Abstract
Background & aims
To examine the link between dietary insulin index (DII) and load (DIL) and sleep duration/quality for the first time.
Methods
This cross-sectional study conducted on data from the recruitment phase of Yazd Health Study (YaHS)-Yazd Nutrition Study (TAMYZ), prospective study in Yazd, central Iran. Data on demographic characteristics, dietary intakes, sleep quantity and quality, and potential confounders were gathered by interview. Sleep quality and its components (insufficient sleep, delay in falling asleep, medication use for sleep, and sleep disorder) were assessed by a modified version of Pittsburgh questionnaire. The link between DII/DIL and low sleep quality and short/long sleep duration was studied using multivariable logistic regression.
Results
In total, 5925 individuals aged 20 to 70 were eligible to take part in the current study. After adjustment for all potential confounders, participants in the highest DIL score tertile had a lower chance for sleep disorder (OR = 0.38; 95%CI: 0.17–0.85, Ptrend = 0.02) and delay in falling asleep (OR = 0.66; 95%CI: 0.42–1.03, Ptrend = 0.05) compared to those in the lowest tertile. The DII was also linked to a lower chance for sleep disorder (OR = 0.61; 95% CI: 0.39–0.93, P trend = 0.02). The DIL was inversely associated with sleep medication use and delay in falling sleep in men and women, respectively (P < 0.05). Moreover, DII was linked to a decreased odds of sleep disorder in women (P < 0.05). The associations were observed in those with overweight or obesity but not in those without overweight (P < 0.05).
Conclusion
Higher DIL and DII might be associated with sleep quality and its components. Prospective investigations are needed in the future to confirm these findings.
Similar content being viewed by others
Introduction
Sleep is vital to human health, as insufficient sleep duration and/or quality is considered a serious public health problem, worldwide [1]. A study in 12 US states found that 35.3% of participants slept less than 7h a day, on average [2].Obesity [3], diabetes [4,5,6], cardiovascular disease [7, 8], cancer [4, 5, 9], depression [10] and mortality [11, 12] have all been linked to insufficient sleep quantity and quality.
Sleep quality and duration have been proven to be influenced by dietary intakes [13]. Consumption of milk and dairy products [14] fruit and vegetables [15] was associated with improved sleep quality. Dietary carbohydrates have also been examined to affect sleep time and quality [16]. Carbohydrates increase plasma tryptophan concentrations [17], which serves as a precursor for brain serotonin, a sleep-inducing agent [18]. Not only the quantity of carbohydrates, but also its quality is regarded important in influencing sleep quality. Dietary glycemic load and its insulinemic potential may also be important [17, 19,20,21]. For instance, consumption of high GI foods reduced sleep onset latency [17], increased sleep duration [19], and promoted usual sleep quality [20]. Dietary insulin index and dietary insulin load have recently been proposed as ways to measure postprandial insulin responses to all dietary components, including amino acids and lipids. These indices are determined by adding the product of the food insulin index, the energy content, and the frequency with which food items are consumed. They are thought to be a better predictor of insulin demand than the dietary glycemic index and dietary glycemic load [22].
In Iran, determining the relationship between dietary insulin index and dietary insulin load and sleep length and quality is very important, where people are eating high amounts of carbohydrates) roughly 60% of total caloric intake) mostly in the form of refined grains in their diet [23, 24]. Furthermore, a high prevalence of low sleep duration and quality are reported in Iranians [25]. To the best of our knowledge, there was no previous study on the contribution of dietary insulin index and dietary insulin load to sleep duration/quality, particularly in the Middle East.
The current study aimed to examine the association between dietary insulin index and dietary insulin load with sleep quality and quantity among a large sample of Iranian adults living in the center of the country.
Participants and methods
Study design and population
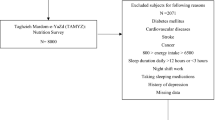
The abstract of this study has already been presented in a congress [26].The current cross-sectional analysis was conducted on the data from recruitment phase of Yazd Health Study (YaHS), a prospective cohort study on 9962 adults’ aged 20 to 70 residents in Yazd, Iran, between 2014 and 2016. Assessment of dietary intakes of these individuals was done in another study named Taghzieh Mardom Yazd (TAMYZ), which we used its data in the current analysis too. Details about YaHS-TAMYZ studies have already been published [27]. All participants in the YaHS-TAMYZ study signed informed consent In the current analysis, during the screening process, participants were excluded if they had missing data on sleep quantity or had missed responding more than 70 items of dietary intake data (n = 850) or unexplained energy intake (< 800 kcal/d or > 6000kcal/d) (n = 864), reported less than 3h or more than 12h of sleep per night (n = 250), were pregnant (n = 104) and reported to have major depression (n = 91). Those who had a history of chronic conditions at the time of the baseline survey, such as diabetes, cancer, or cardiovascular disease, were also eliminated (n = 1908).
Dietary intake assessment
Participants in the YaHS study had their dietary intakes assessed separately using a semi-quantitative food frequency questionnaire (FFQ) consisting of 178 food items, whose validity and reliability had been measured and confirmed in a prior study [28]. The FFQ used in this study was a 178 item questionnaire that contained 168 food items commonly consumed in Iran and 10 questions related to the consumption of traditional foods in Yazd. Participants were asked to respond to a 10-choice frequency response section for each food item, ranging from "never or less than once a month" to "10 or more times per day" for each food item. For each food item, five different portion sizes were asked based on Iranian’s standard serving size. Participants had to answer two questions in this questionnaire: the frequency of food consumption and the amount of food consumed at each time of consumption. Using household measures, the quantity and frequency of daily intake of all food items were converted to grams per day [29]. United States Department of Agriculture's (USDA) food composition database was used to calculate each participant's energy and nutrient intakes [30].
Calculation of dietary insulin index and load
The dietary insulin index was computed using previously published estimations by Brand-Miller et al., which are based on the insulin index of dietary items [31, 32]. The food insulin index (FII) is the difference between the area under the curve after ingestion of a 1000-kJ portion of the test food and the area under the curve after ingestion of a 1000-kJ portion of the reference food during a 2-h period. The FII of similar food items based on the similarity between their energy, carbohydrate, fiber, fat, and protein content was applied in the current investigation for food items that were not available in the food list released by Brand-Miller et al. (Supplementary Table 1). First, the insulin load of each food was determined using the following formula to determine dietary insulin load (DIL):
DIL was calculated for each subject by adding up the insulin load of consumed foods. The dietary insulin index (DII) was then computed for each participant by dividing DIL by total calorie intake.
Assessment of sleep quantity and quality
Data on regular sleep quantity was collected by an interview utilizing a nocturnal sleep questionnaire in the YaHS study. Sleep duration was divided into three categories: fewer than 5 h (short sleep duration), 5 to 8 h (normal sleep duration), and more than 8 h (long sleep duration) [33, 34]. The sleep quality of the study participants was obtained through an abbreviated form of the Pittsburgh questionnaire. The validity and reliability of the complete Pittsburgh questionnaire for the Iranian population has been confirmed [35]. The sleep quality score in the complete Pittsburgh questionnaire is between 0 and 21. In the present study, due to the use of the abbreviated form of the Pittsburgh questionnaire, the average sleep quality score was between 0 and 11, which was calculated based on the following questions: 1. How many minutes does it take to fall asleep from the time you go to bed? [Less than 15 min (0 score), 16–30 min (1 score), 31–60 min (2 scores), more than 1 h (3 scores)]. 2. How many times in the last 30 days have you been unable to fall asleep within half an hour?[None (0 points), less than once per week (1 point), once or twice per week (2 points), three or more times per week (3 points)]. The sum of these two questions, which is a number between 0 and 6, is known as the delay in falling asleep, and the delay in failing asleep was categorized as follows: score 0 (got 0), score 1 to 2 (got 1), score 3 to 4 (got 2), score 5 to 6 (got 3). 3. How many hours a night do you sleep? [More than 7 h (0 score), 6 to 7 h (1 score), 5 to 6 h (2 scores) and less than 5 h (3 scores)]. 4. How many times in the past month have you taken sleeping pills or sedatives to fall asleep? [None (0 score), once a week (1 score), twice a week (2 scores), three or more times a week (3 scores)]. 5. Did you wake up in the middle of the night or early in the morning? [None (0 score), less than once a week (1 score), once or twice a week (2 scores), three or more times a week (3 scores)] 6. How many times did you have nightmares while sleeping? [None (0 score), less than once a week (1 score), once or twice a week (2 scores), three or more times a week (3 scores)] 7. How many times did you wake up in the middle of the night to go to the bathroom? [None (0 score), less than once a week (1 score), once or twice a week (2 scores), three or more times a week (3 scores)]. Answers to questions 2 and 4 to 7, is known as sleep disorder, were added and converted as follows: score zero (0), score 1 to 9 (score 1), for score 10 to 12 (score 2). Finally, the sleep quality score in this study was generated by adding the scores of the above-mentioned questions and was ranged from 0 to 11 [delay in falling asleep (0–3), night sleep (0–3), sleep medication use (0–3), sleep disorder (0–2)].
Anthropometric measurements
Anthropometric markers such as height and weight were objectively measured in the YaHS-TAMYS study with minimum outfits and no shoes. Body weight was measured to the nearest 0.1 kg using a digital scale and body analyzer (Omron BF511, Omron Inc. Nagoya, Japan). Using a tape measure on a straight wall, height was measured in standing posture to the closest centimeter. The formula for calculating the body mass index (kg/m2) was: weight (kg) divided by height squared (m2).
Assessment of other variables
Age, sex, physical activity expressed as metabolic equivalent hours per week (MET-h/wk), job, BMI, smoking status, education level, marital status, and average duration of watching television and mobile use per day were all collected using predefined questionnaires by professional interviewers and were considered possible confounders. The short form of the standard physical activity questionnaire (IPAQ), which is developed to track the level of physical activity in developing countries, was used to assess physical activity [36]. The abbreviated form of physical activity includes questions related to four levels of physical activity such as intense, moderate, walking, and sitting activities over the past seven days, the intensity, duration, and repetition of which are completed by individuals. In this questionnaire, intensive physical activity is defined as at least a 10-min exercise that generates a significant rise in respiration, heart rate, and sweating. Also, moderate activity in this questionnaire is an activity that has a minimum duration of 10 min and increases the average respiration, heart rate, and sweating. This questionnaire is standard and its reliability and validity have been confirmed [37].
Statistical analysis
Subjects were categorized into tertiles based on their total DII and DIL. For continuous data, mean ± standard error (SE) was used, and for categorical variables, percentage was used. The one-way analysis of variance (ANOVA) and chi-square test were used to examine the difference in quantitative and qualitative variables between tertiles of dietary DII/DIL. The analysis of covariance (ANCOVA) was used to compare dietary nutrient intake between the tertiles of DII/DIL, with adjustments for sex, age and energy intake. Logistic regression in crude and multi-variable adjusted models was done to assess the association between DII or DIL and the chance for developing short (< 5 h) vs. normal (5–8 h), long (> 8 h) vs. normal (5–8 h) sleep duration, and insufficient sleep (< 5 h) vs. sufficient sleep (≥ 5 h), low sleep quality (total score 8 and more) vs. normal sleep quality (total score 7 and less), delay in falling asleep (delay in falling asleep score 3) vs. low delay in falling asleep (delay in falling asleep score 2 and less), using medication for sleep [sleep medication use three or more times a week (score 3)] vs. low medication use for sleep [sleep medication use two or less times a week (score 2 or less)], have sleep disorder (sleep disorder score 2) vs. no sleep disorder (sleep disorder score 1 or less). The first tertiles of DII and DIL were used as a reference group to calculate odds ratios (ORs) and 95% confidence intervals (CIs). The first model was adjusted for age, gender, and total calorie intake. In model 2, additional adjustments were performed for BMI (continuous), education (categorizing into Primary school and less/High school/ Diploma and Graduate Diploma/ Bachelor student/ Master student and Doctorate), physical activity (continuous), marital status (single/ married/ widowed or divorced), smoking status (never smoker/ current smoker/ ex-smoker) job situation (categorizing into unemployed/ government employee/ manual worker/ self-employed), anxiety (continuous), stress (continuous), caffeine (continuous), duration of cellphone use (continuous) and watching television & movie (continuous). In addition, in model 3, additional adjustments were made for the usage of sleeping medications (more than twice a week). SPSS software was used for all statistical analyses (version 26.0; SPSS Inc, Chicago IL). Statistical significance was defined as a P value < 0.05.
Results
This study included a total of 5925 participants, ranging in age from 20 to 70 years old (3035 males and 2890 females). Table 1 shows the overall characteristics of participants across dietary DII and DIL tertiles. Participants in the top tertile of DII were more likely to be married, current smoker, and were less likely to be educated, physically active, have stress, and use cellphone compared with those in the bottom tertile (P < 0.05). Moreover, participants in the highest tertile of DIL were more likely to be male, physically active, and use cellphone compared with those in the lowest tertile (P < 0.05). Also mean BMI and distribution of participants in terms of age categories, educated, job status, marital status (married) were significantly different across DIL tertiles (P < 0.05 for all).
Age, sex, and energy adjusted dietary intakes of selected foods and nutrients across tertiles of DII and DIL are shown in Table 2. Participants in the highest tertile of DII had a higher intake of total carbohydrates, thiamine, whole grains, refined grains, fruits, caffeine, and a lower intake of total energy, total fat, saturated fat, monounsaturated fat, poly-unsaturated fat, total protein, simple sugar, magnesium, vitamin E, vitamin B6, vitamin B12, folic acid, calcium, zinc, riboflavin, high-fat dairy products, low-fat dairy products, processed meats, red meats, nuts, legumes, vegetables compared with those in the lowest tertile (P < 0.05). Higher DIL was associated with greater intakes of total energy, total protein, total carbohydrate, vitamin C, thiamine, riboflavin, calcium, iron, zinc, whole grains, refined grains, legumes, red meats, processed meats, fruits, vegetables and, a lower intake of total fat, saturated fat, monounsaturated fat, poly-unsaturated fat, vitamin E, B6, folic acid, nuts (P < 0.05).
Table 3 presents the association between the DII and DIL and odds of developing sleep abnormalities in crude and multivariable-adjusted models in the whole population (n = 5925). In the fully adjusted model, subjects in the highest tertile of DIL had lower odds of sleep disorder compared with those in the lowest tertile (OR: 0.38; 95% CI: 0.17–0.85; P trend = 0.02). Also we found a significant inverse association, such that subjects in the highest tertile of DIL had lower odds of delay in falling asleep compared with those in the lowest tertile (OR = 0.66; 95% CI: 0.42–1.03, P trend = 0.05). Individuals in the highest tertile of DIL were less likely to use medications for sleep (OR: 0.60; 95% CI: 0.43–0.84, P trend = 0.002) compared with those in the lowest tertile in the crude model. However, after taking confounders into account, these associations became non-significant. In the crude or multivariable-adjusted models no other significant association was observed between DIL and sleep abnormalities (P > 0.05). In terms of DII, participants in the top tertile of DII had lower odds of sleep disorder compared with those in the bottom tertile in crude model (OR = 0.67; 95% CI: 0.46–0.97, P trend = 0.02). Even after adjusting for all possible confounders, this association remained significant. (OR = 0.61; 95% CI: 0.39–0.93, P trend = 0.02).
The interaction term between DII and gender was not significant for any outcomes but in terms of DIL and gender, the interaction was significant just for “delay in falling asleep” P = 0.02 not other outcomes. The association between DII and DIL and sleep abnormalities, based on sex, is indicated in Tables 4 and 5. Men in the highest tertile of DIL had lower odds of sleep medication use compared with those in the lowest tertile, in crude model (OR = 0.60; 95% CI: 0.36–1.00, P trend = 0.04). Even after adjusting for all possible confounders, this association remained significant. (OR = 0.28; 95% CI: 0.08–0.91, P trend = 0.01) (Table 4).
After adjusting for all confounders, women in the top tertile of DIL had lower odds of delay in falling asleep compared with those in the first tertile (OR = 0.41; 95% CI: 0.22–0.75; P trend = < 0.01) (Table 5). Although a significant inverse association was seen between DIL and odds of sleep medication use in crude model among women (OR = 0.63; 95% CI: 0.41–0.97; P trend = 0.03), the association was non-significant after controlling for all potential confounders (OR = 1.25; 95% CI: 0.49–3.22; P trend = 0.74). In terms of DII, women in the highest tertile had a lower odds of sleep disorder compared with those in the lowest tertile in the fully adjusted model (OR: 0.56; 95% CI: 0.32–0.97; P trend = 0.04).
The interaction term between DII and BMI was significant for “sleep quality” P = 0.007 and “Had to use medication for sleep “ P = 0.04 also in terms of DIL and gender, the interaction was significant for “sleep quality” P = 0.009 and “Had to use medication for sleep “ P = 0.05 too. The interaction term between DII/DIL and BMI for other outcomes wasn’t significant. Findings from stratified analysis based on BMI status are provided in Tables 6 and 7. In crude or multivariable-adjusted models, there was no significant association with sleep abnormalities among normal-weight persons (BMI 25 kg/m2) (P > 0.05). However, among overweight or obese people (participants with BMI ≥ 25 kg/m2), we found an inverse significant association between DIL and odds of sleep disorder (OR = 0.28; 95% CI: 0.10, 0.78, P trend = 0.01), and delay in falling asleep (OR = 0.50; 95% CI: 0.28, 0.89, P trend = 0.02). We also observed a significant association between DIL and odds of medication use in the crude model (OR = 0.47; 95% CI: 0.31, 0.73, P-trend ≤ 0.001). However, this association disappeared when potential confounders were adjusted. In terms of DII, greater DII was significantly associated with lower odds of low sleep quality (OR = 0.35; 95% CI: 0.16, 0.78, P trend = 0.01), sleep disorder (OR = 0.43; 95% CI: 0.24, 0.75, P trend = 0.002); and sleep medication use (OR = 0.62; 95% CI: 0.39, 0.99, P trend = 0.05) after adjustment for all possible confounders. Also, individuals with the highest DII had a higher chance for having long-sleep duration compared to those in the lowest tertile (OR = 1.43; 95% CI: 1.04, 1.97, P trend = 0.03).
Discussion
As far as we know, the current study is the first investigation on the association between dietary insulin index/load and sleep quality and quantity. Our findings revealed that participants with greater DIL were less likely to have sleep disorder. In terms of DII, participants in the top tertile of DII had lower odds of sleep disorder compared with those in the bottom tertile. However, men in the top tertile of DIL had lower odds of sleep medication use compared with those in the bottom tertile. In addition, women with greater dietary insulin load diet were less likely to have delay in falling asleep. Overweight or obese participants (BMI ≥ 25 kg/m2) with the highest DII had greater odds for having long-sleep duration, lower odds of sleep medication use and low sleep quality than those with the lowest DII. In terms of DIL, overweight or obese participants (BMI ≥ 25 kg/m2) with greater DIL were less likely to have delay in falling asleep.
Insufficient sleep duration and/or quality is a global public health problem [1]. Several factors including dietary intakes [13] can affect the quality and quantity of sleep. Dietary carbohydrates construct a major part of human diet. Although previous research has investigated at the relationship between dietary GI and GL and sleep quality/quantity [17, 21], no research has looked at the relationship between DIL and DII and sleep disturbances. Data on the relationship between dietary carbohydrate consumption and sleep duration, on the other hand, is conflicting. While some studies have demonstrated unfavorable effect of higher carbohydrate intake on sleep duration [38, 39], others showed a positive relationship between carbohydrate consumption and sleep length [40, 41]. We observed that participants with greater dietary insulin load were less likely to have delay in falling asleep in the current study. In line with our findings, Afaghi et al. observed a significant reduction in mean sleep onset latency with a high-GI diet consumed 4 h before bedtime, compared with a low-GI meal, on 12 healthy young men [17]. There was no evidence of a link between DII and DIL and sleep duration (short or long sleep duration). This was in agreement with the study of Daniel et al., who did a cross-sectional study on nine basketball male athletes and reported no significant effect of low GI diet on sleep duration [42]. In contrast, Yoneyama et al. reported an inverse association between a high dietary GI and risk of poor sleep [20]. Gangwisch et al. reported that high-GI diets might be a risk factor for insomnia [43]. In a cross-sectional study with a large sample of Iranian individuals, Mohammadi et al. found a significant relationship between dietary GL, but not GI, and sleep duration [21]. Other studies have found a similar favorable connection with dietary GL and sleep duration [19]. As previously stated, the focus of all published investigations has been on dietary GI and GL rather than DIL and DII and sleep quality/quantity. Dietary GI and GL do not account for other components that influence insulin secretion, whereas DII and DIL measure postprandial insulin responses to all dietary elements, including amino acids and lipids.
As a hypothesis, insulin raises the ratio of plasma tryptophan to other large neutral amino acids (LNAAs) like leucine, isoleucine, phenylalanine, valine, and tyrosine by stimulating the absorption of LNAAs into skeletal muscle, with the exception of tryptophan [44]. Furthermore, plasma glucose concentrations and the ratio of tryptophan to other LNAAs have a positive dose–response relationship [45]. There is a competitive transfer of these amino acids through the blood–brain barrier, increasing the ratio of tryptophan to other LNAAs leads to increased tryptophan transfer and consequently the synthesis of 5-hydroxytryptamine in the brain [44]. It is suggested that additional tissue-specific enzymes can convert this 5-hydroxytryptamine to serotonin and melatonin. Eventually, a rise in melatonin production is related with a decrease in delay in falling asleep [46].
In the present study, we found a sex and BMI dependent associations between DII and DIL and sleep quality and quantity. The fundamental mechanism for this gender disparity is unknown; however, the role of sex hormones in sleep quality could explain it in part. Another factor for the sex difference could be because women's stated dietary intakes are more accurate than men's [47]. One of the possible reasons for the difference in findings by BMI status might be the relationship between obesity and sleep quality and quantity. Obese people's sleep disorders, such as obstructive sleep apnea and obesity hypoventilation syndrome, would inevitably influence the quantity and quality of their sleep [48]. Moreover, people with obesity are more likely to report insomnia or trouble sleeping than normal weight individuals [49].
The current study has a number of advantages. As far as we know this is the first study examining the association between dietary insulinemic potential and sleep quality and quantity. Additionally, we examined these associations in a large sample of adults and we considered several possible confounders in our investigation. BMI and sex-stratified analysis was done. In addition, strengths of this study are the use of a validated FFQ for dietary assessment. However, this investigation had several limitations. The main limitation is that the study design was cross-sectional, which prevents us from inferring causality. Therefore, further analysis on the prospective data of YaHS-TAMYZ or other studies are required to confirm our findings. The dietary intakes, sleep quality, and sleep duration were all measured using self-reported data, which are susceptible to measurement errors. However, such measurement errors would attenuate the risk estimates; as a result, the associations observed in this study could be substantially stronger. The DII and DIL were computed using internationally available data. This should be considered while interpreting our findings.
In conclusion, we found evidence indicating that participants with greater dietary insulin load and dietary insulin index were less likely to have sleep disorder. We found no evidence of a link between dietary insulinemic potential and sleep quantity. More research, particularly with a prospective design, in other populations is needed to confirm these findings.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, [author initials], upon reasonable request.
Abbreviations
- DII:
-
Dietary Insulin Index
- DIL:
-
Dietary Insulin Load
- BMI:
-
Body Mass Index
References
Chattu VK, Manzar MD, Kumary S, Burman D, Spence DW, Pandi-Perumal SR. The global problem of insufficient sleep and its serious public health implications. Healthcare (Basel). 2018;7(1):1.
Centers for Disease Control and Prevention (CDC). Unhealthy sleep-related behaviors--12 States, 2009. MMWR Morb Mortal Wkly Rep. 2011;60(8):233–8.
Sun W, Huang Y, Wang Z, Yu Y, Lau A, Ali G, et al. Sleep duration associated with body mass index among Chinese adults. Sleep Med. 2015;16(5):612–6.
Kakizaki M, Kuriyama S, Sone T, Ohmori-Matsuda K, Hozawa A, Nakaya N, et al. Sleep duration and the risk of breast cancer: the Ohsaki Cohort Study. Br J Cancer. 2008;99(9):1502–5.
Jiao L, Duan Z, Sangi-Haghpeykar H, Hale L, White DL, El-Serag HB. Sleep duration and incidence of colorectal cancer in postmenopausal women. Br J Cancer. 2013;108(1):213–21.
Sanchez C, Killgore W, Gehrels J, Alfonso-Miller P, Grandner M. 0127 nighttime snacking: prevalence and associations with poor sleep, health, obesity, and diabetes. Sleep. 2018;41(suppl_1):A49–50.
Xie D, Li W, Wang Y, Gu H, Teo K, Liu L, et al. Sleep duration, snoring habits and risk of acute myocardial infarction in China population: results of the INTERHEART study. BMC Public Health. 2014;14(1):531.
Hoevenaar-Blom MP, Spijkerman AMW, Kromhout D, van den Berg JF, Verschuren WMM. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: The MORGEN Study. Sleep. 2011;34(11):1487–92.
Song C, Zhang R, Wang C, Fu R, Song W, Dou K, et al. Sleep quality and risk of cancer: findings from the English longitudinal study of aging. Sleep. 2021;44(3):zsaa192. https://doi.org/10.1093/sleep/zsaa192.
Nakata A. Work hours, sleep sufficiency, and prevalence of depression among full-time employees: a community-based cross-sectional study. J Clin Psychiatry. 2011;72(5):605–14.
Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009;18(2):148–58.
Yin J, Jin X, Shan Z, Li S, Huang H, Li P, et al. Relationship of sleep duration with all-cause mortality and cardiovascular events: a systematic review and dose-response meta-analysis of prospective cohort studies. J Am Heart Assoc. 2017;6(9): e005947.
Peuhkuri K, Sihvola N, Korpela R. Diet promotes sleep duration and quality. Nutrition research (New York, NY). 2012;32(5):309–19.
Komada Y, Okajima I, Kuwata T. The effects of milk and dairy products on sleep: a systematic review. Int J Environ Res Public Health. 2020;17(24):9440.
Jansen EC, She R, Rukstalis MM, Alexander GL. Sleep duration and quality in relation to fruit and vegetable intake of US young adults: a secondary analysis. Int J Behav Med. 2021;28(2):177–88. https://doi.org/10.1007/s12529-020-09853-0.
Lindseth G, Lindseth P, Thompson M. Nutritional effects on sleep. West J Nurs Res. 2013;35(4):497–513.
Afaghi A, O’Connor H, Chow CM. High-glycemic-index carbohydrate meals shorten sleep onset. Am J Clin Nutr. 2007;85(2):426–30.
Hartmann E. Effects of L-tryptophan on sleepiness and on sleep. J Psychiatr Res. 1982;17(2):107–13.
Diethelm K, Remer T, Jilani H, Kunz C, Buyken AE. Associations between the macronutrient composition of the evening meal and average daily sleep duration in early childhood. Clinical nutrition (Edinburgh, Scotland). 2011;30(5):640–6.
Yoneyama S, Sakurai M, Nakamura K, Morikawa Y, Miura K, Nakashima M, et al. Associations between rice, noodle, and bread intake and sleep quality in Japanese men and women. PLoS One. 2014;9(8):e105198-e.
Mohammadi M, Nadjarzadeh A, Mirzaei M, Fallahzadeh H, Haghighatdoost F, Sakhaei R, Abolhosseini H, Salehi-Abargouei A. Dietary glycemic index and glycemic load in association with sleep duration: results from a large sample of Iranian adults. Clin Nutr ESPEN. 2021;46:471–6.
Nimptsch K, Brand-Miller JC, Franz M, Sampson L, Willett WC, Giovannucci E. Dietary insulin index and insulin load in relation to biomarkers of glycemic control, plasma lipids, and inflammation markers. Am J Clin Nutr. 2011;94(1):182–90.
Bahreynian M, Esmaillzadeh A. Quantity and quality of carbohydrate intake in Iran: a target for nutritional intervention. Arch Iran Med. 2012;15(10):648–9.
Heidari Z, Feizi A, Azadbakht L, Mohammadifard N, Maghroun M, Sarrafzadegan N. Usual energy and macronutrient intakes in a large sample of Iranian middle-aged and elderly populations. Nutr Diet. 2019;76(2):174–83.
Tirgari B, Azzizadeh Forouzi M, Iranmanesh S, Khodabandeh Shahraki S. Predictors of sleep quality and sleepiness in the Iranian adult: a population based study. JCHR. 2013;1(3):144–52. http://jhr.ssu.ac.ir/article-1-64-fa.html.
https://jnfh.mums.ac.ir/article_20168_b9d6747d9d41bf23fe3c06cde7657c97.pdf?
Mirzaei M, Salehi-Abargouei A, Mirzaei M, Mohsenpour MA. Cohort Profile: The Yazd Health Study (YaHS): a population-based study of adults aged 20–70 years (study design and baseline population data). Int J Epidemiol. 2018;47(3):697–8.
Zimorovat A, Moghtaderi F, Amiri M, Raeisi-Dehkordi H, Mohyadini M, Mohammadi M, et al. Validity and reproducibility of a semiquantitative multiple-choice food frequency questionnaire in Iranian adults. Food Nutr Bull. 2022;43(2):171–88. https://doi.org/10.1177/03795721221078353.
Ghafarpour MH, Houshiar-Rad A, Kianfar H, Ghaffarpour M. The manual for household measures, cooking yields factors and edible portion of food.
Haytowitz DB, Ahuja JKC, Wu X, Somanchi M, Nickle M, Nguyen QA, et al. USDA National Nutrient Database for Standard Reference, Legacy Release. Nutrient Data Laboratory, Beltsville Human Nutrition Research Center, ARS, USDA; 2019. https://doi.org/10.15482/USDA.ADC/1529216. Accessed 22 July 2023.
Holt SH, Miller JC, Petocz P. An insulin index of foods: the insulin demand generated by 1000-kJ portions of common foods. Am J Clin Nutr. 1997;66(5):1264–76.
Bao J, de Jong V, Atkinson F, Petocz P, Brand-Miller JC. Food insulin index: physiologic basis for predicting insulin demand evoked by composite meals. Am J Clin Nutr. 2009;90(4):986–92.
Ferrie JE, Shipley MJ, Cappuccio FP, Brunner E, Miller MA, Kumari M, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30(12):1659–66.
Burazeri G, Gofin J, Kark JD. Over 8 hours of sleep–marker of increased mortality in Mediterranean population: follow-up population study. Croat Med J. 2003;44(2):193–8.
Chehri A, Nourozi M, Eskandari S, Khazaie H, Hemati N, Jalali A. Validation of the Persian version of the Pittsburgh sleep quality index in elderly population. Sleep Sci. 2020;13(2):119–24.
IPAQ. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)— Short Form. Available at: http://www.ipaq.ki.se. Accessed: 23 July 2004.
Karimzadeh Shirazi K, Niknami SAD, Heydarnia AR, Wallace LM, Torkamaan G, Faghihzadeh S. Effects of a TTM-based osteoporosis preventive physical activity education, on increasing muscle strength and balance in women aged 40–65. Hakim Res J. 2007;10(2):3442.
Sanlier N, Sabuncular G. Relationship between nutrition and sleep quality, focusing on the melatonin biosynthesis. Sleep Biol Rhythms. 2020;18(2):89–99.
Martinez SM, Tschann JM, Butte NF, Gregorich SE, Penilla C, Flores E, et al. Short sleep duration is associated with eating more carbohydrates and less dietary fat in Mexican American children. Sleep. 2017;40(2):zsw057.
Liu X, Wu D, Qi X, Niu Y, Li W, Lu Y, et al. The associations between carbohydrate and protein intakes with habitual sleep duration among adults living in urban and rural areas. Clinical nutrition (Edinburgh, Scotland). 2018;37(5):1631–7.
Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, Redline S. The association of sleep duration with adolescents’ fat and carbohydrate consumption. Sleep. 2010;33(9):1201–9.
Daniel NVSZI, Estadella D, Garcia MC, Padovani RC, Juzwiak CR. Effect of the intake of high or low glycemic index high carbohydrate-meals on athletes’ sleep quality in pre-game nights. An Acad Bras Cienc. 2019;91(1): e20180107.
Gangwisch JE, Hale L, St-Onge MP, Choi L, LeBlanc ES, Malaspina D, et al. High glycemic index and glycemic load diets as risk factors for insomnia: analyses from the Women’s Health Initiative. Am J Clin Nutr. 2020;111(2):429–39.
Höglund E, Øverli Ø, Winberg S. Tryptophan metabolic pathways and brain serotonergic activity: a comparative review. Front Endocrinol. 2019;10:158.
Pan RM, Mauron C, Glaeser B, Wurtman RJ. Effect of various oral glucose doses on plasma neutral amino acid levels. Metabolism Clin Exp. 1982;31(9):937–43.
van Geijlswijk IM, Korzilius HP, Smits MG. The use of exogenous melatonin in delayed sleep phase disorder: a meta-analysis. Sleep. 2010;33(12):1605–14.
Marks GC, Hughes MC, van der Pols JC. Relative validity of food intake estimates using a food frequency questionnaire is associated with sex, age, and other personal characteristics. J Nutr. 2006;136(2):459–65.
Ryan S, Crinion SJ, McNicholas WT. Obesity and sleep-disordered breathing—when two ‘bad guys’ meet. QJM. 2014;107(12):949–54.
Pearson NJ, Johnson LL, Nahin RL. Insomnia, trouble sleeping, and complementary and alternative medicine: Analysis of the 2002 national health interview survey data. Arch Intern Med. 2006;166(16):1775–82.
Acknowledgements
The authors would like to thank the participants of the YaHS-TAMYZ cohort studies, and authorities of Tehran University of Medical Sciences and Shahid Sadoughi University of Medical Sciences for their excellent cooperation.
Funding
The present study was funded by Tehran University of Medical Sciences. YaHS was funded by Shahid Sadoughi University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
MM2 establishes YaHS study. PS, AE, ASA conceived and designed the study, PS, ASA, MM1 performed the statistical analysis, data interpretation. PS drafted the manuscript. AE, ASA, MM2 critically reviewed the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
YaHS-TAMYZ study was approved by the research Council of Shahid Sadoughi University of Medical Sciences and the Ethics Committee of Shahid Sadoughi University of Medical Sciences No. 17/1/73941. All methods were performed in accordance with the relevant guidelines and regulations by research Council of Shahid Sadoughi University of Medical Sciences. The current study was also approved by the ethics committee of Tehran University of Medical Sciences’ ethics committee. All participants in the YaHS-TAMYZ study signed informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplementary Table 1. Food insulin index (FII) of all 178 FFQ food items according to theprevious studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sarsangi, P., Mohammadi, M., Salehi-Abargouei, A. et al. Dietary insulinemic potential, sleep quality and quantity in Iranian adults: Yazd health study and TAMYZ study. BMC Nutr 9, 92 (2023). https://doi.org/10.1186/s40795-023-00745-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40795-023-00745-6