Abstract
Introduction
In Ethiopia, malaria is one of the major public health and socioeconomic problems, though tremendous efforts have been made. Currently, the country has a plan to eliminate malaria by 2030. To achieve this plan, epidemiological studies associated with malaria prevalence with gender, age groups, species types, and seasons are essential. Therefore, the aim of this study was to assess the prevalence of malaria from 2013 to 2021 in Addis Zemen town, Northwest Ethiopia.
Methods
A retrospective study was conducted at assess the trend of malaria prevalence over the last nine years using recorded blood smear reports in the laboratory logbook from governmental health institutions. Trends in malaria cases and the proportion of genders, age groups, species, and seasons over time were compared. The data were analyzed using the SPSS-23 software package.
Results
The overall malaria prevalence between 2013 and 2021 was 10.4%. From all confirmed cases, the minimum and maximum prevalence of malaria cases were recorded in 2018 (2%) and 2016 (33.2%) years, respectively. The infectious rate of males (59.3%) was significantly higher than that of females (40.7%) (p < 0.0001). In all survey periods, all age groups were infected by malaria parasites; the majority of the cases were between 15 and 45 years (57%) older than others. Statistically, a greater proportion of P. falciparum (80.1%) was recorded than P. vivax (18.5%) (p < 0.0001). Malaria cases were occurring throughout each month. The relative highest peaks of total malaria cases were observed during the months of September, October, and November. Seasonally, the highest infection rate was observed during spring (40.20%) compared to other seasons.
Conclusions
In conclusion, the study revealed that malaria transmission remained high, which affected males more than females and potentially reproductive ages. Two of the most important Plasmodium species were identified and found during all reviewed months and years, though P. falciparum was the most prevalent. Hence, the problem can be alleviated by using season-based long-lasting insecticide treated nets, regularly overseeing ongoing irrigation activity, overseeing the reduction of the water level of the Sheni River, health education, and providing immediate patient treatment.
Similar content being viewed by others
Introduction
Malaria is a complex disease caused by protozoan parasites belonging to the genus Plasmodium that mosquitoes transmit through blood feeding on human hosts [1,2,3]. Of the five Plasmodium species, only Plasmodium falciparum and Plasmodium vivax have worldwide distribution [4] and are the predominant species in sub-Saharan Africa (SSA) [5].
Malaria is also one of the most important vector-borne diseases that cause morbidity and mortality throughout the world [6, 7] and one of the major diseases of people living in poverty within developing countries [3, 8]. Although tremendous efforts have been made, it continues to pose a serious challenge to countries in the SSA. Based on the WHO report, there were an estimated 241 million malaria cases in 2020, compared to 227 million cases in 2019. About 95% of all malaria cases were in the WHO African Region (Angola, Burkina Faso, Kenya, and Ethiopia). Over 20-year periods (from 2000 to 2020), the population at risk of malaria in SSA nearly doubled because of the presence of antimalarial drug resistance, mosquito resistance to insecticides, and invasive vector species (Anopheles stephensi) [9]. In 2020, there were an estimated 627,000 malaria deaths worldwide compared to 558,000 deaths in 2019. About 47,000 additional malaria deaths were due to disruptions in the provision of malaria prevention, diagnosis, and treatment during the pandemic [9].
In Ethiopia, malaria is the leading health problem because three-fourths (75%) of the total area of the country is malarious, and more than two-thirds (approximately 68%) of the total population lives below 2,000 m below sea level [10, 11]. In the country, the nature of malaria transmission is seasonal and unstable, with major transmission occurring from September to mid-December, following the main rainy season (June-August), and minor transmission occurring during March-May [12,13,14,15,16,17,18]. Besides, it varies with elevations, temperatures and rainfall [19,20,21].
Recently, in Ethiopia, malaria morbidity and mortality rates have shown a significant reduction over time. The number of confirmed malaria cases and death rate decreased by 47% and 58%, respectively, between 2016 and 2019 [5]. These results are obtained due to early diagnosis, quick treatment of cases, prevention and control of malaria among pregnant women using intermittent preventive therapy, and vector control methods including long-lasting insecticidal nets (LLINs) and indoor residual sprayings (IRSs) [5, 21]. However, retrospective data shows that the overall trend of malaria prevalence in many SSA-countries like Ethiopia is inadequate [3, 5]. In particular, this is true in Addis Zemen town; the overall trend of malaria prevalence has not studied so far [22], except for the species composition and ecology of anophelines [23]. Epidemiological data from known health facilities laboratory registration books over long periods of time is vital to designing timely and appropriate interventions. That is, generating evidence-based data on prevalence and the distribution of malaria over sex, age, and season from the registration books in the years is so essential. Such information is essential to evaluating the impact of previous intervention strategies as well as to designing evidence-based interventions. Hence, this study aimed to assess the prevalence of malaria in the past nine years at two health facilities in Addis Zemen Town, Northwest Ethiopia.
Methods
Study area description
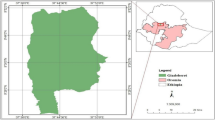
This study was conducted in Addis Zemen town, South Gondar, Amhara region, northwestern Ethiopia. Addis Zemen town is the capital of Libo-Kemkem district (Fig. 1). The district is situated at 11° 54′ 36” N and 37° 15′ 36” E with an elevation of 2,000 m above sea level. This town is located 656 km away from Addis Ababa (the capital city of the Ethiopia), 76 km away from Bahir Dar (the capital city of the Amhara region), and 61 km away from Debre Tabor town (the capital city of North Gondar administrative zone). According to the Addis Zemen Town Administration Office (Unpub. Report, 2021), the total population of the town was 40,798 (male = 19,601 and female = 21,197).
Map of study area: (a) Ethiopia, (b) Libo-Kemekem district, (c) Addis Zemen town, (d) Sheni river [22]
Addis Zemen town covers about 16.86 km2, which is divided into 4-kebeles (the smallest administrative unit). The town receives a unimodal rainfall of approximately 1,300 mm per year, mostly between June and August. The mean annual temperature is 19.7 °C. In the town, there was about 5-health facilities (2-government, 1-hospital and 1-health center; and 3-private medium clinics). Besides, there were 2 drugstores. In the town, there is the Sheni River, which is used for irrigation, swimming, washing clothes, and extracting sand for town residents [22]. Previous investigations have shown that the town of Addis Zemen has been considered a malaria spot area because large numbers of malaria cases have been recorded in the years [24, 25]. In this town, one of the carriers responsible for malaria in Ethiopia has been identified, Anopheles gambiae s.l [23].
Study design and population
A retrospective study was conducted to determine the nine (from 2013 to 2021) years of malaria prevalence from governmental health institutions (Addis Zemen Hospital and Health Center) found in Addis Zemen Town. The town has been divided into four ‘kebeles’. The study population included only residents of the four ‘Kebeles’ (the smallest administrative unit) in the town who had visited the health institutions during the study period and were only suspected and diagnosed with malaria. Therefore, this study was unable to discuss asymptomatic individuals, and this can be considered a limitation of this study.
Data collection
Nine-year (2013–2021) retrospective data on malaria prevalence’s were collected from the blood film malaria registration laboratory logbooks at Addis Zemen hospital and Addis Zemen health center, Addis Zemen town, Amhara Region, Northwestern Ethiopia. In these health facilities, peripheral smear examination of a well-prepared and well-stained blood film has been used as the gold standard for confirming the presence of the malaria parasite as per the WHO protocol [26]. In Ethiopia, the staining techniques and blood film examinations for malaria parasite detection are conducted according to a standard operating procedure in each hospital and health center throughout the country [13].
Data quality control
The retrospective data was taken from the primary record books of the Addis Zemen hospital and health center. The information about every member of the community was registered during their visit to the above health institutions. All necessary data were collected independently by four well trained health workers and cross-checked with each other, and finally confirmed by all authors. Moreover, external quality assurance was done by Amhara Regional Health Bauru. All incomplete data were excluded.
Data analysis
The data was entered in Microsoft Excel data sheets, cross-checked, and analyzed using the SPSS-23 software package. Descriptive statistics were employed to calculate frequencies and percentages of overall malaria prevalence, trends of malaria transmission in terms of seasons, years, sex, age groups, and species of malaria parasite. Tables and figures were used to describe the analyzed data (sex, age, and season, Plasmodium species). For the chi-square test, a p-value < 0.05 was taken as statistically significant.
Results
Annual malaria case trends
During the past nine years, from January 2013 to December 2021, a total of 44,289 malaria suspected cases were examined using a microscope in Addis Zemen Hospital and Addis Zemen Health Center. Of these, 4,626 (10.4%) were positive for malaria in both health institutions. The number of suspected malaria cases gradually increased from 2013 to 2016 and then decreased until 2018. It then began to rise, peak in 2020, and then gradually decline (Table 1). Similarly, the number of positive malaria cases showed a gradual increment from 2013 (3.9%, 181/4626) to 2016 (33.2%, 1534/4626) and then decreased until 2018 (2%, 94/4626). It then began to rise, and a peak in 2020 (20.4%, 942/4626), and then gradually declined. From all confirmed cases, the minimum and the maximum prevalence of malaria cases were recorded in 2018 (2%, 94/4626), and 2016 (33.2%, 1534/4626) years, respectively (Table 1).
Malaria incidence rate by sexes
Figure 2 illustrates the total number of confirmed malaria cases by gender in the last nine years. In all confirmed malaria cases, 59.3% and 40.7% were males and females, respectively. Statistical data indicate that the overall proportion of positive cases among men was significantly higher than that of confirmed malaria cases among women (χ2 = 295.527, d.f. = 8, p < 0.0001). In both sexes, the lowest and highest proportion of cases occurred in 2018 and 2016, respectively (Fig. 2).
Distribution of malaria cases by age groups
The distribution of malaria cases in relation to age is shown in Fig. 3. In all survey periods, all age groups were infected by malaria parasites; the majority of the cases were between 15 and 45 years (57%, n = 2635) as compared with age less than 5 (15.2%, 705), between 5 and 14 (18.3%, n = 847), and greater than 45 (9.5%, n = 439) years. The lowest malaria prevalence was seen people older than 45 years.
In connection with Plasmodium spp., both P. falciparum and P. vivax were affected in all age groups. However, P. falciparum was the predominant parasite in all age groups compared P. vivax. Particularly, a higher number of P. falciparum (59.3%) was observed in age groups ranging from 15 to 45 than the rest. Similarly, a higher proportion of P. vivax (47.3%) cases were reported when compared with other age groups (Fig. 3).
Distribution of Plasmodium species
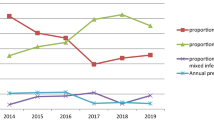
Throughout the reviewed periods (2013–2021), only two species of Plasmodium (P. falciparum and P. vivax) were found in the study area. The overall prevalence of P. falciparum and P. vivax was 80.1% (3705/4526) and 18.5% (858/4626), respectively. Moreover, 1.4% (63/4626) of mixed infections (P. falciparum + P. vivax) were observed. If compared the average 9-year proportion, P. falciparum was fourfold and fifty-eightfold more dominant than P. vivax and mixed infection, respectively (Fig. 4).
Statistically, a greater proportion of P. falciparum was recorded than P. vivax (χ2 = 330.079, d.f. = 8, p < 0.0001) in every surveyed year. In both sexes, the lowest and highest proportion of cases occurred in 2018 and 2016, respectively. The prevalence of P. falciparum slightly increased from 2013 and reached its peak level in 2016, then declined and reached the lowest level in 2018 (1.6%). Again, it showed an alarmingly high increment up to 2020 (23.3%). The prevalence of P. vivax also showed a similar tendency (Fig. 4).
Seasonal and monthly variation of malaria cases
Malaria cases were occurring throughout each month (Fig. 5), and there was a statistically significant average monthly variation (χ2 = 60.904, d.f. = 11, p < 0.0001). The mean monthly case was 385.5 (range 195–728). The relative highest peaks of total malaria cases were observed during the months of September, October, and November, and the lowest peaks were observed during the months of March and April. In particular, the highest P. falciparum cases were recorded in October and November, and the minimum cases were recorded in April and then March. Likewise, highest P. vivax cases were documented during the month of November, October, and September, and the fewest cases were observed in the months of April, January, and February (Fig. 5).
The seasonal distribution of malaria cases is presented in Fig. 6. The prevalence of malaria occurred in all seasons across the nine years. The highest and lowest cases of malaria were observed during spring (40.20%, 1859/4626) and autumn (17.40%, 807/4626), respectively. In all seasons, the proportion of P. falciparum was very high in comparison with P. vivax (Fig. 6). In particular, more cases of P. falciparum and P. vivax were detected in the spring, and summer; while the least number was found during autumn and winter, respectively.
Discussions
Annual malaria case trends
Malaria is one of the most important vector-borne diseases that causes morbidity and mortality throughout the world [8]. Worldwide cases of acute illness due to malaria are estimated to be 300–500 million; however, much of the burden is laid on African children under five years of age [27] and pregnant women [1, 27]; even the health problem is worsening in SSA countries. In Ethiopia, malaria is the leading health problem due to three-fourths of the total area of the country is malaria, and more than two-thirds of the total population lives below 2,000 m of altitude [10, 11].
Potentially, this study district is one of the malaria hotspots/ hubs in Ethiopia [24, 25]. In the present study, the overall slide-positive rate of malaria was 4,626 (10.4%) in nine-year period. This result was lower as compared with other reports such as 39.6% [28], 48% [29], 33.8% [30], 21.8% [31], and 21.8% [32] done in various parts of Ethiopia. However, it was higher than 7.15% [14], 8.4% [33], 5.4% [34], and 5% [35], which were reported elsewhere in Ethiopia. This difference could be due to the presence of studies time variation, variations in geographical locations and climate, differences in population awareness about malaria bed net application, the skill of the laboratory personnel to detect and identify malaria parasites, differences in insecticide application in the districts, expansion of irrigation, insecticide and drug resistance, and differences in health-seeking behavior of suspected individuals [26, 31, 36, 37].
The Ethiopian Ministry of Health gave great attention to achieving zero indigenous malaria in districts with annual parasite incidence less than 10 and preventing the reintroduction of malaria in districts reporting zero indigenous malaria cases by 2025 via ensuring early diagnosis and prompt treatment, strengthening vector control, improving malaria surveillance and response systems, etc. [5]. However, in the current study, malaria cases were recorded throughout the year, though fluctuation was a characteristic. The highest peaks of total malaria cases were observed in 2016 (33.2%), and the lowest peaks were observed in 2018 (2%). This result agreed with a study conducted at a Bichena primary hospital, higher and lower malaria cases were recorded in similar years [38]. Similarly, a 7-year retrospective study conducted in Dembia district by Addisu et al. [31]. indicated that the highest prevalence was observed in 2016 (30.2%), followed by 2015 (24%). However, it contradicts the Ethiopian 2011 malaria indicator survey, which reported a malaria prevalence of 1.3%. This makes our finding very higher than the 2011 Ethiopian malaria indicator report [39]. This contradiction might be associated with the employed sample size, the difference in malaria control and prevention activities implemented by stakeholders, climatic factors (rainfall and temperature), the expansion of irrigation, insecticides and drug resistance. Moreover, in this study, the second peak malaria case was seen in 2020 (20.4%). The rebound-back of malaria cases this year, probably linked to the recent impact of COVID-19 on malaria control and elimination efforts. The spreading of disease across the country was collapsing the country’s malaria control and prevention strategy [5]. Consistent with this, Eshetu et al. [18]. reported the second peak malaria case in the year 2020 in Maksegnit health center, central Gondar zone, Ethiopia.
Malaria incidence rate by sexes
During this survey, males were more affected by malaria parasites than females (p < 0.0001). This finding is in line with many studies conducted in different parts of Ethiopia [14, 18, 29, 31, 35, 38, 40], India [41, 42], and South Africa [43].
The presence of more infectious males than females in the present study is probably related to male work experiences and lifestyle (e.g., day labor). Males were often engaged in early night outdoor agricultural activities because irrigation activity is common in Addis Zemen. Moreover, males usually sleep outdoors to look after farms. Therefore, all these practices made males have a higher chance of exposure to be infected by the anopheles malaria vector as compared to female counterparts, who are mostly at home for taking care of children and homework purposes and protected from such infective bites. Evidence showed that much greater mosquito human-biting activities occurred outdoors than indoors during the early parts of the night, suggesting higher outdoor malaria transmission potential in Ethiopia [44]. To the reverse of our finding, a higher proportion of malaria-positive females were reported than males in Ethiopia [34] and Mozambique [45]. This variation could be coupled with the presence of low immune status of the females compared with males [46]. The presence of female house-tasks/ responsibilities/ or cooking the evening meal outdoors or reaching childbearing age or waking up before sunrise to fetch water may also put them at great risk of malaria infection [47, 48].
Distribution of malaria cases by age groups
Regarding age, all groups were found to be infected with the malaria parasite; however, the majority of the cases were between 15 and 45 years (57%) followed by between 5 and 14 (18.3%). This was in agreement with a study conducted in Kola Diba [28], Kombolcha [14], Wolkite Health Center [49], Bichena Health Center [38], and Maksegnit Health Center [18]. However, in contrast to this finding, the study conducted in Wolita [50] and Metema Hospital [51] showed high malaria positivity in 5–14-years older than the rest age groups. The presence of more malaria infected between the 15–45 age groups in Addis Zemen town is probably linked to the regular practice of irrigation activity in the town and its surroundings [52,53,54]. All these practices are more exposed to productive age groups and males to anopheles mosquito bites, which can transmit Plasmodium parasites.
Distribution of Plasmodium species
This study revealed the prevalence of P. falciparum (80.1%), P. vivax (18.5%), and mixed infections (1.4%). The existence of these two parasites is comparable with the previous documents made in Ethiopia, which were the most common parasites across the country [28, 29, 31, 38, 55]. P. falciparum was the most predominant species than P. vivax, which it exceeded about fourfold. This finding was in agreement with other previous studies conducted in Metema (P. falciparum, 90.7% and P. vivax, 9%) [51], Kola Diba (P. falciparum, 75% and P. vivax, 25%) [56], Woreta town (P. falciparum, 69.7% and P. vivax, 26.5%) [29], and selected Amhara region (Central, North and West Gondar zones) (P. falciparum, 73.4% and P. vivax, 26.6%) [55]. A previous report from WHO [57] supported this reality too, P. falciparum and P. vivax accounted for 70% and 30% of all laboratory-confirmed cases, respectively in Ethiopia. The exclusive predominance of P. falciparum over P. vivax could be that the P. falciparum parasite can multiply rapidly by involving more than one parasite in a single red blood cell, colonizing all ages of the red blood cells without any selection, parasite-infected red blood cells can accumulate in various organs, and the availability of P. falciparum-infected cases in communities [58, 59]. Furthermore, the drug resistance nature [60,61,62], misdiagnosed and inappropriate therapy [63], and gaps of programme performance could be the other possible reasons for such dominance.
Contrary to the current study, many other studies reported a higher proportion of P. vivax, than P. falciparum in many parts of Ethiopia [64,65,66,67,68,69]. Additionally, in Butajira P. vivax was 62.5% [70], in Hallaba health center P. vivax was 70.41% [71], in Adim Tullu district P. vivax was 84.6% [72], and in Wolkite health center P. vivax was 69.7% [49], Ethiopia. The possible explanation for this trend shifts from P. falciparum to P. vivax might be due to the public health importance of P. vivax, i.e., frequently overlooked and left in the shadow of the enormous problems (headache, shivering, appetite loss, anemia, nausea, vomiting) caused by P. falciparum [63]. In addition, the prevention and control activities of malaria as guided by the national strategic plan (2006–2010) mainly focus on P. falciparum because it is assumed to be more prevalent and fatal malaria in the country Ethiopia [73]. Another possible reason might be climate variability [65].
In our study, the proportion of both P. falciparum and P. vivax showed a slight increment from 2013 and reached a peak level in 2016, and then declined and reached the lowest level in 2018. Again, they showed an alarming rise in 2020. Consistent with this finding, Minwuyelet & Aschale [38] reported high and low prevalence levels of P. falciparum and P. vivax in Bichena primary hospital in 2016 (6.81%, 2.67%) and 2018 (2.13%, 1.65%), respectively. On the contrary, other retrospective studies [16, 32, 55, 74, 75] done in various parts of Ethiopia indicated that there was an overall decline in malaria incidence associated with a decrement of P. falciparum and P. vivax. The lack of smooth reduction in the present study could be connected with COVID-19 [5] and the absence of better practices of malaria prevention and control strategies, i.e., the use of LLINs alone, IRS alone, or the use of improper insecticide-treated bed nets [76]. Besides, in Addis Zemen town irrigation is common and farmers may spend most of their time in the field, which exposes them to mosquito bites [77].
Seasonal and monthly variation of malaria cases
Malaria cases were occurring throughout the months of each year and showed significant variation (χ2 = 60.904, d.f.=11, p < 0.0001). The highest peaks of total malaria cases were observed during September, October, and November, and the lowest peaks were observed during March and April. In particular, the highest P. falciparum cases were recorded in September, October, and November and the minimum cases were recorded in April and then March. Likewise, the highest P. vivax cases were documented during September, November, October, and the least cases were observed in April, January, and February. Our findings are consistent with other studies conducted in Bichena Primary Hospital [38], Woreta Health Center [34], and Boricha district [32]. Moreover, other previously made studies [14,15,16] in various parts of Ethiopia also documented the presence of increased total malaria cases in September, October, and November. These months are considered the peak malaria transmission period in Ethiopia after the heavy rain in July and August [5, 78].
Regarding the seasonal distribution of malaria, in our findings, the highest and the lowest cases of malaria were observed during spring (40.20%) and autumn (17.40%), respectively. In line with this, Getacher et al. [15], Alkadir et al. [16], and Minwuyelet & Aschale [38] reported highest and lowest peak of total malaria cases in spring and autumn in Bichena primary hospital and Ataye district, Ethiopia, respectively. Moreover, Alemu et al. [65]. in Jimma town, Gemechu et al. [79]. in Sibu Sire district, Alelign et al. [29]. in Woreta town, and Gebretsadik et al. [14]. in Kombolcha health center, Ethiopia, had reported peak malaria cases in spring than in other seasons. This season is preferable for mosquito breeding because it provides appropriate temperature and enough rainfall for them. In Ethiopia, the peak malaria transmission occurs between September and December following the June to September long rains [73, 78]. Different from this result, Eshetu et al. [18]. reported highest malaria cases in Autumn than in other seasons in Maksegnit Health Center. The review of Alelign et al. [29]. also indicated that Autumn was the second peak malaria season in Woreta town. This would be an important indication that the area needs due attention and further concerted malaria interventions.
Conclusions
The study indicated that malaria remains one of the most important causes of morbidity in our study area with a high slide positivity rate (10.4%). Malaria cases were occurring in all months and throughout the nine years, but the malaria transmission was peaking from September to November, coinciding with the major malaria transmission season in Ethiopia. In addition, cases of malaria were more frequent in men than in women, and the age group between 15 and 45 was very affected, which represents the productive segment of the Ethiopian population [80]. Two of the most important Plasmodium species were identified, P. falciparum and P. vivax; however, P. falciparum is the most predominant. These species were found in all the reviewed months and years in the study area. This could be a great challenge to the success of the ongoing malaria elimination program in Ethiopia. In this study, malaria transmission remains high, especially in the spring season. Hence, proper season-based LLINs (duration and timing of warm and wet climate) use and regular supervision of the ongoing irrigation activity, pocket water management due to Sheni River water reduction, health education, and immediate patient treatment are needed to alleviate the problem.
Data availability
All relevant data are available on the manuscript. If necessary and reasonably requested, all used data in the manuscript are available in the hand of corresponding author.
Abbreviations
- WHO:
-
World Health Organization
- SSA:
-
sub-Saharan African
- MoH:
-
Ministry of Health
- LLINs:
-
Long Lasting Insecticide Treated Nets
- IRSs:
-
Indoor Residual Sprayings
- m.a.s.l.:
-
meter above sea level
- SPSS:
-
Statistical Package for the Social Science
References
Heggenhougen HK, Hackethal V, Vivek P. The behavioral and social aspects of malaria and its control. WHO TDR/STR/SEB/ 03.1; 2003.
Ramirez JL, Garver LS, Dimopoulos J. Challenges and approaches for mosquito targeted malaria control. Curr Mol Med. 2009;9(2):116–30.
WHO. World Malaria Report-2014. NLM classification: WC 765. WHO; 2014.
Mendis K, Sina BJ, Marchesini P, Carter A. The neglected burden of Plasmodium Vivax malaria. Am J Trop Med Hyg. 2001;64:97–106.
MoH. National malaria elimination strategic plan: 2021–2025. Addis Ababa, Ethiopia; 2020.
Paaijmans KP. Weather, water and malaria mosquito larvae: the impact of meteorological factors on water temperature and larvae of the afro-tropical malaria vector. Anopheles gambiae Giles; 2008.
Ebi KL. Managing the changing health risks of climate change. By Elsevier B.V. Curr Opin Environ Sustainabi. 2009; 1:107–10.
Wimberly MC, Chuang T, Henebry JM, Liu Y, Alemayehu M, Paulos G et al. A computer system for forecasting malaria epidemic risk using remotely-sensed environmental data. Managing resources of a limited planet, 6th biennial meeting, Leipzig, Germany; 2012.
WHO. World malaria report 2021. Geneva World Health Organization; 2021.
Ayele DG, Zewotir TT, Mwambi HG. Prevalence and risk factors of malaria in Ethiopia. Malar J. 2012;11(195):1–8.
Pennas T, Girma S. Essential malaria actions: Ethiopia: Case study. USAID and President Malaria Initiative; 2012.
MoH. Ethiopia national malaria indicator survey 2007: Technical report. Ethiopia Ministry of Health. Addis Ababa, Ethiopia; 2008.
MoH. National strategic plan for malaria prevention, control and elimination in Ethiopia, 2011– 2015. Addis Ababa: Ministry of Health of Ethiopia; 2010.
Gebretsadik D, Getacher DF, Fiseha M. Eight-year trend analysis of malaria prevalence in Kombolcha, South Wollo, north-central Ethiopia: a retrospective study. Parasit Vectors. 2018;11:55.
Getacher FD, Gebretsadik D, Gebreweld A. Analysis of the trend of malaria prevalence in Ataye, North Shoa, Ethiopia between 2013 and 2017. Malar J. 2018;17:323.
Alkadir S, Gelana T, Gebresilassie A. A five-year trend analysis of malaria pre valence in Guba district, Benishangul-Gumuz regional state, western Ethiopia: a retrospective study. Trop Dis Travel Med Vaccines. 2020;6:18.
Teshome TS, Tesfaye AD. Five-year trend analysis of malaria prevalence in Shewarobit, Amhara Regional State, North-central Ethiopia. Pan Afr Med J. 2021;40:237.
Eshetu T, Muhamed B, Awol M, Kassa Z, Getu M, Derso A et al. A retrospective analysis of malaria trends in Maksegnit health center over the last seven years, Northwest Ethiopia: 2014- 2020. J Parasitol Res. 2022. Retrieved from: https://doi.org/10.1155/2013/704730.
Graves PM, Richards FO, Ngondi J, Emerson PM, Shargie EB, Endeshaw T, et al. Individual, household and environmental risk factors for malaria infection in Amhara, Oromia and SNNP regions of Ethiopia. Trans R Soc Trop Med Hyg. 2009;103:1211–20.
Connor SJ, Dinku T, Wolde-Georgis T, Bekele E, Jima D. A collaborate epidemic early warning and response initiative in Ethiopia; 2010.
Alelign A, Dejene T. Current status of malaria in Ethiopia: evaluation of the burden, factors for transmission and prevention method. Acta Parasitol Globalis. 2016;7(1):01–6.
Workineh L, Mekuria S, Kiros T, Hailemichael W, Eyayu TA. Retrospective study of malaria trend in Libo-Kemkem District over the last five years: North West Ethiopia. Infect Drug Resist. 2021;14:3683–91.
Kindu M, Aklilu E, Balkew M, Gebre-Michael T. Study on the species composition and ecology of anophelines in Addis Zemen, South Gondar, Ethiopia. Parasit Vectors. 2018;11:215.
Midekisa A, Senay G, Henebry GM, Semuniguse P, Wimberly MC. Remote sensing-based time series models for malaria early warning in the highlands of Ethiopia. Malar J. 2012;11:165.
Asmamaw T, Alemu A, Unakal C. Prevalence of malaria and HIV among pregnant women attending antenatal clinics at Felege Hiwot referral hospital and Addis Zemen health center. Int J Life Sc Biotech Pharm Res. 2013;2:81–91.
WHO. World malaria report 2016. WHO, Geneva. CCBY-NC-SA 3.0 IGO; 2016.
Dawit G, Temesgen Z, Mwambi HG. The risk factor indicators of malaria in Ethiopia. Malar J. 2013;5(7):335–47.
Alemu A, Muluye D, Mihret M, Adugna M, Gebeyaw M. Ten-year trend analysis of malaria prevalence in Kola Diba, North Gondar, Northwest Ethiopia. Parasit Vectors. 2012;5:173.
Alelign A, Tekeste Z, Petros B. Prevalence of malaria in Woreta town, Amhara region, Northwest Ethiopia over eight years. BMC Public Health. 2018;18(990):5–6.
Tesfa H, Bayih AG, Zeleke AJ. A 17-year trend analysis of malaria at Adi Arkay, north Gondar Zone, Northwest Ethiopia. Malar J. 2018;17(1):155.
Addisu Y, Tegegne Y, Mihiret Y, Setegn A, Jejaw AZ. A 7-year trend of malaria at primary health facilities in Northwest Ethiopia. J Parasitol Res. 2020;1–5.
Dabaro D, Birhanu Z, Yewhalaw D. Analysis of trends of malaria from 2010 to 2017 in Boricha District, Southern Ethiopia. Malar J. 2020;19:88.
Feleke DG, Gebretsadik D, Gebreweld A. Analysis of the trend of malaria prevalence in Ataye, North Shoa, Ethiopia between 2013 and 2017. Malar J. 2018;17(1):323.
Derbie A, Alemu M. Five years’ malaria trend analysis in Woreta health center, Northwest Ethiopia. Ethiop J Health Sci. 2017;27(5):665–472.
Yimer M, Hailu T, Mulu W, Abera B, Ayalew W. A 5-year trend analysis of malaria prevalence within the catchment areas of Felegehiwot referral hospital, Bahir Dar City, northwest-Ethiopia: a retrospective study. BMC Res Notes. 2017;10:239.
Alemu M, Tadesse D, Hailu T, Mulu W, Derbie A, Hailu T et al. Performance of laboratory professionals working on malaria microscopy in Tigray, North Ethiopia. J Parasitol Res. 2017; e9064917.
Gari T, Lindtjorn B. Reshaping the vector control strategy for malaria elimination in Ethiopia in the context of current evidence and new tools: opportunities and challenges. Malar J. 2018;17:454.
Minwuyelet A, Aschale Y. Analysis of five-year trend of malaria at Bichena primary hospital, Amhara Region, Ethiopia. J Parasitol Res. 2021. Retrieved from https://doi.org/10.1155/2021/6699373.
MoH. Ethiopia national malaria indicator survey 2011. Technical Summary; 2012.
Karunamoorthi K, Bekele M. Changes in malaria indices in an Ethiopian health center: a five year. Health Scope. 2012;1(3):118–26.
Koh KH, Chew PH, Kiyu A. A retrospective study of malaria infections in an intensive care unit of a general hospital in Malaysia. Singa Med J. 2004;45(1):28–36.
Kumar A, Valecha N, Jain T, Dash AP. Burden of malaria in India: retrospective and prospective view. Am J Trop Med Hyg. 2017;77(6):69–78.
Ngomane L, de Jager C. Changes in malaria morbidity and mortality in Mpumalanga Province, South Africa (2001–2009): a retrospective study. Malar J. 2012;11:19.
Kenea O, Balkew M, Tekie H, Gebre-Michael T, et al. Human-biting activities of Anopheles species in south-central Ethiopia. Parasit Vectors. 2016;9:527.
Temu A, Coleman M, Abilio P, Kleinschmidt I. High prevalence of malaria in Zambezia, Mozambique: the protective effect of IRS versus increased risks due to pig-keeping and house construction. PLoS ONE. 2012;2(7):e31409.
WHO Global malaria programme. World malaria report 2014. WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland; 2014.
Quaresima V, Agbenyega T, Oppong B, Awunyo JA et al. Are malaria risk factors based on Gender? A Mixed-Methods Survey in an Urban Setting in Ghana. Trop Med Infect Dis. 2021; 6,161. Retrieved from: https://doi.org/10.3390/tropicalmed6030161.
Okiring J, Epstein A, Namuganga JF, Kamya EV et al. Gender difference in the incidence of malaria diagnosed at public health facilities in Uganda. Malar J. 2022; 21:22. Retrieved from: https://doi.org/10.1186/s12936-022-04046-4.
Solomon A, Kahase D, Alemayehu M. Trend of malaria prevalence in Wolkite health center: an implication towards the elimination of malaria in Ethiopia by 2030. Malar J. 2020;19:112.
Legesse D, Haji Y, Abreha S. Trend analysis of malaria occurrence in Wolaita zone, southern Ethiopia: retrospective cross-sectional study. Malar Res Treat. 2015. Retrieved from: https://doi.org/10.1155/2015/123682.
Ferede G, Worku A, Getaneh A, Ahmed A et al. Prevalence of malaria from blood smears examination: A seven-year retrospective study from Metema hospital, Northwest Ethiopia. Malar Res Treat. 2013. Retrieved from: https://doi.org/10.1155/2013/704730.
Keiser J, Utzinger J, Tanner M. The effect of irrigation and large dams on the burden of malaria on global and regional scale. Am J Tr Med Hyg. 2005;72(4):392–406.
UNDP. Discussion paper: gender and malaria. December 2015. Rederived from: https://www.undp.org/sites/g/files/zskgke326/files/publications/Discussion%20Paper%20Gender_Malaria.pdf.
The conversation. Agriculture is linked with malaria in complex ways: evidence from 16 African countries. 2022. Retrieved from: https://theco nvers ation. com/ agric ulture- is- linked- with- malar ia- in-compl ex- ways- evide nce- from- 16- afric an- count ries- 179881.
Lankir D, Solomon S, Gize A. A five-year trend analysis of malaria surveillance data in selected zones of Amhara region, Northwest Ethiopia. BMC Public Health. 2021;20:1175.
Alemu A, Fuehrer HP, Getnet G, Tessema B, et al. Plasmodium Ovale Curtisi and Plasmodium ovale Wallikeri in North-West Ethiopia. Malar J. 2013;12(346):1–7.
WHO. World malaria report 2011.WHO. Geneva. (NLM classification: WC 765; 2011.
Beeson J, Brown G. Pathogenesis of Plasmodium Falciparum malaria: the roles of parasite adhesion and antigenic variation. CMLS. 2002;59(2):258–71.
Paul AS, Egan ES, Duraisingh MT. Host-parasite interactions that guide red blood cell invasion by malaria parasites. Curr Opin Hematol. 2015;22(3):220–6.
Schiltzer M. Antimalarial drugs– what is in use and what is in the pipeline? Arch Pharm Chem Life Sci. 2008;341:149–63.
Dondorp AM, Fairhurst RM, Slutsker L, Macarthur JR, et al. The threat of artemisinin-resistant malaria. New Engl J Med. 2011;365:1073–5.
Sinha S, Medhi B, Sehgal R. Challenges of drug-resistant malaria. Parasite. 2014;21(61):1–9.
Baird JK. Neglect of Plasmodium Vivax malaria. Trends Parasitol. 2007;23:533–9.
Woyessa A, Gebre-Michael T, Ali A. An indigenous malaria transmission in the outskirts of Addis Ababa, Akaki Town and its environs. Ethiop J Health Dev. 2004;18:2–7.
Alemu A, Abebe G, Tsegaye W, Golassa L. Climatic variables and malaria transmission dynamics in Jimma town, South West Ethiopia. Parasit Vectors. 2011;4(30):1–11.
Solomon T, Yeshambel B, Takele T, Girmay M, et al. Malaria pattern observed in the highland fringe of Butajira, Southern Ethiopia: a ten-year retrospective analysis from parasitological and metrological data. MWJ. 2012;3:5.
Sena L, Deressa W, Ali A. Analysis of trend of malaria prevalence in South- West Ethiopia: a retrospective comparative study. Malar J. 2014;13:188.
Molla E, Ayele B. Prevalence of malaria and associated factors in Dilla town and the surrounding rural areas, Gedeo Zone, southern Ethiopia. J Bacteriol Parasitol. 2015;6(242):1–7.
Yimer F, Animut A, Erko B, Mamo H. Past five-year trend, current prevalence and house- hold knowledge, attitude and practice of malaria in Abeshge, South-Central Ethiopia. Malar J. 2015;14(230):1–6.
Tesfaye S, Belyhun Y, Teklu T, Medhin G, et al. Malaria pattern observed in the highland fringe of Butajira, Southern Ethiopia: a ten-year retrospective analysis from parasitological and metrological data. MWJ. 2012;3(5):1–6.
Tefera G. Prevalence of malaria and associated factors among patients attending at Hallaba health center, southern Ethiopia. Immun Infect Dis. 2014;2(3):25–9.
Gari T, Kenea K, Loha K, Deressa W, et al. Malaria incidence and entomological malaria incidence and entomological findings in an area targeted for a cluster randomized controlled trial to prevent malaria in Ethiopia: results from a pilot study. Malar J. 2016;15(145):1–12.
MoH. National five-year strategic plan for malaria prevention and control in Ethiopia 2006– 2010. Ethiopia, Ministry of Health. Addis Ababa, Ethiopia; 2006.
Hawaria D, Getachew H, Zhong G, Demissew A, et al. Ten years’ malaria trend at Arjo– Didessa sugar development site and its vicinity, Southwest Ethiopia: a retrospective study. Malar J. 2019;18:1452.
Shamebo T, Petros B. Trend analysis of malaria prevalence in Halaba special district, Southern Ethiopia. BMC Res Notes. 2019;12:190.
Damien BG, Kesteman T, Dossou-Yovo GA, Dahounto A et al. Long-Lasting insecticide- treated nets combined or not with indoor residual spraying may not be sufficient to eliminate malaria: a case-control study, Benin, West Africa. Trop Med Infect Dis. 2023, 8(10)475. Retrieved from: https://doi.org/10.3390/tropicalmed8100475.
Kibret S, Alemu Y, Boelee E, Tekie H, Alemu D, Petros B. The impact of a small-scale irrigation scheme on malariatransmission in Ziway area, Central Ethiopia. Trop Med Int Health. 2009;15(1):41–50.
MoH. Guideline for malaria epidemic prevention and control in Ethiopia. (2nd edt). Addis Ababa: Ministry of Health; 2004.
Gemechu T, Samuel A, Yewhalaw D. Ten years trend analysis of malaria prevalence and its correlation with climatic variables in Sibu Sire District, east wollega zone, Oromia regional state, Western Ethiopia: a retrospective study. Sci Technol Arts Res J. 2015;4(4):99–105.
Megquier S, Belohlav K. Ethiopia’s Key. Young People and the Demographic Dividend; 2014. Retrieved from: https://www.prb.org/wp-content/uploads/2015/01/ethiopia-demographic.
Acknowledgements
The authors would like to thank Addis Zemen hospital and health center staffs for providing the required data. We also acknowledge Debre Tabor University for financing this study. Our gratitude also extends to the data collectors.
Funding
This research was financed by Debre Tabor University.
Author information
Authors and Affiliations
Contributions
T.A. conceived the study, involved in the data collection, analyzed data statistically, prepared the first draft of this manuscript; L.Z. conceived the study; G.A. involved in the data collection. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Official letter was collected from the directorate of Research and Publication of the University of Debre Tabor. And then, permission was obtained from the head of the Addis Zemen health office before the data collection.
Consent
Not applicable.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Adugna, T., Zelalem, L. & Alelign, G. Blood smears examination and prevalence of malaria in Addis Zemen Town, Northwest Ethiopia (2013–2021): a retrospective study. Trop Dis Travel Med Vaccines 10, 12 (2024). https://doi.org/10.1186/s40794-024-00219-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40794-024-00219-y