Abstract
Background
In Ethiopia, there are different pocket studies that assessed adolescent undernutrition which came up with inconsistent and inconclusive findings. Therefore, estimating the pooled prevalence and associated factors of the adolescent undernutrition using meta-analysis is crucial in Ethiopia.
Methods
A systematic review of eligible articles was conducted using preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines. A comprehensive searching of the literature was made in Pub Med, Scopus, Google, Google Scholar, Cochrane Library and CINAHL. The quality of the articles included in the review was assessed using the Newcastle-Ottawa Scale (NOS) for non-randomized studies in meta-analyses. The pooled prevalence and odds ratio of the associated factors with their 95% confidence interval was computed using STATA version 14 software.
Results
Twenty-two studies were included in the meta-analysis with a total of 17,854 adolescents. Using the random-effects model analysis, the pooled prevalence of stunting and underweight was 20.7% (95% CI: 16.08, 25.33) and 27.5% (95% CI: 17.9, 57.14), respectively. Rural residence, having family size≥5, households with an unprotected water source for drinking and food-insecure household were significant associated factors for adolescent stunting. Early adolescent age (10–14 years), family size≥5, food-insecure household, lack of latrine, WHO diet diversity score < 4, mother educational status (with no formal education) were significant associated factors for adolescent underweight.
Conclusion
Adolescent undernutrition remains one of the most important public health problems in Ethiopia. Almost a quarter of Ethiopian adolescents were affected by stunting and underweight. Large family size, rural residence and unprotected source of drinking water were the associated factors for adolescent stunting. Similarly, large family size, early age of adolescent, lack of latrine, low dietary diversity score, mother illiteracy, and food insecure household were the associated factors for adolescent underweight. It would be good to give high emphasis on adolescent undernutrition and it is important to address the above mentioned associated factors during adolescent nutritional interventions in Ethiopia.
Similar content being viewed by others
Background
An adolescent is defined as a person aged 10–19 years which is a period of gradual transition from childhood to adulthood [1]. Adolescence period is a critical period of physical growth and development. This period is very sensitive to malnutrition due to the increased physiologic need for nutrition. Adolescent malnutrition affects not only themselves but also their future generation. Underweight is low Body Mass Index (BMI) for age i.e. < − 2 standard deviation (SD). Stunting is the low height for age i.e. <−2SD. Adolescents are key to break the cycle of the intergenerational cycle of malnutrition, poverty, and food insecurity. Investing in this group of population will ensure longer-term sustainable results for reducing poverty, food insecurity, fertility, and malnutrition. However, this group has received minimal attention in the developing countries including Ethiopia. As a result, adolescent undernutrition is widespread problem prominently in economically developing countries including Ethiopia [1,2,3,4,5]. The prevalence of adolescent undernutrition in Ethiopia is very high and it increases over time. For example, according to the 2005 and 2011 Ethiopian demographic and health survey report, the prevalence of underweight in adolescent girls was 32 and 36%, respectively [6, 7]. In Ethiopia, overweight or obesity among adolescents is rare. Early marriage and subsequent pregnancy, lack of educational attainment, poor access to safe water and sanitation, lack of health services targeted for adolescents, lack of knowledge on the existing adolescent health services, cultures and low utilization of family planning methods were reported as a contributing factor to adolescent undernutrition. The consequences of adolescent undernutrition include delayed growth, retarded intellectual development, goiter, increase risk of infection, blindness, anemia and inadequate bone mineralization. In adolescent girls, future consequences of stunting include increased risk of adverse reproductive outcomes. E.g. risk for low birth weight, cephalo-pelvic disproportion, dystocia, and cesarean section. Again low body mass in adolescent girls is associated with reduced bone mass in early adulthood and may result in postmenopausal osteoporosis and its sequel [8,9,10].
Current efforts and investments in the first 1000 days of life that focuses on preventing stunting in children will be realized if there is improved adolescent nutrition. So far, most of the interventions have either focused on children aged 0–5 years or on pregnant or lactating women. However, not much attention has been paid to adolescents’ nutrition in developing countries. Today’s well-nourished adolescents will have optimal skills, talents, energies, and more of they will be healthy and responsible citizens and parents by tomorrow. To improve adolescent undernutrition and to break the intergenerational cycle of malnutrition, there should be attention and evidence-based interventions for adolescent undernutrition [11]. Notably, there are different studies which assessed adolescent undernutrition and associated factors in Ethiopia. However, they came up with inconsistency and inconclusive findings. They documented the prevalence of stunting in this group ranging from 7.2 to 40.1% [12, 13] and prevalence of underweight in this group from 5.8 to 80.8% [12, 14]. Moreover, there is no study or review that estimated the pooled prevalence of adolescent undernutrition and associated factors. Therefore, this systematic review and meta-analysis were conducted to estimate the pooled prevalence and associated factors of adolescent undernutrition, which could help policymakers and health professional for decision making.
Methods
Study design and search strategy
A systematic review of eligible articles was conducted using preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines [15]. A comprehensive search of articles published in English from January 2000 through November 2017 was made from Pub Med, Scopus, Google, Google Scholar, Cochrane Library and CINAHL. In addition to these databases, the reference lists of the included studies were scanned to find potential articles. The search was performed using key terms such as adolescent undernutrition (including stunting, underweight), adolescent malnutrition, prevalence of adolescent undernutrition, magnitude of adolescent undernutrition, assessment of adolescent undernutrition, adolescent nutritional disorders, associated factors, risk factors, determinants and Ethiopia separately and/or in combination using the Boolean operator like “OR” or “AND”. Two of the authors made the search independently. Articles retrieved from the databases were exported to Endnote version X6 to facilitate the article selection process and manage citation.
Study selection and eligibility criteria
This review and meta-analysis included studies conducted in Ethiopia with the primary objective of adolescent undernutrition and associated factors. Studies published in English and conducted at both facility and community levels were included. Studies conducted in adolescents who had diseases like HIV/AIDS, renal diseases and others were excluded. Before including the studies in the final review, they were assessed for inclusion criteria using the title, abstract and a full review of the studies. When the prevalence or associated factors were not reported, we contacted the authors, and if they did not respond or told us that the required data were not available, we excluded the study from the review. When one population was reported in more than one publication, only the most recent one or with maximum information was included in the review to avoid sample overlapping.
Outcome measure
The primary outcome of this review was the prevalence of adolescent undernutrition i.e. stunting and underweight. According to the WHO 2006 reference data, adolescent height-for-age, and Body Mass Index (BMI) for age below − 2 Standard Deviation (SD) is stunting and underweight respectively [16]. The second outcome of this review was the associated factors of adolescent undernutrition. If one factor was identified as an associated factor for adolescent undernutrition in two and above studies (articles) then that associated factor was included in this review and meta-analysis but not considered if it identified only from one study. Age, diet diversity score (DDS), family size, household food insecurity, residence, sex and water protection were included in the analysis of associated factors for adolescent stunting. Age, diet diversity score (DDS), family size, father education status, household food insecurity, latrine, mother education status and sex were included in the analysis of associated factors for adolescent underweight.
Quality assessment and data extraction
Two reviewers independently assessed the quality of the studies by adopting the specific protocol. The criteria proposed in the Newcastle-Ottawa Scale (NOS) for non-randomized studies were used to assess the quality of studies [17]. The following parameters were assessed: sampling strategy, inclusion/exclusion criteria, sample size, cut-offs and reference for the assessment of adolescent undernutrition status, criteria to identify undernutrition and covariates included in statistical models to identify associated factors. The final scoring system comprised 10 criteria for rating different quality elements for each study (Table 1). Any discrepancy between the two reviewers was resolved through discussion and by involving a third reviewer. A predefined data extraction format was used to collect information on the name of the author/s, year of publication, study period, study design, sample size, residence (rural, urban, both), age of study participants, sex of study participants, prevalence of stunting, prevalence of underweight and associated factors.
Publication bias and heterogeneity
To assess the existence of publication bias, funnel plots were scattered and tested for asymmetry. Egger’s test was computed [18]. A p-value< 0.05 was used to declare the statistical significance of publication bias. After a detailed examination of the studies by the authors, I2 test statistics were used to check the heterogeneity of studies. I2 statistics described the total variation across studies. I2 test statistics of < 50, 50–75% and > 75% was declared as low, moderate and high heterogeneity respectively [19].
Statistical methods and analysis
Statistical analysis was carried out using STATA version 14. Initially, data were entered into Microsoft Excel and then exported to STATA version 14 for further analysis. The effect size of the meta-analysis was the prevalence of stunting, underweight and odds ratio of the associated factors. Random effect model was used as a method of analysis [12]. We identified associated factors for adolescent undernutrition that met the meta-analysis eligibility criteria, by looking at the adjusted ORs and 95% CIs reported in each study. Subgroup analysis was conducted by regions of the country and residence of the study participants. The effect of selected associated factors which include; Age, diet diversity score (DDS), family size, household food insecurity, residence, sex and water protection for adolescent stunting, and diet diversity score (DDS), family size, father education status, household food insecurity, latrine, mother education status and sex for adolescent underweight was analyzed using separate categories of meta-analysis. The findings of the review and meta-analysis were presented using tables, forest plots and Odds Ratio (OR) and 95% confidence intervals (CI).
Results
Study searches and selection
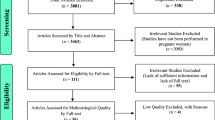
In the initial search, we found a total of 2100 records from different electronic search databases which include; Pub Med (595), Google (563), Google Scholar (490), Scopus (205), CINAHL (150) and Cochrane Library (97). From this, 250 duplicate records were removed and 1800 records were excluded after screening by title and abstracts. We assessed the full texts of 50 remaining records for eligibility, and 28 records were further excluded by the inclusion and exclusion criteria. Finally, 22 studies were considered for the final review and meta-analysis [7, 12,13,14, 20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] (Fig. 1). Of the 22 studies, 16 and 21studies were used to estimate the pooled prevalence of stunting and underweight respectively.
Characteristics of the studies and systematic review
All the studies included in this review were cross-sectional studies. A total of 17,854 adolescents were included in the analysis. The included studies reported sample size ranging from 211 [21] to 3724 [7]. Thirteen (59.1%) of the included studies were conducted in both urban and rural areas [7, 13, 14, 20, 22, 28, 30, 32,33,34,35,36,37]. One of the studies was conducted in Addis Ababa, the capital city of Ethiopia [12] and one study was conducted at the national level [7], 5 studies were from Eastern Ethiopia, 4 studies were from Western Ethiopia, 5 were from Northern Ethiopia and 6 were from Southern Ethiopia. The highest prevalence of stunting was reported from Western Ethiopia (40.1%), and the least was from central Ethiopia (Addis Ababa) (7.2%). The highest prevalence of underweight was reported from western Ethiopia (80.8%), while the least was from central Ethiopia (Addis Ababa) (5.8%) (Table 1).
Prevalence of adolescent undernutrition
Sixteen studies were included in the analysis to estimate the pooled prevalence of adolescent stunting. The heterogeneity among the 16 studies used to estimate the pooled prevalence of adolescent stunting was very high (I2 = 97.3% and p < 0.001). Using the random-effects model, the pooled prevalence of adolescent stunting was 20.7% (95% CI: 16.08, 25.33). Twenty-one studies were included to estimate the pooled prevalence of underweight. Heterogeneity among the studies used to estimate the pooled prevalence of adolescent underweight was very high (I2 = 99.6% and p < 0.001). The pooled prevalence of adolescent underweight was 27.5% (95% CI: 17.9, 57.14). A subgroup analysis by region and residence in Ethiopia was computed to compare the prevalence of adolescent undernutrition across different areas and participant characteristics. Thus, the pooled prevalence estimated for adolescent stunting was high in western Ethiopia (29.4% (95% CI: 13.39, 45.25)), and the least was in central Ethiopia (Addis Ababa) (7.2% (95% CI: 5.63, 8.77)). Similarly, the subgroup analysis by residence showed that adolescent stunting was high in rural areas (26.5, 95%CI: 20.62, 32.38). The estimated pooled prevalence of adolescent underweight was high in western Ethiopia (44.5, 95%CI: 3.65, 85.38) and least in central Ethiopia (Addis Ababa) (5.8, 95%CI: 4.43, 7.17). Another subgroup analysis showed that adolescent underweight was high in rural areas (41.34, 95%CI: 8.32, 74.37) (Figs. 2, 3, 4 5 and 6).
The associated factor for adolescent undernutrition
Eleven studies were included in the analysis of associated factors for adolescent stunting. Six associated factors for adolescent stunting were included in the analysis. The pooled odds ratios were ranging from 0.87 to 3.39. Heterogeneity wasn’t observed among studies evaluating a residence, family size≥5, unprotected water source for drinking, and food-insecure households. From these associated factors, adolescent age and sex were not statistically significant factors for adolescent stunting but residence, family size≥5, unprotected water source for drinking and food-insecure households were statistically significant factors (Table 2).
Similarly, 16 studies were included in the analysis of associated factor for adolescent underweight. Eight associated factors were included in the analysis. The pooled odds ratios were ranging from 0.69 to 4.1. Heterogeneity was observed among studies evaluating adolescent age, family size, food-insecure household, sex, latrine availability, diet diversity score (DDS), and mother educational status. Thus, weights were calculated using the random-effects analysis. Early adolescent age (10–14 years)), family size≥5, lack of latrine, WHO diet diversity score < 4, mothers with no formal education were statistically significant factors for adolescent underweight (Table 3).
Publication bias
We assessed the funnel plot for asymmetry by visual inspection for stunting, underweight and the associated factors. The funnel plot appeared symmetrical and found no publication bias and Egger’s test was also computed for stunting, underweight and associated factors similar to the funnel plot, it revealed evidence of no publication bias (Figs. 7 and 8) (Tables 2 and 3).
Discussion
Undernutrition negatively affects adolescents by affecting their ability to learn and work at maximum productivity, increases the risk of poor obstetric outcomes, affects sexual maturation and growth, intergenerational cycle of undernutrition, and preventing the attainment of normal bone and teeth strength [38].
This review was conducted to estimate the pooled prevalence and associated factors of adolescent undernutrition in Ethiopia. Quality of the studies included in this review was assessed using the Newcastle-Ottawa Scale (NOS) for non-randomized studies. Studies with > 5 score (out of 10 scores) were classified as good quality studies and those with ≤5 were poor quality studies. All studies had a score of > 5. In this review, the random-effects model was used for meta-analysis, considering the likelihood of significant heterogeneity amongst studies. The pooled prevalence of adolescent stunting was 20.7% (95%CI: 16.1, 25.3). Subgroup analysis using region and residence showed marked differences in the prevalence of adolescent stunting. High prevalence of stunting was observed from studies conducted in western Ethiopia and rural areas; 29.4 and 26.5% respectively. Stunting prevalence was low in central Ethiopia (Addis Ababa) and in the urban areas. The difference in the prevalence between the regions and residences could be due to the differences in socio-demographic and economic characteristics, and the number of studies included in each category of analysis. The stunting prevalence of this review is lower when we compared with the result report from studies conducted in South East Asia countries (Bangladesh (48%), Myanmar (39%)), India (54%) [39, 40] and Indonesia (23.6%) [41]. The prevalence of stunting in this review is within the range of the prevalence reported from Latin America and Caribbean countries (7–43%) [42]. The difference might be due to the difference in sampling and study period, cultural and dietary practices, access and utilization of health services.
In this review, the pooled prevalence of adolescent underweight was 27.5% (95%CI: 17.9, 37.1). Prevalence of adolescent underweight was high in western Ethiopia and in the rural areas; 44.5 and 41.3% respectively. Low prevalence of adolescent underweight was observed in central Ethiopia (Addis Ababa) and urban areas. The difference in adolescent underweight between regions as well as residences could be due to the recurrent drought and insufficient food production in western Ethiopia, especially in the rural areas. Drought, insufficient food production along with the global increased food prices can result in food insecurity and adolescent underweight [1]. According to the Ethiopia Demographic Health Survey Reports (EDHS), adolescent underweight in 2000, 2005, and 2011 was 38.4, 32.5, and 36% respectively [43]. The pooled adolescent underweight of this review (27.5%) is somewhat less than the underweight estimated in EDHS. Again underweight prevalence of this review is lower as compared to underweight prevalence studied in Bangladesh (67%), Myanmar (32%) and India (49%) [39, 40] but it is higher than the underweight prevalence reported from Latin America and Caribbean countries (3–22%), Zambia (13.7%), Nigeria (18.6%), Tanzania (21%) [42, 44,45,46]. The possible explanation for the differences in prevalence could be due to the differences in socioeconomic status, study period and access and utilization of health care services. Prevalence of adolescent underweight in this review is in line with the underweight prevalence reported from seven African countries (12.6–31.9%) [47].
In this review, the effect sizes of associated factors for adolescent undernutrition were estimated. The odds of having stunted adolescents were 2.25 times higher in households with a family size of ≥5 members. Likewise, a household with a family size of ≥5 members was 2.95 times higher in underweight than non-underweight adolescents. This finding is in line with a study conducted in sub-Saharan countries [48]. There is increased sharing of the available food among the large household members causing inadequate consumption of food. In addition to this, large family size usually found in uneducated parents who are more likely to accept and practice food taboo that affecting adolescent nutritional status [46]. Food insecure households were at increased risk of adolescent undernutrition. Food insecurity is one of the underline causes for undernutrition which can result in chronic nutritional problems in adolescent and cause long term negative effects in life [49].
Residence (rural) and unprotected source of drinking water were other significant associated factors for adolescent stunting. This can be explained by the inequalities in access to medical services, socio-economic status and health information in urban and rural settings. The unprotected source of drinking water is a vehicle for intestinal parasites and other communicable diseases which causes poor nutritional status. A similar finding was found in a study conducted in sub-Saharan countries [48]. Because of repeated infections, there is depressed immunity and making the severity and duration of diseases more severe and cause poor nutritional status of adolescents [41]. Age of adolescent (early age adolescent) was identified as an associated factor for adolescent underweight. There is faster growth and development in the early age of adolescent (10–14 years) as compared to late adolescent (15–19 years). Hence, if the requirement for achieving their maximum need for growth and development is not fulfilled, they will be prone to develop underweight [1]. Lack of latrine is statistically significant factor for adolescent underweight (AOR = 2.2, 95%CI: 1.1,4,4, p-value = 0.03). In areas where there is a scarcity of proper latrine utilization, contamination and infections will be common. Poor environmental sanitation and barefoot walking may serve as a means for parasitic infection [2, 3]. Improving dietary quality is important for increasing micronutrient intake [50]. In this review, WHO diet diversity score < 4 was higher among underweight adolescents as compared to the counterpart (AOR = 1.95 95%CI: 1.3, 2.9, p-value = 0.001). Low WHO DDS reflects inadequate dietary intake which can result in undernutrition [10].
Mother educational status was found as an associated factor for adolescent underweight. Educated mothers are cautious of what their family eats than uneducated mothers. Educational attainment of mother could lead to higher income and may imply a higher availability of food and household resources. It might be positively associated with higher nutritional awareness as well as better caring practices of an adolescent. Educated mothers can allocate family resources for nutrition and have health decision-making power which ultimately affects the nutritional status of the adolescent [1, 41]. This review used a comprehensive search strategy and more than two reviewers were involved in each step of the review process. PRISMA guideline was strictly followed during the review process and in order to explore the source of heterogeneity, a subgroup analysis was performed.
This review, however, has certain limitations like all the studies included were cross-sectional which could affect the temporal relationship between the assessed associated factors and outcome of interest. The number of studies for estimation of the effect size of associated factors was small which could affect the generalization of the findings. Study participants were not proportional in sex (female participants were higher in number).
Conclusion
Adolescent undernutrition remains one of the most important public health problems in Ethiopia. Almost a quarter of Ethiopian adolescents were affected by stunting and underweight. Large family size, rural residence and unprotected source of drinking water were the associated factors for adolescent stunting. Similarly, large family size, early age of adolescent, lack of latrine, low dietary diversity score, mother illiteracy, and food insecure household were the associated factors for adolescent underweight. It would be good to give high emphasis on adolescent undernutrition and it is important to address the above mentioned associated factors during adolescent nutritional interventions in Ethiopia.
Availability of data and materials
All data regarding this review are contained and presented in this review document.
Abbreviations
- AOR:
-
Adjusted Odds Ratio
- CI:
-
Confidence interval
- CINAHL:
-
Cumulative Index of Nursing and Allied Health Literature
- DDS:
-
Diet diversity score
- EDHS:
-
Ethiopian demographic and health survey
- PRISMA:
-
Preferred Reporting Items for Systematic Review and Meta-Analysis
- WHO:
-
World Health Organization
References
World Health Organization. Nutrition in adolescence-issues and challenges for the Health Sector; 2005. p. 1–123.
Afework M. Multiple micronutrient deficiencies in adolescent school girls from Tigray, Northern Ethiopia; 2009.
Robert E, Cesar G, Susan P, Zulfi QARA, Parul C, de Onis M, Maternal and Child Nutrition Study Group, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:472–51.
Manyanga T, El-Sayed H, Doku DT, Randall JR. The prevalence of underweight, overweight, obesity and associated risk factors among school-going adolescents in seven African countries. BMC Public Health. 2014;14(887):1–12.
Public Health at Glance. Adolescent nutrition. 2003.
Central Statistical Agency (Ethiopia) and ORC Macro. Demographic and health survey. Addis Ababa and Calverton: Central Statistical Agency and ORC Macro; 2005.
Central Statistical Agency (Ethiopia) and ICF International. Ethiopia demographic and health survey. Addis Ababa and Calverton: Central Statistical Agency and ICF International; 2012.
World Health Organization. Nutrition for health and development (NHD): a global agenda for combating malnutrition. 2000.
USAID and SPRING. Review of programmatic responses to adolescent and Women’s nutritional needs in low and middle-income countries. 2015.
UNICEF, Open University, AMREF and Federal Democratic Republic of Ethiopia Ministry of Health. Adolescent and youth reproductive health: blended learning module for the health extension program; 2008. p. 1–150.
Rukhsana H, Suman B. Adolescent nutrition: a review of the situation in selected southeast Asian countries; 2006.
Yoseph G, Solomon S, Balem D, Gessessew B. Nutritional status of adolescents in selected government and private secondary schools of Addis Ababa, Ethiopia. Int J Nutr Food Sci. 2014;3(6):504.
Herrador Z, Sordo L, Gadisa E, Moreno J, Nieto J, Benito A, et al. Cross-sectional study of malnutrition and associated factors among school-aged children in rural and urban settings of Fogera and Libo Kemkem districts, Ethiopia. PLoS One. 2014;9(9):e105880 PubMed PMID: 25265481. Pubmed Central PMCID: 4179248.
Huruy A, Tefera B, Legesse N. Socio-demographic factors associated with underweight and stunting among adolescents in Ethiopia. Pan Afr Med J. 2015;20:252 Pub Med PMID: 26161175. Pub Med Central PMCID: 4484194.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(5):393–5.
World Health Organization. Multicentre Growth Reference Study Group. WHO child growth standard’s: length/height-for-age, weight-for-age, weight for length, weight-for-height and body mass index-for-age: methods development. Geneva: World Health Organization; 2006.
Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses: Ottawa Hospital Research Institute; 2000. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ. 2003;327(7414):557.
Abdulkadir A, Tefera B, Habtemu J. Nutritional status and associated factors among primary school adolescents of pastoral and agropastoral communities, Mieso Woreda, Somali region, Ethiopia: a comparative cross-sectional study. J Public Health Epidemiol. 2016;8(11):297–310.
Afework M, Fitsum H, Barbara S, Gideon K, Vincent L, Zenebe A, Mekonen Y, Girmay G. Nutritional status of adolescent girls from rural communities of Tigray, Northern Ethiopia. Ethiop J Health Dev. 2009;23(1):5–11.
Ahmed Y, Tomas B. Nutritional status and associated risk factors among adolescent girls in Agarfa High School, Bale Zone, Oromia region, south East Ethiopia. Int J Nutr Food Sci. 2015;4(4):445–52.
Tolessa D, Mektie W, Aderajew N. Nutritional status and associated factors among school adolescent in Chiro Town, West Hararge, Ethiopia. Gazi Med J. 2015;21(1):32.
Dessalegn A, Jemal H, Gebrekiros G, Desta H. Magnitude of a double burden of malnutrition and its associated factors among selected in-school adolescents: evidence from South Ethiopia. Int J Nutr Metab. 2017;9(4):30–7.
Gebregyorgis T, Tadesse T, Atenafu A. Prevalence of thinness and stunting and associated factors among adolescent school girls in Adwa town, North Ethiopia. Int J Food Sci. 2016;2016:1–8.
Hadush G, Omer S, Huruy A. Assessment of nutritional status and associated factors among school-going adolescents of Mekelle city, northern Ethiopia. Int J Nutr Food Sci. 2015;4(1):118–24.
Roba KT, Abdo M, Wakayo T. Nutritional status and associated factors among school adolescent girls in Adama city, Central Ethiopia. J Nutr Food Sci. 2016;06(03):1–8.
Hunegnaw M, Takele T, Teresa K. Malnutrition and its correlates among rural primary school children of Fogera District, Northwest Ethiopia. J Nutr Disord Ther. 2013;03(02):1–7.
Mekonnen T, Semere S, Tesfaye A, Abdurrahman K. Nutritional status and associated factors of adolescent school girls, Goba town, Southeast Ethiopia. Global J Med Res. 2016;16:1):1–9.
Melaku YA, Zello GA, Gill TK, Adams RJ, Shi Z. Prevalence and factors associated with stunting and thinness among adolescent students in northern Ethiopia: a comparison to World Health Organization standards. Arch Public Health. 2015;73:44 Pub Med PMID: 26516456. Pub Med Central PMCID: 4624644.
Mesert Y, Jemal H, Hailu K, Fleming Fallon L Jr. Socioeconomic and demographic factors affecting body mass index of adolescents students aged 10-19 in ambo (a rural town) in Ethiopia. Int J Biomed Sci. 2010;6(4):321–6.
Teji K, Dessie Y, Assebe T, Abdo M. Anaemia and nutritional status of adolescent girls in Babile District, eastern Ethiopia. Pan Afr Med J. 2016;24:62 Pub Med PMID: 27642403. PubMed Central PMCID: 5012790.
Berheto TM, Mikitie WK, Argaw A. Urban-rural disparities in the nutritional status of school adolescent girls in the Mizan district, southwestern Ethiopia. Int Electron J Rural Remote Health Res Educ Pract Policy. 2015:1–10.
Wassie M, Gete A, Yesuf M, Alene G, Belay A. Predictors of nutritional status of Ethiopian adolescent girls: a community-based cross-sectional study. BMC Nutrition. 2015;1(1):1–7.
Wolde M, Berhan Y, Chala A. Determinants of underweight, stunting and wasting among school children. BMC Public Health. 2015;15:8 PubMed PMID: 25595201. Pubmed Central PMCID: 4308904.
Yayehyirad Y, Abel G, Wondessen N, Dejene H, Tadesse A, Frehiwot M, et al. Undernutrition and its associated factors among adolescent girls in the rural community of Aseko district, eastern Arsi zone, Oromia region, eastern Ethiopia, 2017. Int J Clin Obstet Gynecol. 2017;1(2):17–26.
Yebyo H, Weres ZG, Gesesew H, Miruts KB. Assessment of adolescents’ under nutrition level among school students in eastern Tigray, Ethiopia: a cross-sectional study. J Nutr Food Sci. 2015;05(05):1–6.
Tesfalem T, Pragya S, Debebe M. Prevalence and associated factors of overweight and obesity among high school adolescents in urban communities of Hawassa, southern Ethiopia. Curr Res Nutr Food Sci Jour. 2012;1(1):23–36.
World Health Organization. Adolescent nutrition: a review of the situation in selected south-east Asian countries. 2006.
Amitava P, Amal K, Arunangshu S, Prakash C. Prevalence of undernutrition and associated factors: a cross-sectional study among rural adolescents in West Bengal, India. Int J Pediatr Adolesc Med. 2017;4:9e18.
Sitti P, Andi I, Ida R, Abdul R. Low socioeconomic status among adolescent schoolgirls with stunting in Maros district, South Sulawesi in Indonesia. Int Proc Chem Biol Environ Eng. 2016;95. https://doi.org/10.7763/IPCBEE.2016.V95.13.
Pan American Health Organization. Underweight, short stature and overweight in adolescents and young women in Latin America and the Caribbean. 2008.
Government of the Federal Democratic Republic of Ethiopia, National Nutrition Program two. 2016.
Bupe B. Nutritional status among female adolescents aged (15 – 19 years) in Zambia: why it matters. Horizon journal of medicine and medical. Sciences. 2015;1(1):001–7.
Chukwuma B, Anthony C, Kenechi A, Kevin C, Chinomnso C, Irene A, et al. Prevalence and determinants of adolescent malnutrition in Owerri, Imo state, Nigeria. Int J Sci Healthc Res. 2016;1(3):53–63.
Lorraine S, Parke E, Helen S, F. James L. Household Food Security Is Inversely associated with undernutrition among adolescents from Kilosa (Tanzania). J Nutr. 2012;142(9):1741–7.
Taru M, Hesham E, David T, Jason R. The prevalence of underweight, overweight, obesity and associated risk factors among school-going adolescents in seven African countries. BMC Public Health. 2014;14:887. https://doi.org/10.1186/1471-2458-14-887.
Susan K, Guy P, Grace E, Bart V. Determinants of stunting and overweight among young children and adolescents in sub-Saharan Africa. Food Nutr Bull. 2014;35(2):167–78.
James E. Adolescent health and development (AHD): a resource guide for World Bank operations staff and government counterparts; 2004.
Allen LH. Interventions for micronutrient deficiency control in developing countries: past, present, and future. J Nutr. 2003;133:3875S–8S.
Acknowledgments
We would like to express our gratitude and appreciation to all staffs of Adigrat University (College of Medicine and Health Sciences) for their help. Again we would like to acknowledge participants of the studies and the authors of the studies included in this review.
Funding
No fund was received for this review.
Author information
Authors and Affiliations
Contributions
KBT involved in study design, selection of articles, data extraction, statistical analysis, and manuscript writing. AKB, GG, and AG are also involved in data extraction, statistical analysis, and manuscript writing. All authors read, revised and approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Berhe, K., Kidanemariam, A., Gebremariam, G. et al. Prevalence and associated factors of adolescent undernutrition in Ethiopia: a systematic review and meta-analysis. BMC Nutr 5, 49 (2019). https://doi.org/10.1186/s40795-019-0309-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40795-019-0309-4