Abstract
Background
A dietary pattern with a high glycemic index (GI) and glycemic load (GL) can be a precursor to sleep disorders that link to many chronic diseases. We aimed to assess the association of dietary GI and GL with the risk of insomnia in Iranian adults.
Methods
A total of 111 newly diagnosed insomnia cases and 333 controls aged 18–60 years were included in this case–control study. The participants’ dietary intakes were collected using a valid and reliable food frequency questionnaire. The diagnosis of insomnia in subjects was performed by a neurologist using the Insomnia Severity Index (ISI) questionnaire. Multivariable logistic regression models, adjusted for the potential confounders, were used to determine the risk of insomnia according to tertiles of dietary glycemic indices.
Results
The mean (SD) age and BMI of the study population (78.6% female) were 31.8 (10.0) years and 24.70 (3.62) kg/m2, respectively. The median (IQR) of dietary GI and GL in subjects was 62.7 (57.0–68.6) and 213.5(167.4–268.5), respectively. Based on the multivariable-adjusted model, after controlling for age, sex, physical activity, obesity, smoking, socioeconomic score, general health questionnaire (GHQ) score, and dietary energy intake, the odds of insomnia were increased across tertiles of dietary GL[(OR:2.72,95%CI:1.10–6.70),(Ptrend = 0.017)], however, no significant association was observed between high GI diet and insomnia risk [(OR:1.38,95%CI:0.77–2.47),(Ptrend = 0.298)].
Conclusions
Our results revealed that greater adherence to dietary pattern with high GL could be increased the odds of insomnia in Iranian adults.
Similar content being viewed by others
Background
Insomnia is the most prevalent type of sleep disorders. It is mainly described as poor sleep quality and quantity and weak daily performance. According to sex, place of living, and explanation of the condition, 10–35% of the adult population is affected by sleep disturbances, and 5–10% meet the insomnia DSM criteria [1,2,3,4]. Based on recent studies, the prevalence of sleep disorders in the general population, from consistent problems to periodic patterns, is 9 and 27%, respectively [5]. Insomnia is associated with decreased ability to do tasks, reduced life quality, and increased unfavorable mental and medical outcomes [6]. Also, it has been suggested that insomnia symptoms could be related to an increased risk of mortality [7], cardiovascular diseases [8], and injuries [9].
Recently, studies have mostly focused on the association of nutritional factors with sleep duration and quality [10, 11]. However, results on the role of nutrients in the progression of insomnia symptoms are limited and lower understood. The manipulation of carbohydrate intakes in dietary patterns has received more attention over the years regarding its effect on multiple aspects of sleep. Carbohydrate can be an important nutrient for sleep because it acts as a primary main source of energy for all cells and may be associated with brain function and sleep-related hormonal regulation [12]. An interventional study showed that high carbohydrate (HC) diets significantly decreased slow wave sleep (SWS) and increased rapid eye movement (REM) sleep compared with a low carbohydrate diet or a normal balanced diet [13]. In another study [14], the same changes in SWS observed after consuming an HC test meal. However, some studies revealed that subjects suffering from insomnia had low carbohydrate intakes [12, 15,16,17].
Moreover, a study conducted on the effect of the glycemic index (GI) on sleep architecture demonstrated significant higher subjective ratings of sleepiness and shortened sleep onset latency after consuming a high-GI meal four h before bedtime compared with a low-GI meal [18]. To the best of our knowledge, evidence on the association between dietary GI and glycemic load (GL) and risk of insomnia symptoms is limited to a large prospective study conducted on postmenopausal women [19]. The Gangwisch et al. study reported that a high-GI dietary pattern may be the main factor for the increased risk of insomnia in women, however, higher adherence to a diet rich in fiber, fruit, and vegetables can reduce the risk of insomnia [19].
Dietary GI and GL are essential indicators for assessing the dietary carbohydrate quality and quantity, which helps us more accurately assess this macronutrient's role in predicting chronic disease risks such as insomnia. Therefore, the present study aimed to identify the possible relationship between dietary GI and GL and the risk of insomnia in Iranian adults.
Methods and materials
Study population
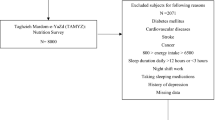
The current case–control study was conducted on 444 individuals (111 newly diagnosed insomnia patients and 333 controls aged 18–60 years) referred to Isfahan health centers between July 2016 and August 2017. The cases were recruited among patients with moderate or severe primary insomnia diagnosed by a neurologist using the Insomnia Severity Index (ISI) questionnaire [20, 21]. The participants for the control group were selected among individuals without clinically obvious insomnia. The process of assigning individuals to two control and case groups was as follows, in the health centers of Isfahan city, the referring subjects who were eligible for the study according to the inclusion and exclusion criteria were asked to answer the questions of the ISI questionnaire [20]. According to the ISI questionnaire score, individuals were classified into four levels: severe, moderate, mild, and no insomnia, in terms of the risk of primary insomnia. Next, individuals with moderate and severe risk were categorized as cases, and individuals with mild risk and no insomnia were categorized as controls. Using a simple sampling method, the sampling process continued until the number of cases reached 111 subjects and the number of control reached 333 subjects.
The exclusion criteria of the present study include having heart failure, chronic kidney disease, severe joint diseases, restless leg syndrome (based on self-reported medical history), severe mental disorders [screened by General Health Questionnaire (GHQ) 28 questionnaire], apnea, and sleep-related respiratory disorders (screened by Stop questionnaire), any recent stressful events such as death or a serious illness of a family member, divorce, alcohol consumption, stimulants and sedative drugs intakes, supplement intakes for more than three days a week, shift work, pregnancy, and lactation. We also excluded the individuals who did not respond to more than 35 food items in the food frequency questionnaire (FFQ) or whose caloric intake was outside the range of 800 to 4200 kcal.
Measurements
Assessment of primary insomnia
The presence of primary insomnia in individuals was determined using a brief, reliable, valid self-reporting questionnaire, the ISI [20], which was previously validated among Iranian population [21]. The ISI is a seven-item questionnaire, investigating the nature and symptoms of sleep problems using a Likert-type scale, a type of scale in which respondents specify their level of agreement or disagreement on a symmetric agree-disagree scale for a series of statements. The content of this questionnaire corresponds closely to ICD-10-CM F51.01 and DSM-IV criteria for insomnia. It assesses the individuals’ current understanding of symptom severity [difficulty in initiating sleep (DIS), difficulty in maintaining sleep (DMS), and early morning awakening (EMA)], sleep-related satisfaction, and daytime impairment by seven questions with a 5-point Likert scale (0–4) with a total score of 28. A score of 0–7 represents that individuals have no clinically significant insomnia. The score of 8–14 shows sub-threshold insomnia in participants. The score ranged from 15–21 indicates clinical insomnia with moderate severity, and 22–28 indicating severe clinical insomnia [20]. We classified the case and control groups based on Smith and Trinder's study [22], which suggests a cut-off score of 14 to distinguish individuals with insomnia from normal controls.
Dietary assessment
In the current study, participants' dietary intakes were collected using a validated and reproducible 168-item semi-quantitative FFQ [23]. A list of typical Iranian foods with standard serving sizes was included in our FFQ. Individuals were asked to report their average dietary intake during the previous year by choosing one of the following categories: never or less than once a month, 3–4 times per month, once a week, 2–4 times per week, 5–6 times per week, once daily, 2–3 times per day, 4–5 times per day, and six or more times a day. Portion sizes of each food item were converted into grams using standard Iranian household measures [24]. The daily energy and nutrient intakes were computed based on the United States Department of Agriculture’s (USDA) Food Composition Table (FCT). For some local foods unavailable in USDA FCT [25], we used the Iranian FCT [24]. Finally, we used the Nutritionist IV software, modified for Iranian foods, to determine the energy, macronutrients, and micronutrients based on participants’ dietary intakes.
Glycemic indices
For each carbohydrate-containing food, GI is described as the area under the blood glucose response curve over two hours after eating the food relative to that after consuming the equivalent amount of carbohydrate as glucose. The international table of GI and list of the GI of Iranian foods [26, 27] were used to obtain the GI value of each food item. The total dietary GI and GL were determined as the following:
Anthropometrics’ measurements
We measured the participants’ weight to the nearest 100 g with minimum clothes and without shoes using digital scales (model 707, Seca, Hamburg, Germany). A stadiometer (model 208 Portable Body Meter Measuring Device; Seca) was used to measure height to the nearest 0.5 cm in the standing position while the individuals were unshod. Body mass index (BMI) was calculated as weight (kilograms) divided by height (meters2).
Assessment of other variables
Data on age, sex, marital status, socioeconomic status (SES), medical history, drug use, and smoking status were obtained using a demographic questionnaire. SES score was calculated based on three variables, including household size (≤ 4, > 4 people), education levels (academic and non-academic education), and acquisition (house ownership or not). For each of these variables, participants were given a score of 1 (if their family members were ≤ 4, were academically educated or owned a house) or given a score of 0 (if their family members were > 4, or had non-academic education, or leasehold property). Then, the total SES score was computed by summing up the assigned scores (minimum SES score of 0 to maximum score of 3). An SES score of 3 equated to high, 2 was moderate, and 1 or 0 was low. Through face-to-face interviews, physical activity levels were measured using the international physical activity questionnaire (IPAQ). All results of the IPAQ were expressed as Metabolic Equivalents per week (METs/week) [28].
Considering that severe depression and apnea may lead to secondary insomnia, individuals were screened based on the GHQ28 and Stop questionnaires. GHQ28 had 4 sub-scales for measuring physical complaints, severe depression, anxiety and insomnia, and social dysfunction [29]. Each sub-scale includes 7 questions with 4 replies with a cut-off point of 6 for each sub-scale and a general cut point of 24. Based on this valid and reliable questionnaire, individuals who scored more than 6 for the subscale of depression were excluded from the study. The Stop questionnaire, considered for assessing the obstructive sleep apnea (OSA) risk, consists of 4 questions with two answers: yes or no, which are scored as 0 and 1. Based on the cut-off point for this questionnaire, individuals with a score equal to or greater than 2 were considered at high risk for OSA [30] and were excluded from the study.
Statistical analysis
Statistical analysis was performed using Statistical Package Software for Social Science, version 21 (SPSS Inc., Chicago, IL, USA). The variables' normality was checked using the Kolmogorov–Smirnov test and histogram chart. Baseline characteristics and dietary intakes were expressed as mean ± SD or median (25–75 interquartile range) for quantitative variables and percentages for qualitative variables. Independent sample t-test and chi-square were used for testing the differences between cases and controls for continuous and categorical variables, respectively (Table 1). Participants were categorized into tertiles based on the dietary GI and GL. The general and dietary data were expressed across tertiles of dietary GL. Linear regression and chi-square tests were used to test the trend of continuous and categorical variables across tertiles of dietary GL (Table 2). We used the logistic regression analysis to determine the odds of insomnia across dietary GI and GL tertiles. The analysis was adjusted for potential confounders, including age and sex, obesity, physical activity, smoking, SES, dietary intake of energy, and GHQ score. The odds ratio (OR) with a 95% confidence interval (CI) of insomnia across tertiles of dietary GI and GL were reported (Table 3), and p-values < 0.05 were considered statistically significant.
Results
The mean (SD) age and BMI of the participants (78.6% female) was 31.8 (10.0) years and 24.70 (3.62) kg/m2, respectively. The median (IQR) of dietary GI and GL were 62.7 (57.0–68.6) and 213.5(167.4–268.5), respectively. Based on Table 1, our findings showed that participants in the cases group had a higher BMI, age, GHQ score, and ISI score than the control group (P < 0.05). Also, the protein intake in the case group was significantly lower than in the control group (P < 0.05). However, our findings indicated no significant difference in physical activity, % of smoking, % of females, SES score, and the intakes of energy, total fats, and carbohydrates among participants based on case and control groups.
Table 2 reported the study participants' general characteristics and dietary intake based on tertiles of the GI score. Participants in the highest tertile of GI were low smoked and had low SES and a lower intake of protein (% of energy) compared to those in the lowest tertile of dietary GI (P < 0.05). However, there were no significant differences in other variables, including age, gender distribution, % of obesity and overweight, physical activity, GHQ, ISI score, and intake of energy, fats, and carbohydrate across the tertile of dietary GI.
General characteristics and nutrient intakes of the study population across tertiles of dietary GL are shown in Table 3. Participants in the highest tertile of GL were significantly low-active and had lower fat and protein intake compared to those in the lowest tertile of GL (P < 0.05). Also, participants in the highest tertile of GL had a higher percentage of males, ISI score, and higher intake of energy and carbohydrate intake compared to those in the lowest tertile of GL (P < 0.05). However, there were no significant differences in other variables.
The association between the higher dietary GI and GL and the risk of insomnia is reported in Table 4. In the crud model, there was no significant relationship between GI (OR = 1.10; 95% CI: 0.66–1.84, P for trend = 0.676) and GL (OR = 1.32; 95% CI: 0.79–2.20, P for trend = 0.195) and odds of insomnia. Also, in the age and sex-adjusted model, no association was found between GI (OR = 1.10; 95% CI: 0.65–1.86, P for trend = 0.704) and GL (OR = 1.34; 95% CI: 0.78–2.30, P for trend = 0.209) and risk of insomnia. In the multivariable-adjusted model, after adjustment for age, sex, obesity, smoking, physical activity, dietary energy intake, SES, and GHQ score, the odds of insomnia were increased across tertiles of dietary GL [(OR: 2.72, 95% CI: 1.10–6.70), (P for trend = 0.017)], however, no significant association was found between high dietary GI and risk of insomnia [(OR: 1.38, 95% CI: 0.77–2.47), (P for trend = 0.298)].
Discussion
This case–control study suggested that higher adherence to a high GL diet, characterized by higher intakes of foods with added sugars, refined starchy foods, and sweetened beverages, may be associated with an increased risk of insomnia, whereas no significant association was found between high GI and risk of insomnia.
Evidence on the role of carbohydrate intakes, low carbohydrate diet, or dietary GI and GL on the risk of insomnia lacks sufficient consensus on this topic [15, 17,18,19]. A cross-sectional study conducted on the Japanese adult population has suggested that a carbohydrate intake of less than 50% of total energy can be associated with some insomnia symptoms such as difficulty maintaining sleep [15], however, Kalam et al. study reported that higher adherence to an alternate day fasting combined with a low carbohydrate dietary pattern did not affect sleep quality, duration, and severity of insomnia [17]. Furthermore, another study indicated that consuming a carbohydrate-based high-GI meal with a simple manipulation of food intake (the type of rice) has led to a remarkable shortening of sleep onset latency in young adult subjects [18]. Our findings are nearly comparable with the results of only a large prospective study investigating the association of dietary GI with the risk of insomnia symptoms among postmenopausal women [19]. The Gangwisch et al. study suggested that individuals with a high-GI dietary pattern may be more prone to the development of insomnia. Also, they declared that greater adherence to the dietary pattern, characterized by higher intakes of fiber, fruit, and vegetables, may decrease the risk of incident insomnia [19]. However, we observed remarkable results only in the relationship between high dietary GL and the risk of insomnia, but in the case of high dietary GI, although the reported ORs showed a possible positive effect of this dietary index in increasing the prevalence of insomnia, these results are not statistically meaningful.
The results of studies indicate that both the quantity and quality of carbohydrate intakes are maybe important and effective in reducing insomnia symptoms such as sleep quality and duration (sleep onset latency, difficulty maintaining sleep, difficulty initiating sleep, etc.). Some studies declared that lower carbohydrate intake might worsen insomnia symptoms [15, 16]. However, in another study, the quantity of carbohydrate intake in the diet is not effective in sleep quality, sleep duration, and severity of insomnia in obese adults [17]. Regarding the importance of carbohydrate quality in predicting insomnia symptoms, our study and the Gangwisch et al. study [19] mostly suggested that adhering to a diet with a high GI or high GL can be an important risk factor exacerbating the symptoms of insomnia.
In the current study, the dietary GL (OR: 2.72) had a remarkable power in predicting the increased risk of insomnia, whereas the association of dietary GI with risk of insomnia is not statistically significant; Some reasons can explain the non-significance of the results related to the dietary GI and the risk of insomnia; first, we must note that the reported OR for insomnia based on the dietary GI is clinically remarkable (OR: 1.38), which shows that a high GI diet can predict an increase in the risk of sleep disorders such as insomnia, but in our study, due to the small sample size and insufficient power of the study, these results are statistically insignificant. Also, GI explains how carbohydrates may affect blood glucose levels, whereas GL takes into consideration every component of the food as a whole, giving a more real-life picture of a food's impact on your blood glucose levels [31,32,33]. In other words, the GL more accurately measures how certain foods will impact your blood glucose levels and insulin secretion by considering the number of carbohydrates in an average serving [34, 35]. As a result, a food pattern with a high GL is likely to lead to mental disorders and sleep disorders through hyperglycemia, increasing basal insulin, and creating a steep insulin peak [36, 37]. However, the high GI food components of individuals in the form of a dietary pattern can be more effective in increasing blood sugar, insulin levels and insulin-related disorders when the GL level of the individual's diet is also high.
Although the conducted studies according to carbohydrates quality and insomnia and its related variables such as sleep onset latency, sleep duration, or continuation indicated ambiguous findings, it seems that a high GL and GI diet serves as a risk factor for increased risk of insomnia [15,16,17, 19]; Some reasons and mechanisms may explain this negative effect of a high GI and GL diet on sleep disorders. A higher intake of high GL and GI foods in a meal is the main determinant of elevated postprandial glucose levels and the glycemic response, leading to substantial blood glucose fluctuations and postprandial hyperglycemia [36]. Postprandial hyperglycemia from high dietary GI and GL results in compensatory hyperinsulinemia [36]. Hyperinsulinemia drastically reduces the plasma glucose concentration, which may compromise brain glucose, ∼70 mg/dl [38]. This significant change in plasma glucose level stimulates the secretion of anti-insulin hormones, including autonomic counter-regulatory hormones such as glucagon, epinephrine, corticosteroids, and growth hormone [39]. Increased secretion of these stress hormones leads to symptoms such as heart palpitations, brain stimulation, anxiety, sweating, tremor, paresthesia, irritability, and increased appetite in the body that can affect various aspects of sleep and lead to producing arousal from sleep and reducing sleep efficiency [39,40,41]. Indeed, hyperglycemia in response to a consuming high GI and GL diet or higher carbohydrates intake can initially lead individuals to drowsy and help them to fall asleep [42]; however, the compensatory hyperinsulinemia and counter-regulatory hormone responses can consequently cause insomnia and arousal [40, 41]. Also, sweetened beverages and added sugars foods are important characteristics of a high GI and GL diet may have an adverse effect on sleep quality because higher intakes of simple sugars can contribute to dysregulation of the intestinal microbiome, a maladaptive microbiota imbalance that can remarkably be effective in various aspects of sleep [43]. Furthermore, higher adherence to dietary pattern with high GI and GL may be led to an increase in the stimulation of inflammatory immune responses [44]; these immune responses can play an essential role in increment the risk of insomnia by inducing the production of anti-inflammatory cytokines that act as sleep inhibitors.
The current study has some strengths. To the best of our knowledge, this study is the first study to investigate the association of dietary GI and GL with the risk of insomnia symptoms in the Middle East and North Africa region peoples by controlling the effects of potential main confounding factors. Also, we focused on subjects with a specific sleep disorder considering both genders. We also used validated questionnaires to collect data on the study population's food intake and physical activity levels. However, some limitations of this study deserve to mention. Our study has a case–control design, which does not allow for determining causal relationships. Also, recall bias is unavoidable due to the use of FFQ to collect nutritional information in this study as a case–control study, however, the use of a validated questionnaire minimizes this error. This study, as a case–control study, could be typically prone to selection bias; however, we selected the study population from the health centers of Isfahan city, which were representative of the adults of Isfahan city, so we minimized this error.
Furthermore, we have determined the sleep problems based on self-reported data as a subjective scale, leading to possible misreports and may affect the findings. It seems that objective measurements such as objective measurements, such as polysomnography or multiple actigraphy for the diagnosis of sleep disorders, could have minimized these errors. Finally, despite adjusting several covariates, the current study cannot control all potential confounding, and the effects of some residual confounding factors and unknown confounders may have occurred.
Conclusions
In conclusion, the current study reported that higher adherence to a dietary pattern with high GL is related to higher odds of primary insomnia. However, no statistically significant association was observed between a high GI diet and the risk of insomnia. It is suggested that more studies with large sample sizes and prospective designs, such as population-based cohort studies, are performed to examine our hypothesis of the relationship between dietary GI and GL and the odds of insomnia. Also, future population-based observational studies could examine the importance of the effect of the timing of the consumption of carbohydrate foods in modulating sleep at night.
Availability of data and materials
The datasets analyzed in the current study are available from the corresponding author on reasonable request.
Abbreviations
- CI:
-
Confidence interval
- DSM:
-
Diagnostic and Statistical Manual of Mental Disorders
- FCT:
-
Food Composition Table
- FFQ:
-
Food frequency questionnaire
- GHQ:
-
General Health Questionnaire
- GI:
-
Glycemic index
- GL:
-
Glycemic load
- HC:
-
High carbohydrate
- IPAQ:
-
International physical activity questionnaire
- ISI:
-
Insomnia Severity Index
- METs:
-
Metabolic Equivalents
- OSA:
-
Obstructive sleep apnea
- OR:
-
Odds ratio
- REM:
-
Rapid eye movement
- SWS:
-
Slow wave sleep
- SES:
-
Socioeconomic status
- USDA:
-
United States Department of Agriculture
References
Linton SJ, Kecklund G, Franklin KA, Leissner LC, Sivertsen B, Lindberg E, et al. The effect of the work environment on future sleep disturbances: a systematic review. Sleep Med Rev. 2015;23:10–9.
Jansson-Fröjmark M, Linton SJ. The course of insomnia over one year: a longitudinal study in the general population in Sweden. Sleep. 2008;31(6):881–6.
Morin CM, LeBlanc M, Daley M, Gregoire J, Merette C. Epidemiology of insomnia: prevalence, self-help treatments, consultations, and determinants of help-seeking behaviors. Sleep Med. 2006;7(2):123–30.
Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111.
Voyer P, Verreault R, Mengue PN, Morin CM. Prevalence of insomnia and its associated factors in elderly long-term care residents. Arch Gerontol Geriatr. 2006;42(1):1–20.
Léger D, Bayon V. Societal costs of insomnia. Sleep Med Rev. 2010;14(6):379–89.
Parthasarathy S, Vasquez MM, Halonen M, Bootzin R, Quan SF, Martinez FD, et al. Persistent insomnia is associated with mortality risk. Am J Med. 2015;128(3):268-75.e2.
Sofi F, Cesari F, Casini A, Macchi C, Abbate R, Gensini GF. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol. 2014;21(1):57–64.
Laugsand LE, Strand LB, Vatten LJ, Janszky I, Bjørngaard JH. Insomnia symptoms and risk for unintentional fatal injuries—the HUNT study. Sleep. 2014;37(11):1777–86.
St-Onge M-P, Mikic A, Pietrolungo CE. Effects of diet on sleep quality. Adv Nutr. 2016;7(5):938–49.
Dashti HS, Scheer FA, Jacques PF, Lamon-Fava S, Ordovás JM. Short sleep duration and dietary intake: epidemiologic evidence, mechanisms, and health implications. Adv in Nutr (Bethesda, Md). 2015;6(6):648–59.
Vlahoyiannis A, Giannaki CD, Sakkas GK, Aphamis G, Andreou E. A systematic review, meta-analysis and meta-regression on the effects of carbohydrates on sleep. Nutrients. 2021;13(4):1283.
Phillips F, Crisp A, McGuinness B, Kalucy E, Chen C, Koval J, et al. Isocaloric diet changes and electroencephalographic sleep. The Lancet. 1975;306(7938):723–5.
Yajima K, Seya T, Iwayama K, Hibi M, Hari S, Nakashima Y, et al. Effects of nutrient composition of dinner on sleep architecture and energy metabolism during sleep. J Nutr Sci Vitaminol. 2014;60(2):114–21.
Tanaka E, Yatsuya H, Uemura M, Murata C, Otsuka R, Toyoshima H, et al. Associations of protein, fat, and carbohydrate intakes with insomnia symptoms among middle-aged Japanese workers. J Epidemiol. 2013;23(2):132–8.
Tan X, Alén M, Cheng SM, Mikkola TM, Tenhunen J, Lyytikäinen A, et al. Associations of disordered sleep with body fat distribution, physical activity and diet among overweight middle-aged men. J Sleep Res. 2015;24(4):414–24.
Kalam F, Gabel K, Cienfuegos S, Ezpeleta M, Wiseman E, Varady KA. Alternate day fasting combined with a low carbohydrate diet: effect on sleep quality, duration, insomnia severity and risk of obstructive sleep apnea in adults with obesity. Nutrients. 2021;13(1):211.
Afaghi A, O’Connor H, Chow CM. High-glycemic-index carbohydrate meals shorten sleep onset. Am J Clin Nutr. 2007;85(2):426–30.
Gangwisch JE, Hale L, St-Onge M-P, Choi L, LeBlanc ES, Malaspina D, et al. High glycemic index and glycemic load diets as risk factors for insomnia: analyses from the Women’s Health Initiative. Am J Clin Nutr. 2019;111(2):429–39.
Morin CM, Belleville G, Bélanger L, Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–8.
Yazdi Z, Sadeghniiat-Haghighi K, Zohal MA, Elmizadeh K. Validity and reliability of the Iranian version of the insomnia severity index. Malays J Med Sci. 2012;19(4):31–6.
Smith S, Trinder J. Detecting insomnia: comparison of four self-report measures of sleep in a young adult population. J Sleep Res. 2001;10(3):229–35.
Asghari G, Rezazadeh A, Hosseini-Esfahani F, Mehrabi Y, Mirmiran P, Azizi F. Reliability, comparative validity and stability of dietary patterns derived from an FFQ in the Tehran Lipid and Glucose Study. Br J Nutr. 2012;108(6):1109–17.
Azar M, Sarkisian E. Food Composition Table of Iran. National Nutrition and Food Research Institute, Shaheed Beheshti University of Medical Sciences, Tehran. 1980.
USDA. Food composition table. Available from: https://fdc.nal.usda.gov/.
Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr. 2002;76(1):5–56.
Taleban F, Esmaeili M. Glycemic index of Iranian foods: guideline for diabetic and hyperlipidemic patients (in persian). Tehran: National Nutrition and Food Technology of Iran, Shahid Beheshti University of Medical Science; 1999;1:1–16.
Moghaddam MB, Aghdam FB, Jafarabadi MA, Allahverdipour H, Nikookheslat SD, Safarpour S. The Iranian version of International Physical Activity Questionnaire (IPAQ) in Iran: content and construct validity, factor structure, internal consistency and stability. World Appl Sci J. 2012;18(8):1073–80.
Nourbala AA, Bagheri YS, Mohammad K. The validation of general health questionnaire-28 as a psychiatric screening tool. Hakim Res J. 2009;11(4):47–53.
Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. J Am Soc Anesthesiol. 2008;108(5):812–21.
Bell SJ, Sears B. Low-glycemic-load diets: impact on obesity and chronic diseases. Crit Rev Food Sci Nutr. 2003;43(4):357–77.
Monro JA, Shaw M. Glycemic impact, glycemic glucose equivalents, glycemic index, and glycemic load: definitions, distinctions, and implications. Am J Clin Nutr. 2008;87(1):237s-s243.
Vlachos D, Malisova S, Lindberg FA, Karaniki G. Glycemic Index (GI) or Glycemic Load (GL) and dietary interventions for optimizing postprandial hyperglycemia in patients with T2 diabetes: a review. Nutrients. 2020;12(6):1561.
Haghighatdoost F, Azadbakht L, Keshteli AH, Feinle-Bisset C, Daghaghzadeh H, Afshar H, et al. Glycemic index, glycemic load, and common psychological disorders. Am J Clin Nutr. 2016;103(1):201–9.
Vrolix R, van Meijl LE, Mensink RP. The metabolic syndrome in relation with the glycemic index and the glycemic load. Physiol Behav. 2008;94(2):293–9.
Bao J, Atkinson F, Petocz P, Willett WC, Brand-Miller JC. Prediction of postprandial glycemia and insulinemia in lean, young, healthy adults: glycemic load compared with carbohydrate content alone. Am J Clin Nutr. 2011;93(5):984–96.
Brand-Miller JC. Glycemic load and chronic disease. Nutr Rev. 2003;61(suppl_5):S49–55.
Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, et al. Hypoglycemia and diabetes: a report of a workgroup of the American diabetes association and the Endocrine Society. Diabetes Care. 2013;36(5):1384–95.
Alsahli M, Gerich JE. Hypoglycemia. Endocrinol Metab Clin North Am. 2013;42(4):657–76.
Banarer S, Cryer PE. Sleep-related hypoglycemia-associated autonomic failure in type 1 diabetes: reduced awakening from sleep during hypoglycemia. Diabetes. 2003;52(5):1195–203.
Gais S, Born J, Peters A, Schultes B, Heindl B, Fehm HL, et al. Hypoglycemia counterregulation during sleep. Sleep. 2003;26(1):55–9.
St-Onge M-P, Mikic A, Pietrolungo CE. Effects of diet on sleep quality. Adv Nutr (Bethesda, Md). 2016;7(5):938–49.
Opp MR, Toth LA. Neural-immune interactions in the regulation of sleep. Front Biosci. 2003;8(2):768–79.
Krueger JM, Majde JA. Humoral links between sleep and the immune system. Ann NY Acad Sci. 2003;992(1):S9-20.
Acknowledgements
This study is related to the project NO. 1399/ 64299 from Student Research Committee, Shahid Beheshti University of Medical Sciences (SBMU), Tehran, Iran. We also appreciate the Student Research Committee and Research & Technology Chancellor in SBMU for their financial support of this study. The authors express their appreciation to all of the participants of this study.
Conflict of interests
The authors declared there is no conflict of interest.
Funding
This project was funded by grant NO. 1399/ 64299 from Student Research Committee, Shahid Beheshti University of Medical Sciences (SBMU), Tehran, Iran. The funding body has no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
HF, MKJ, and FT conceptualized and designed the study. EM, HT, and HF analyzed and interpreted the data. HF, AN, and SS drafted the initial manuscript. PM supervised the project. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed written consents were obtained from participants. All procedures performed in studies involving human participants adhered to the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The ethics research committee approved the study’s protocol of the Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Farhadnejad, H., Sadat, S., Jahromi, M.K. et al. The association of dietary glycemic index and glycemic load with the risk of insomnia in the adult population. BMC Nutr 9, 28 (2023). https://doi.org/10.1186/s40795-023-00689-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40795-023-00689-x