Abstract
Background
Malnutrition and sickle cell anemia (SCA) result in high childhood mortality rates. Although maternal depression is an established risk factor for malnutrition in younger children, little is known about its impact on treatment response in children with malnutrition. We aimed to determine the relationship, if any, between maternal depression scores and malnutrition treatment outcomes in older children with SCA.
Methods
We conducted a planned ancillary study to our randomized controlled feasibility trial for managing severe acute malnutrition in children aged 5–12 with SCA in northern Nigeria (NCT03634488). Mothers of participants completed a depression screen using the Patient Health Questionnaire (PHQ-9).We used a multivariable linear regression model to describe the relationship between the baseline maternal PHQ-9 score and the trial participant’s final body mass index (BMI) z-score.
Results
Out of 108 mother-child dyads, 101 with maternal baseline PHQ-9 scores were eligible for inclusion in this analysis. At baseline, 25.7% of mothers (26 of 101) screened positive for at least mild depression (PHQ-9 score of 5 or above). The baseline maternal PHQ-9 score was negatively associated with the child’s BMI z-score after 12 weeks of malnutrition treatment (β=-0.045, p = 0.041).
Conclusions
Maternal depressive symptoms has an impact on malnutrition treatment outcomes. Treatment of malnutrition in older children with sickle cell anemia should include screening for maternal depression and, if indicated, appropriate maternal referral for depression evaluation and treatment.
Trial Registration
The trial was registered at clinicaltrials.gov (#NCT03634488) on January 30, 2018, https://clinicaltrials.gov/study/NCT03634488.
Similar content being viewed by others
Background
Malnutrition is an ongoing global public health concern, with sub-Saharan Africa accounting for 33% of all malnourished children globally [1]. Children with sickle cell anemia (SCA) are at an increased risk of malnutrition due to their high protein turnover rate, resulting in higher daily nutrient requirements [2,3,4]. Sub-Saharan Africa is home to over 75% of all newborns with SCA, with Nigeria accounting for over 30% of children born with SCA worldwide [5]. Children with SCA and malnutrition are at a higher risk for hospitalization [6] and mortality [7]. Addressing the double burden of malnutrition and SCA is crucial for decreasing childhood mortality, especially in sub-Saharan Africa, where the majority of affected children reside.
Maternal mental health, particularly depression, is an established risk factor for childhood malnutrition during the antenatal and postpartum periods [8,9,10,11,12,13]. Maternal depression is critical not only in the peripartum period but also as the child grows into adolescence and young adulthood. Maternal depression significantly impacts the health of older children and is commonly associated with reduced healthcare seeking [14], increased childhood illnesses [15], and poorer child diet quality [16]. Strong evidence indicates parents of children with SCA have a higher prevalence of depressive symptoms [17, 18], and mothers, in particular, are more likely to experience a lower quality of life [19].
Building on the prior evidence of the influence of maternal depression on the development of malnutrition, we tested the hypothesis that participants in a malnutrition trial whose mothers score higher on the depression screening tool, the Patient Health Questionnaire-9 (PHQ-9) [20], will experience less improvement in their body mass index (BMI) z-scores following treatment for malnutrition.
Methods
Study design
The planned ancillary study was completed as a prospective cohort study within a randomized controlled feasibility trial for managing severe acute malnutrition in children with SCA conducted in Nigeria (SAMS trial, NCT03634488) [21]. The SAMS trial included participants aged 5–12 years old with laboratory-confirmed SCA (HbSS or HbS-beta0 thalassemia) and uncomplicated severe acute malnutrition, as determined by a BMI z-score <-3.0 according to the World Health Organization growth reference [22]. Participants were recruited from Aminu Kano Teaching Hospital and Murtala Mohammed Specialist Hospital in Kano, Nigeria. Participants were randomly allocated to receive either age-based supplemental ready-to-use therapeutic food (RUTF; 500-1,000 daily calories) alone or a combination of RUTF and moderate fixed-dose hydroxyurea (20 mg/kg per day) for a 12-week treatment period. A total of 108 participants completed the trial. The main findings from the SAMS trial have been previously published [21]. This planned ancillary study and the main SAMS trial adhere to CONSORT reporting guidelines [23].
The Institutional Review Boards at Aminu Kano Teaching Hospital, Murtala Mohammed Specialist Hospital, and Vanderbilt University Medical Center approved the SAMS trial. All participants’ legal guardians provided written informed consent before screening and enrollment, and children over seven years of age provided assent. The study activities were carried out in accordance with the principles of the Declaration of Helsinki.
Study sample
Within the cohort of participants who completed the SAMS trial (n = 108), our analysis focused on mother-child dyads where the mothers underwent baseline depression screening utilizing the PHQ-9. The exclusion criteria were primary caregivers who were not mothers, mothers of participants who did not complete the 12-week trial, and mothers who did not complete a PHQ-9 assessment at their initial visit. We decided to limit the PHQ-9 score analysis to only mothers to assess the impact of maternal depression on malnutrition treatment. This decision was based on the current body of evidence, which predominantly examines the correlation between maternal depression and nutritional status [8,9,10].
Data collection
During the 12-week SAMS trial, participants presented to the clinic once for screening, followed by a visit to evaluate for refeeding within 5 days of study initiation, then every four weeks. At the initial visit, baseline medical history, demographics, physical examinations, and anthropometric measurements were conducted. Subsequently, at each follow-up visit, interim illness history, physical examinations, and anthropometric measurements were systematically performed per the study protocol [21]. Head of household education was used as a proxy for socioeconomic status.
In addition, at study enrollment and every subsequent clinic visit, the mother of the trial participant was assessed for depression using the PHQ-9, a publicly available depression screening tool [20, 24]. Each of the 9 criteria for depression per the Diagnostic and Statistical Manual of Mental Disorders: DSM-5 is evaluated on a scale of 0 (not at all) to 3 (nearly every day) [20]. The PHQ-9 can be scored as either a continuous variable, ranging from 0 to 27, where higher scores represent more severe depression, or can be interpreted categorically [24]. The PHQ-9 scores are categorized based on the severity of the depressive symptoms and are organized as follows: minimal (score of 0–4), mild (score of 5–9), moderate (score of 10–14), moderately severe (score of 15–19) or severe (score of 20–27) [20]. Additionally, the PHQ-9 has previously been validated to accurately identify individuals with depression in Nigeria [25]. Research staff were trained in the pre-trial period to administer the PHQ-9 verbally in Hausa, the most common local language. Translated versions of PHQ-9 have been previously validated in sub-Saharan African communities with consistent psychometric properties [26, 27]. The PHQ-9 yields similar results when administered verbally compared to when self-administered [28]. Mothers were referred to a local psychiatrist if their PHQ-9 score reached 9 or above during any clinic visit throughout the SAMS trial, as per the study protocol and prearranged agreement between the study team and the psychiatrist.
Data analysis
Data were collected and managed with Research Electronic Data Capture (REDCap) hosted at Vanderbilt University Medical Center [29, 30]. We summarized continuous variables as means and standard deviations or as medians and interquartile ranges for variables not normally distributed. Categorical variables and prevalence were reported as counts and percentages. For comparisons between groups, a chi-square test or Fisher’s exact test was used for percentages, a t-test for means, and a Mann-Whitney U test for medians. A Pearson correlation was used to assess the relationship between baseline PHQ-9 scores and change in BMI z-scores. We used a linear regression model with covariates in the final model from the SAMS trial to study whether PHQ-9 scores at baseline were associated with the final BMI z-score. We used a two-sided P value of < 0.05 as evidence of a significant result for our a priori hypotheses. SPSS version 29.0.1 (IBM, Armonk, NY) was used for analysis.
Results
Baseline characteristics and depression scores for mother-child dyads
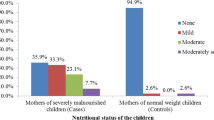
Among the 108 participants who completed the SAMS trial, 93.5% (101 mother-child dyads) met inclusion criteria, with exclusions based on missing baseline maternal PHQ-9 scores (n = 4) and the primary caregiver not being the mother (n = 3). Almost all mothers with known marital status (93.8%, n = 91 of 97) were married, with a median of 6 (IQR: 4.0–8.0) children in the household. The median PHQ-9 score at baseline for the whole cohort was 3 (IQR: 1–5). At baseline, 24.8% of mothers (n = 25) screened positive for mild depression (score of 5–9), and 1.0% of mothers (n = 1) screened positive for moderate depression (score of 10–15). Otherwise, there were no significant differences in baseline demographic and clinical information between mother-child dyads by whether mothers had a baseline PHQ-9 score of 5 or above (Table 1).
There was no significant association between baseline maternal depression score and baseline child anthropometrics.
All children were severely wasted per study criteria with a mean baseline BMI z-score of -3.7. There was also no significant difference between baseline BMI, weight-for-age, and height-for-age z-scores between women with or without at least mild baseline depression scores (PHQ-9 score of 5 or above; Table 2).
Higher maternal depression scores at the study baseline predict lower BMI z-scores at the study endpoint.
Higher levels of baseline maternal depression were associated with a smaller change in BMI z-scores over the 12-week trial period (r=-0.26, p = 0.009; Fig. 1). We then constructed a multivariable linear regression model for the final BMI z-score, including the maternal baseline PHQ-9 score and the previously identified significant covariates from the SAMS trial (child’s age and baseline BMI z-score) [21]. In the multivariable model, the baseline PHQ-9 score was associated with the final BMI z-score (β=-0.045, p = 0.041, Table 3; Fig. 2), and the baseline BMI z-score remained significantly associated with the final BMI z-score (β = 0.666, p < 0.001), while age was no longer significantly associated with the final BMI z-score (β=-0.041, p = 0.125).
Discussion
Maternal depression is an established risk factor for infant-child malnutrition. To our knowledge, for the first time, we demonstrate that the higher a mother’s depressive screening score, the lower her child’s BMI z-score at the end of malnutrition treatment. The baseline maternal depressive symptoms influence the extent of this improvement. Our findings highlight the complex interplay between maternal depressive symptoms and the outcomes of malnutrition treatment in older children with SCA.
Limited studies have explored the effects of maternal mental health on the outcomes of malnutrition treatment in children. For instance, a cross-sectional study conducted seven years after malnutrition treatment failed to establish a significant link between maternal mental health and the current nutritional status of the children [31]. Similarly, another study focused on maternal distress during admission for nutritional rehabilitation found no significant association with child weight gain at a 4-week post-discharge follow-up [32]. Methodological variations, such as differences in follow-up times, could potentially account for the divergent findings in these studies compared to ours. The assessment of maternal distress during inpatient admission may have primarily captured acute distress related to the admission itself because maternal distress levels appear to be consistent regardless of whether the child was admitted for nutritional rehabilitation or other reasons [33].
Observational studies linking maternal depression to the development of childhood malnutrition support our finding of the impact of maternal depression on malnutrition treatment outcomes. Children under 5 years old of mothers with depression are more likely to develop malnutrition [10, 13, 34, 35]. In Nigeria, length and weight were lower in the first 6 months of life in children of depressed mothers compared to children of non-depressed mothers [36]. This phenomenon can be at least partially explained by attachment theory, suggesting that a mother’s depressive symptoms may hinder her capacity to respond to her child’s cues, thus affecting both the child’s physical and emotional needs [34, 37, 38]. Maternal depression has also been linked to suboptimal childhood feeding practices [38,39,40]. Together, these observations underscore the importance of addressing maternal depression as a crucial factor in improving the nutritional status of children.
Limitations of this prospective cohort study, performed as a planned ancillary study to a randomized controlled feasibility trial, include a new onset international conflict that may have limited the food to northern Nigeria, decreasing the response to malnutrition treatment [41,42,43]. The trial was not designed to test the hypothesis that maternal depression affects the response to malnutrition treatment; thus, our results should be considered hypothesis-generating. We did not evaluate the father’s depression score, which may contribute to the change in the BMI z-score of the trial participant. However, a priori, we had no evidence that the father’s PHQ-9 score would be associated with treatment response during the trial. Additionally, we did not have a measure of maternal education, which impact should be further explored in future studies. Strengths of our study include the high retention rate, detailed data on the children’s response to the interventions, and the innovative approach in examining maternal depressive symptoms as a novel risk factor for suboptimal response to malnutrition treatment.
Conclusions
In children over 5 years old with SCD in northern Nigeria, a higher screening maternal depression score at the start of malnutrition therapy leads to less improvement in a child’s nutritional status. Based on the results of this study, we have implemented maternal depression screening as standard of care for routine visits in our evaluation of children with uncomplicated severe acute malnutrition. Higher PHQ-9 scores in mothers of children with uncomplicated severe acute malnutrition are further evaluated to determine the mother’s mood and whether she would be amenable to a referral for a formal evaluation.
Data availability
The deidentified individual participant data analyzed during this study are available upon request from the corresponding author until 2028, approximately 5 years after publication. After 2028, institutional resources may not be available to provide the data. Requests for data should be directed to lauren.klein@vumc.org, and requestors will need to prepare and sign a data transfer agreement between Vanderbilt University Medical Center and their respective institutions.
Abbreviations
- BMI:
-
Body mass index
- DSM-5:
-
Diagnostic and Statistical Manual of Mental Disorders
- PHQ-9:
-
Patient Health Questionnaire 9
- REDCap:
-
Research Electronic Data Capture
- RUTF:
-
Ready-to-use therapeutic food
- SCA:
-
Sickle cell anemia
References
Akombi BJ, Agho KE, Merom D, Renzaho AM, Hall JJ. Child malnutrition in sub-saharan Africa: a meta-analysis of demographic and health surveys (2006–2016). PLoS ONE. 2017;12(5):e0177338.
Hyacinth HI, Adekeye OA, Yilgwan CS. Malnutrition in Sickle Cell Anemia: implications for infection, growth, and Maturation. J Soc Behav Health Sci. 2013;7(1). https://doi.org/10.5590/JSBHS.2013.07.1.02.
Nartey EB, Spector J, Adu-Afarwuah S, Jones CL, Jackson A, Ohemeng A, et al. Nutritional perspectives on sickle cell disease in Africa: a systematic review. BMC Nutr. 2021;7(1):9.
Hibbert JM, Creary MS, Gee BE, Buchanan ID, Quarshie A, Hsu LL. Erythropoiesis and Myocardial Energy Requirements Contribute to the hypermetabolism of Childhood Sickle Cell Anemia. J Pediatr Gastroenterol Nutr. 2006;43(5):680–7.
Joint WHO-March of Dimes Meeting on Management of Birth Defects and Haemoglobin Disorders (2nd: 2006: Geneva S, World Health Organization, March of Dimes. Management of birth defects and haemoglobin disorders: report of a joint WHO-March of Dimes meeting, Geneva, Switzerland, 17–19. May 2006. 2006;27.
Cox SE, Makani J, Fulford AJ, Komba AN, Soka D, Williams TN, et al. Nutritional status, hospitalization and mortality among patients with sickle cell anemia in Tanzania. Haematologica. 2011;96(7):948–53.
Klein LJ, Abdullahi SU, Gambo S, Stallings VA, Acra S, Rodeghier M, et al. Underweight children older than 5 years with sickle cell anemia are at risk for early mortality in a low-resource setting. Blood Adv. 2022;7(11):2339–46.
Ashaba S, Rukundo GZ, Beinempaka F, Ntaro M, LeBlanc JC. Maternal depression and malnutrition in children in southwest Uganda: a case control study. BMC Public Health. 2015;15(1):1303.
Nguyen PH, Friedman J, Kak M, Menon P, Alderman H. Maternal depressive symptoms are negatively associated with child growth and development: Evidence from rural India. Matern Child Nutr. 2018 [cited 2020 Aug 29];14(4). https://doi.org/10.1111/mcn.12621.
Pierce M, Hope HF, Kolade A, Gellatly J, Osam CS, Perchard R, et al. Effects of parental mental illness on children’s physical health: systematic review and meta-analysis. Br J Psychiatry. 2020;217(1):354–63.
Mohammedahmed A, Koko A, Arabi A, Ibrahim M. Maternal depression, a hidden predictor for severe acute malnutrition in children aged 6–59 months: a case-control study at Omdurman Paediatrics Teaching Hospital, Sudan. Sudan J Paediatr. 2020;111–21.
Haithar S, Kuria MW, Sheikh A, Kumar M, Vander Stoep A. Maternal depression and child severe acute malnutrition: a case-control study from Kenya. BMC Pediatr. 2018;18(1):289.
Surkan PJ, Kennedy CE, Hurley KM, Black MM. Maternal depression and early childhood growth in developing countries: systematic review and meta-analysis. Bull World Health Organ. 2011;89(8):607–15.
Minkovitz CS, Strobino D, Scharfstein D, Hou W, Miller T, Mistry KB, et al. Maternal depressive symptoms and children’s receipt of Health Care in the first 3 years of life. Pediatrics. 2005;115(2):306–14.
Anderson LC, Tegegn A, Tessema F, Galea S, Hadley C. Food insecurity, childhood illness and maternal emotional distress in Ethiopia. Public Health Nutr. 2012;15(4):648–55.
Thompson AL, Jahnke JR, Teran E, Bentley ME. Pathways linking maternal mental health and child health in a dual burden context: evidence from Galapagos, Ecuador. Soc Sci Med. 2022;305:115043.
Sil S, Woodward KE, Johnson YL, Dampier C, Cohen LL. Parental psychosocial distress in Pediatric Sickle Cell Disease and Chronic Pain. J Pediatr Psychol. 2021;46(5):557–69.
Moskowitz JT, Butensky E, Harmatz P, Vichinsky E, Heyman MB, Acree M, et al. Caregiving time in sickle cell disease: psychological effects in maternal caregivers. Pediatr Blood Cancer. 2007;48(1):64–71.
van den Tweel XW, Hatzmann J, Ensink E, van der Lee JH, Peters M, Fijnvandraat K, et al. Quality of life of female caregivers of children with sickle cell disease: a survey. Haematologica. 2008;93(4):588–93.
Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med. 2001;16(9):606–13.
Abdullahi SU, Gambo S, Murtala HA, Kabir H, Shamsu KA, Gwarzo G, et al. Feasibility trial for the management of severe acute malnutrition in older children with sickle cell anemia in Nigeria. Blood Adv. 2023;7(20):6024–34.
Onis MD, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007 Sep;85(9):660–7. https://doi.org/10.2471/BLT.07.043497.
Schulz KF, Altman DG, Moher D, the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Trials. 2010;11(1):32.
Kroenke K, Wu J, Yu Z, Bair MJ, Kean J, Stump T, et al. Patient health questionnaire anxiety and Depression Scale: initial validation in three clinical trials. Psychosom Med. 2016;78(6):716–27.
Adewuya AO, Ola BA, Afolabi OO. Validity of the patient health questionnaire (PHQ-9) as a screening tool for depression amongst Nigerian university students. J Affect Disord. 2006;96(1):89–93.
Omoro SAO, Fann JR, Weymuller EA, MacHaria IM, Yueh B. Swahili Translation and Validation of the Patient Health Questionnaire-9 Depression Scale in the Kenyan Head and Neck Cancer Patient Population. Int J Psychiatry Med. 2006;36(3):367–81.
Carroll HA, Hook K, Perez OFR, Denckla C, Vince CC, Ghebrehiwet S, et al. Establishing reliability and validity for mental health screening instruments in resource-constrained settings: systematic review of the PHQ-9 and key recommendations. Psychiatry Res. 2020;291:113236.
Pinto-Meza A, Serrano-Blanco A, Peñarrubia MT, Blanco E, Haro JM. Assessing Depression in Primary Care with the PHQ-9: can it be carried out over the telephone? J Gen Intern Med. 2005;20(8):738–42.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
Chisala MN. Impact of maternal mental health on recovery from severe acute malnutrition in Malawi.
Stewart RC, Bunn J, Vokhiwa M, Umar E, Kauye F, Tomenson B, et al. A prospective study of psychological distress among mothers of children admitted to a nutritional rehabilitation unit in Malawi. Child Care Health Dev. 2011;37(1):55–63.
Colman S, Stewart RC, Macarthur C, Kennedy N, Tomenson B, Creed F. Psychological distress in mothers of children admitted to a nutritional rehabilitation unit in Malawi - a comparison with other paediatric wards. Matern Child Nutr. 2015;11(4):915–25.
Gelaye B, Rondon MB, Araya R, Williams MA. Epidemiology of maternal depression, risk factors, and child outcomes in low-income and middle-income countries. Lancet Psychiatry. 2016;3(10):973–82.
Bennett IM, Schott W, Krutikova S, Behrman JR. Maternal mental health, and child growth and development, in four low-income and middle-income countries. J Epidemiol Community Health. 2015;70(2):168–73.
Adewuya AO, Ola BO, Aloba OO, Mapayi BM, Okeniyi JAO. Impact of postnatal depression on infants’ growth in Nigeria. J Affect Disord. 2008;108(1):191–3.
Jidong DE, Husain N, Ike TJ, Murshed M, Pwajok JY, Roche A, et al. Maternal mental health and child well-being in Nigeria: a systematic review. Health Psychol Open. 2021;8(1):20551029211012199.
Herba CM, Glover V, Ramchandani PG, Rondon MB. Maternal depression and mental health in early childhood: an examination of underlying mechanisms in low-income and middle-income countries. Lancet Psychiatry. 2016;3(10):983–92.
Chee Din MA, Mohd Fahmi Teng NI, Abdul Manaf Z. Maternal depression and child feeding practices: determinants to malnutrition among young children in Malaysian rural area. Womens Health. 2023;19:17455057221147800.
Anato A, Baye K, Tafese Z, Stoecker BJ. Maternal depression is associated with child undernutrition: a cross-sectional study in Ethiopia. Matern Child Nutr. 2020;16(3):e12934.
Oyekanmi S. How the Russia-Ukraine war is damaging Nigeria’s economy. Nairametrics. 2022 [cited 2023 Dec 31]. Available from: https://nairametrics.com/2022/03/15/how-the-russia-ukraine-war-is-damaging-nigerias-economy/.
The Russia-Ukraine crisis. presents threats to Nigeria’s food security, but potential opportunities for the fertilizer, energy sectors| IFPRI: International Food Policy Research Institute. [cited 2023 Dec 31]. Available from: https://www.ifpri.org/blog/russia-ukraine-crisis-presents-threats-nigerias-food-security-potential-opportunities.
Ukraine/Russia. As War Continues, Africa Food Crisis Looms| Human Rights Watch. 2022 [cited 2023 Dec 31]. Available from: https://www.hrw.org/news/2022/04/28/ukraine/russia-war-continues-africa-food-crisis-looms.
Acknowledgements
The authors are incredibly grateful to the research coordinators and study personnel who meticulously gathered data during the trial in Kano, Nigeria. The authors thank Leshana Saint-Jean, Mustafa Nateqi, Jamil Galadanci, and Jennifer Beck-Smith for coordinating administrative tasks to successfully complete this study.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (NIH) under award number R21HD097992. CR was supported by the Vanderbilt-Meharry James Puckette Carter Summer Scholarship Program. LJK was supported by the Fogarty International Center and the National Heart, Lung, and Blood Institute of the NIH under award number D43TW009337, and the National Institute of Diabetes & Digestive & Kidney Diseases of the NIH under award number T32DK007673. Data collection and storage were supported through a grant from the Vanderbilt Institute for Clinical and Translational Research NCATS/NIH UL1 TR000445.
Author information
Authors and Affiliations
Contributions
SUA, SG, SA, VAS, MR, and MRD conceptualized and designed the study. SUA, SG, AH, KAS, GG, BM, and SS conducted the trial. MRD, MR, LJK, and CR had full access to all the data in the study and took responsibility for the data’s integrity and analysis accuracy. LJK, MR, and CR performed all analyses. LJK, MR, and CR wrote the initial manuscript. All authors have previously reviewed the manuscript and approved its submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Boards at Aminu Kano Teaching Hospital, Murtala Mohammed Specialist Hospital, and Vanderbilt University Medical Center approved the SAMS trial. The study included participants exclusively below the age of 16, and their legal guardians granted written informed consent prior to screening and enrollment. Moreover, children aged seven and above also provided assent alongside their guardians’ consent.
Consent for publication
Not applicable.
Competing interests
Dr. DeBaun and his institution sponsor two externally funded research investigator-initiated projects. Clinical studies will have funds provided by Global Blood Therapeutics (GBT). GBT was not a co-sponsor of either study. Dr. DeBaun did not receive any compensation for the conduct of these two investigator-initiated observational studies. Dr. DeBaun is a member of the GBT advisory board for a proposed randomized controlled trial for which he receives compensation. Dr. DeBaun is on the steering committee for a Novartis-sponsored phase II trial to prevent priapism in men. Dr. DeBaun was a medical advisor in developing the CTX001 Early Economic Model and provided medical input as part of an expert reference group for the Vertex/CRIPR CTX001 Early Economic Model in 2020. Dr. DeBaun consulted for the Formal Pharmaceutical company about sickle cell disease in 2021 and 2022. The authors have no other relevant affiliation or financial involvement with any entity or organization with a financial interest in or conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ritter, C., Abdullahi, S.U., Gambo, S. et al. Impact of maternal depression on malnutrition treatment outcomes in older children with sickle cell anemia. BMC Nutr 10, 18 (2024). https://doi.org/10.1186/s40795-024-00826-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40795-024-00826-0