWF). This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
GCB, OD, and AK formed the concept of the study; OD, IP, AK, and GCB performed the literature search; OD, IP, AK, PCD, and GCB performed the data extraction. PCD, OD, IP, and GCB contributed to the data extraction and meta-analysis calculations. IP, OD, and AK assessed the risk of bias; IP, OD, and PCD performed the GRADE analysis; and OD and GCB drafted the manuscript. All authors were involved in the interpretation of the meta-analyses and read, revised, and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Olyvia Donti, Andreas Konrad, Ioli Panidi, Petros Dinas and Gregory Bogdanis declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Search algorithm, risk of bias assessment for randomized controlled trials and control trials, Grading of Recommendations, Assessment, Development and Evaluations (GRADE) analysis and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.
Additional file 2
. Funnel plot for the meta-analysis of the effects of static stretching training on range of motion.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Donti, O., Konrad, A., Panidi, I. et al. Is There a “Window of Opportunity” for Flexibility Development in Youth? A Systematic Review with Meta-analysis. Sports Med - Open 8, 88 (2022). https://doi.org/10.1186/s40798-022-00476-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40798-022-00476-1