Abstract
It is undeniable that plastics are ubiquitous and a threat to global ecosystems. Plastic waste is transformed into microplastics (MPs) through physical and chemical disruption processes within the aquatic environment. MPs are detected in almost every environment due to their worldwide transportability through ocean currents or wind, which allows them to reach even the most remote regions of our planet. MPs colonized by biofilm-forming microbial communities are known as the ‘‘plastisphere”. The revelation that this unique substrate can aid microbial dispersal has piqued interest in the ground of microbial ecology. MPs have synergetic effects on the development, transportation, persistence, and ecology of microorganisms. This review summarizes the studies of plastisphere in recent years and the microbial community assemblage (viz. autotrophs, heterotrophs, predators, and pathogens). We also discussed plastic-microbe interactions and the potential sources of plastic degrading microorganisms. Finally, it also focuses on current technologies used to characterize those microbial inhabitants and recommendations for further research.
Similar content being viewed by others
Introduction
Plastic products are being manufactured at an enormous scale globally as they have become an integral part of daily life. Its market range consists of packaging and wrapping materials, carry bags, building materials, and industrial products. The global estimation of plastic waste generation is about 6.3 billion tons in the past few years [1, 2]. Studies have estimated that the current rate of plastic waste generation could double by 2030 if increased at this rate without any intervention [3]. Indian Plastic Waste Management Amendment Rules, 2021, has defined single-use plastics (SUP) as “a plastic commodity intended to be used once for the same purpose before being disposed of or recycled”. The Indian government has banned most SUPs from 1st July 2022 to mitigate environmental pollution. The European Commission has decided to ban some SUPs, which came into effect on 3rd July 2021. Landfill treatment of plastic wastes results in secondary environmental pollution [4,5,6]. Most plastic wastes (70–80%) are transported through the river to the ocean [7] and dispersed along the coastline, surface water, seafloor, and remote areas far from land [8, 9]. As per recent reports, an estimated 51 trillion plastic fragments of 236,000 tons are present in the marine environment [10]. Lebreton et al. [11] reported that about 91% of mismanaged plastic wastes are transported via watersheds (> 100 km2). Furthermore, > 25% of them have been discarded globally into 14 large (> 1,000,000 km2) riverine watersheds, viz. in Asia (Ganges, Yangtze, and Amur), Africa (Nile, Zambezi, and Congo), North America (Mississippi and Saint Lawrence), Europe (Lena and Volga), South America (Amazon). Recent multilevel dataset of microplastic abundance confirmed the presence of 8.2 × 104 ~ 57.8 × 104 tons (24.4 trillion pieces) of microplastics in the world’s upper oceans [12]. Global scenario of microplastic concentration on major aquatic bodies is mentioned in (Additional file 1: Table S1). Frias et al. [13] defined Microplastics (MPs) as ‘‘Any synthetic polymeric matrix or solid particle, with regular or irregular shape and with size ranging from 1 μm to 5 mm, of either primary or secondary manufacturing origin, which are insoluble in water”. Primary MP fragments are produced in microscopic dimensions [14]. Each year, a projected 1.5 million tons of MPs are released into the ocean [15]. Smaller MP fragments are comparatively more detrimental and difficult to eliminate than larger fragments of similar weight [16, 17]. The MPs formed from the decomposition and weathering of plastic waste in the aquatic environment converted to secondary MPs [14, 18]. The distribution of plastic waste in aquatic bodies is determined by buoyancy. Wind and ocean current facilitates the long distance transportation of floating plastics (for e.g., PE and PP) as they are less dense than seawater [19]. MPs like PVC, on the other hand, are more likely to sink due to greater density [20]. Floating plastics may lose surface hydrophobicity and eventually settle at the seafloor due to an increase in density over time [21]. They are capable of persisting for hundreds to thousands of years due to durability and stability. MPs in the form of fragments, pellets, fibre, foams, and films are most frequently collected from surface water samples [22]. MPs have a larger surface area to volume ratio and provide easier attachment for hydrophobic organic matters [23, 24].
Microplastics' physical attributes include their form, size, colour, density, and crystallinity, which are typically measured using microscopes. These characteristics can have a big impact on how microplastics behave in the environment and how harmful they are to creatures [25]. The size of microplastics is crucial to research since it is the most important property that distinguishes microplastics from conventional pollutants [26]. Microplastic density varies based on the polymers used and the manufacturing technique. Polyethylene (PE), polypropylene (PP), and polyvinylchloride (PVC), for example, have densities of 0.92–0.97 g/cm3, 0.85–0.94 g/cm3, and 1.38 g/cm3, respectively [27]. Lower density microplastics float on the top or suspend in water, where they can be consumed by creatures dwelling in the middle and upper levels [28]. They can also be identified by their colour, which can be utilized to determine their likely origin sources. Translucent, pink, yellow, red, green, blue, brown, black, grey, purple, and white are all prevalent colours for microplastics. Colourful samples are easier to spot in environmental media, whereas dull samples are often overlooked. The hue can also influence how long they stay in the environment and how quickly they degrade. The exposure time of the sample in the environment is represented by different degrees of fading [29, 30]. With increased exposure duration, the likelihood of oxidation and the degree of weathering increases [31]. According to certain studies, the shape of microplastics has an impact on desorption, adsorption, and ecological impacts. Sharp edges suggest a recent incursion into the ecosystem, but smooth edges indicate a long residence time, as numerous environmental pressures can produce relatively smooth edges. Another essential polymer characteristic is crystallinity, i.e., the mass ratio or volume ratio of the crystalline region [32]. The crystallinity of microplastics varies with age due to either breakdown or molecular rearrangement of polymer chains [33, 34]. The chemical composition and surface groups of microplastics are the most important chemical features. Polymers, dyes, additives (plasticizers, antioxidants), and contaminants are all adsorbed on the surface of microplastics. During the production, use, and weathering of plastics, these compounds are easily discharged into the environment [35, 36]. The physical features of polymers, such as porosity, molecular size, and degree of degradation, have an impact on the leaching rate of a chemical component [37]. Additive leakage can be facilitated by surface aging. The effect is obvious, and it is determined by the chemical content and distribution coefficient in the source plastic [38].
Studies have suggested that the adsorption of MPs in the ocean is much higher than that of sediments [39, 40]. MPs act as a unique substrate for microbial attachment due to the presence of organic compounds coupled with the various substances. The abundance of organic matter along with the coarseness of the MPs also offers a habitat for microorganisms to overcome environmental obstacles [41]. These properties make MPs an ideal substrate for microorganisms in the environment [42,43,44]. Plastic debris offers a surface for limiting nutrients like nitrogen, iron, and phosphorus depleting the source of nutrients in the ocean [45]. Floating MPs are a potential transport medium for microbes and even some pathogens, hastening the spread of infectious diseases [46]. The microbial colonizers on the MP surface (biofilm), known as plastisphere [47], are drifted and dispersed by currents and waves to a new habitat [46]. The identified plastisphere biomass contributes to 0.01–0.2% (approximate) of the total microbial biomass on the open ocean surface [45]. The plastisphere biomass in the ocean is probably substantial as recent studies have confirmed that only about 1% of the plastic debris that is released into the marine environment can be estimated [10]. The aquatic ecosystem and food chain are also impacted by MPs. In the ocean, MPs reportedly affected the growth and photosynthesis rate of phytoplankton [48], swimming potential of zooplankton, and hamper their reproductive efficiency [49, 50]. Researchers have also reported substantial quantities of MPs in the sediments and sea surface of the Arctic Ocean, which is supposed to be less affected by anthropogenic activities [51, 52]. MPs are reported in different levels of food web, from fish, shellfish, and coral to Antarctic krill [53,54,55,56]. MPs entering biological systems may cause local inflammation, weight loss and interfere with energy redistribution [57, 58].
In addition to it, the organic pollutants adsorbed on MP surface, such as diazinon, phenanthrene, and nonylphenol also cause ecological damage [59]. Studies have revealed that MPs also adsorb and accumulate pollutants, such as polychlorinated biphenyls (PCBs), alkylphenols bisphenol A (BPA), polybrominated diphenyl ethers (PBDEs), and dichloro-diphenyl-trichloroethane (DDTs), from the surrounding aquatic environment [2, 60, 61]. Different types of MPs with equal surface area and volume exhibits variable adsorption capacity [62]. PEs are capable of adsorbing more organic pollutants [63]. Antibiotics are also adsorbed by MPs, which accelerates the transportation of drug-resistant microbial population [64]. Subsequently, MPs can generate potential biotic and abiotic stress on the aquatic environment [65, 66]. In this review, we provided an understanding of the plastisphere, highlighting its role in plastic degradation. Moreover, these microbes may biologically transform plastic debris into detrimental compounds [67]. This review elucidates the current understanding of the composition of the plastisphere micro-ecosystem, highlighting its role in plastic biodegradation and future perspectives.
Methodology
A relevant literature search was conducted using the following terms, i.e., “plastisphere” OR “plastisphere ecology” OR “plastic degradation” OR “plastisphere characterization” OR “microplastic” OR “microplastic characterization”. Search engines for scientific research articles like Scopus, Web of Science (WoS), and Science Direct were used for this purpose. These searches consisted of papers published up to 5th January, 2022 and overall, 275 selected articles were included for the literature review. The preliminary selection method was based on the title and abstract of the paper. Those which could not be assessed by the title and abstract, were subjected to secondary quality check by scrutinizing the content of the paper. There were 65 studies on plastispheres and 62 studies on microplastics, respectively, 46 studies on plastic biodegradation, and 41 studies on various plastisphere characterization methodologies. The remaining 57 publications covered a variety of related topics such as plastic-microbe interaction, remediation procedures etc. The relevant information was collected and compiled after thoroughly reading the selected papers. There is a fair possibility that some important and relevant works might have been left uncited, specifically those that are not included in the Scopus, WoS, and Science Direct databases and did not use our article search terms.
Plastisphere biodiversity
The term “plastisphere” was introduced by Zettler et al. [47] to describe the diverse microbial community attached to plastic surfaces and distinct from the surroundings. Environmental sampling and laboratory incubation methods are the most preferred key strategies to study the plastisphere. Although reports on benthic aquatic environments are limited due to the expense and difficulty of sampling. Studies have been executed in which plastics are incubated under artificial laboratory setups [68]. These types of experiments are usually appropriate to study the degradation capacity and enzymatic functions of microorganisms. Previous studies of the plastisphere used scanning electron microscopy (SEM) for morphological identification of different species [3]. In contrast, recent studies to investigate plastisphere mostly relied on high-through output sequencing [47]. For example, studies of marine plastisphere used the eukaryotic 18S rRNA gene and the small ribosomal subunit 16S gene (16S rRNA) for Metabarcoding with sequencing technologies such as 454 pyrosequencing and MiSeq Illumina sequencing [69]. The classification of fungal community in plastisphere is performed using Internal Transcribed Spacer (ITS) technique [70, 71]. The hypervariable 16S rRNA locus V3 and V4-V5 locus of prokaryotes are the most commonly used genetic barcodes in metabarcoding studies (Table 1). 18S rRNA is a distinctive target for the identification of microbial eukaryotes in the plastisphere. Fungal-specific primers targeting the Internal Transcribed Spacer (ITS) regions are used for the detection of fungi (Table 2). However, more future studies on the plastisphere are required.
Plastisphere community assemblage
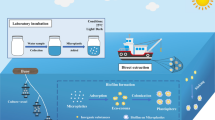
The community diversity of microbes within the plastisphere has been reported in early scanning electron micrographs of biofilms formed on plastics [96]. The term “Biofilm” can be defined as aggregates of cells that are either attached or unattached to a substrate and grow within a matrix consisting of extracellular polymeric substances (EPS) [97]. The process of biofilm formation employs a specific set of genes that are involved in the expression of adhesion, chemotaxis, communication, and substrate transport, enabling the formation of matrix and fluid channels to distribute nutrients between cells [98]. Current SEM studies [47, 87, 88, 92] combined with molecular data established the plastisphere as a surface-based micro-ecosystem (Fig. 1) comprising primary producers (phototrophs), symbionts, predators, and decomposers.
Conceptual illustration of the diverse plastisphere community, presenting a microbial ecosystem inhabiting plastic debris. Community members include phototrophs, heterotrophs, predators, and pathogens. Hypothesis drawn by a metagenomic study [92] on metabolic potential of plastisphere residents concludes that microorganisms found on plastic debris possess discrete sets of genes compared to those in a surrounding aquatic environment. The metabolic capacity and functional diversity of the plastisphere microbial community are not properly explained. Metagenome rRNA gene reads between 40 and 99% obtained from plastic debris mapped to eukaryotic rRNA, though it is unclear if the represented data is the actual abundances as eukaryotic microbial rRNA genes may consist of a significant number of disparate copies [133]
Phototrophs
Several studies on plastics exposed to sunlight have represented phototrophs (e.g., diatoms) as ubiquitous members of the plastisphere. They are often proclaimed as primary and occasionally dominant colonizers of plastic debris [94, 99,100,101,102,103]. Association between primary producers and microbial community plays significant role in aquatic food webs. Interaction between phototrophs and other microorganisms is also evident in the plastisphere [104]. Studies conducted on plastics recovered from the Sargasso Sea reported morphology [105] and amplicon reads [47] of several diatom genera including Pleurosigma sp., Cyclotella meneghiniana, Mastogloia angulata, Mastogloia hulburti, Mastogloia pusilla [105], Amphora sp., Nitzschia sp. and Sellaphora sp.[47]. Furthermore, the genera Amphora, Mastogloia, and Nitzschia were also reported from the Arabian Gulf [86]. These findings imply the tendency of diatom species to inhabit the sunlit plastisphere zone. Now it has been possible to unveil the phototroph genera present in the plastisphere with the help of new chloroplast databases [106] that assigns eukaryotic phototroph data derived from amplicon studies. The collected microplastics from the Pacific and Atlantic gyres along with the data gathered from marine incubation studies validated the presence of diatoms and chlorarachniophytes (protistan phototrophs) within the ocean plastisphere [107, 108]. Amplicon sequencing and microscopic observations characterized diatoms as common members of the plastisphere, whereas metagenomic analysis [92] showed the presence of diatom clades, hinting at their replacement over the time of community maturation. Cyanobacteria are also a representative of the photosynthetic community of plastisphere [92], which is evident from the combined measurement of chlorophyll, respiration measurement, and oxygen production. Several genera of filamentous cyanobacteria, such as Phormidium, Rivularia, and Leptolyngbya have been found on ocean microplastics. Complementary chromatic adaptation (light-harvesting adaptation strategies) of filamentous cyanobacteria makes them capable of overcoming the high and low-light challenges of the oligotrophic open ocean [92]. A PET bottle (polyethylene terephthalate) incubation study conducted in marine waters of Oman [86] reported that 4% of the plastisphere community was occupied by Microcystis (cyanobacteria) indicating a possibility of transportation of harmful algal bloomers to marine waters through the propagules attached to the plastic surface. As microcystin producers like Microcystis are not typical inhabitants of the marine environment, this finding indicates that these colonies might have arisen from plastic sources. It was reported that plastisphere colonizing cyanobacteria exhibit an entirely different light-harvesting mechanism with a higher expression of phycobilisome antenna encoding genes [92]. This suggests that cyanobacteria of plastisphere photosynthesize through the phycobilisome complexes. Phycobilisome proteins are converted to scarcity of nitrogen, and can rapidly restructure once the nitrogen source is accessible [109]. Phycobilisome serves as a light-harvesting complex and nitrogen reservoir for cyanobacteria present in nitrogen scarce environments. High expression of nifD, nifK, and nifH (nitrogen fixing enzymes) was also reported from plastisphere [92].
Photoheterotrophs and heterotrophs
Photoheterotrophic genera Erythrobacter and Roseobacter are commonly present in sunlit zones of the ocean. The bacteriochlorophyll within some of them is capable of fixing CO2 and photosynthesizing without producing oxygen engaging in heterotrophy [110]. Mixotrophic Roseobacter is a less frequently encountered bacteria species [111, 112]. Several previous studies support the concept of heterotrophic utilization of organic substrates by Cyanobacteria [113, 114]. Functional genes (e.g., genes related to mixotrophy) should be taken into consideration when drawing a conclusion rather than comparison based on taxonomic genera represented on plastic debris [89, 90]. An assortment of bacterial isolates derived from cultures containing polyethylene terephthalate (PET) or polypropylene as a sole carbon source, included Firmicutes (e.g., Bacillus spp.) [115], Actinobacteria (e.g., Rhodococcus spp.) [116] and Gammaproteobacteria (e.g., Azotobacter spp. and Pseudomonas spp.) [117, 118]. Some studies [47, 90, 94, 95] precisely addressed fungal sequences growing on plastic debris. The fungal community is commonly associated with decomposition, symbiosis, parasitism, pathogenesis, and predation, playing the role of potential saprotrophs in the plastisphere. Although fungal community diversity in the plastisphere is a comparatively underexplored area, recent reports [94] on fungal assemblages, Ascomycota, Cryptomycota, and Chytridiomycota were dominant members present on polyethylene and polystyrene substrates in fresh and brackish waters, making up to 4% of the total eukaryotic reads.
Predators
SEM and molecular data unveiled a striking symbiotic relationship between Ephelota (a predatory ciliate) and its ectosymbiotic sulfide oxidizing bacteria in the plastisphere [47, 94]. Plastic debris provides the surface attachment space to these suctorian ciliates, which are also common epibionts on copepods. There is more evidence of this type of co-occurrence network [94] that has shown positive associations between Amoebophrya and Suessiaceae on polyethylene surfaces. In addition, Amoebophrya is also a typical parasite found on dinoflagellates. The predatory ciliate Ephelota is commonly found in microplastic samples recovered from freshwater, marine, and brackish water, which may be an important perception of this particular ciliate’s survival potential far away from the coast. A study showed the prevalence of these genera on plastic concerned with seaweed mariculture [119]. Choanoflagellates, Radiolaria, and Micromonas (small flagellates) also belong to the predator community in the plastisphere, which consume bacteria and other organisms.
Pathogens
There are reported threats from toxic chemicals and invasive microbial species associated with plastic [120]. Colonization of plastics by pathogenic microbial genera has recently been a topic of concern since the study has shown Vibrio and many other potential microbial pathogens can get attached to the surface of plastic debris [47]. Plastic samples recovered from tropical [121] and temperate [85, 91, 122,123,124], marine, as well as freshwater [125, 126] environments, reported incidence of various potential pathogens (e.g., Arcobacter spp., Aeromonas salmonicida, and members of the group Campylobacteraceae). Microbial infections in fish, mollusks, and crustaceans [127] cause immense damage to aquaculture practices. One of the most common pathogens of fish is Vibrio spp., which is also responsible for the emergence of antimicrobial resistance [128]. Many of the equipment and gear used in aquaculture facilities (e.g., pens, lines, nets, floats, etc.) are plastic made which provides surface attachment area to potential harmful microbial colonizers). In addition to this, the presence of phototrophs on plastic debris which are responsible for algal blooms has also been reported [102, 129, 130]. Most of the evidence is based on molecular sequencing data and is not enough to confirm pathogenicity or toxicity, and reported abundances of pathogens were relatively low in the majority. Plastic debris can travel a long distance and frequent consumption by aquatic fauna is also evident. Aside from the risk of ingestion [120], studies have shown plastic as a potential transporter of protistan pathogens of corals [131] and fish [132]. Whenever plastic debris passes through wastewater treatment plants and into waterways, there are plenty of chances of human pathogen getting attached to its surface. A recent study [125] disclosed a relatively higher abundance of the human gastrointestinal pathogen (Campylobacteraceae) on MPs from a sewage treatment plant. Despite all the considerations, the role of plastics as means of transportation for the pathogenic microbial community is still in need of further additional investigation.
Ecological succession
Alike any other biofilms, the formation of plastisphere usually comprises attachment, secretion of EPS (extracellular polymeric substances), and proliferation [47]. The physical properties of plastics play a key role in the selection in earlier stages of colonization, and these pioneers subsequently impact the selection of other communities within the plastisphere [134]. Microbial adhesion is affected by several environmental factors like temperature, pH, nutrient availability, and hydrodynamic conditions [135]. These variables can alter the physicochemical features of the MP surface, such as texture, hydrophobicity, and charge [136]. Microbial attachment can be accelerated in aquatic environments by increasing flow velocity, water temperature, or nutrient concentration, as long as these factors do not reach critical values [137]. Biofilm development, for example, has recently been discovered to be a social element of bacterial life. Environmental factors influence whether a cell forms or leaves a biofilm. Cells change their gene expression in response to their surroundings. Environmental influences and gene regulation are linked by second messengers like cAMP and bis-(3′-5′)-cyclic dimeric guanosine monophosphate (c-di-GMP). Cell-to-cell contact is also crucial in the formation of the biofilm. One of the most significant processes is surface attachment, which marks the transition from planktonic to biofilm mode. First, bacterial cells reversibly adhere to the surface at their poles, a process known as a reversible attachment [138, 139]. Cells undergo irreversible attachment after the intracellular second messenger, c-di-GMP, is implicated in transition [140]. Many bacteria synthesize c-di-GMP, which controls EPS synthesis and motility in opposite ways. The environmental concentration of carbon and oxygen regulates the function of these second messengers [141]. Temperature changes substantially impact biofilm shape, including thickness and cell density [142].
Studies on the plastisphere have shown strong shifts of distinct communities throughout the early stage of colonization. However, in mature biofilms, microbial communities are converged over time and remained stable. Although a consistent and stable plastisphere can be achieved within a few weeks, the formation of a mature eukaryotic community may take more time [80]. The hydrophobic property of plastics provides a strong interface to the aquatic environment obstructing the surface attachment of microbes. However, plastic surfaces are hastily covered with organic matter (collectively known as the ecocorona [66]) which facilitates microbial colonization. A preliminary study [143] has revealed that in a marine environment, the process of microbial attachment to plastics happens within minutes. The primary colonization on a pristine plastic surface is an initial proliferation of microbial colonizers devoid of high competition aiming to cover the maximum surface area within a short period. After initial colonization, microbes deviate into specific niches to avoid competing for habitat and resources. The maturing biofilm releases quorum quenching secondary metabolites as well as complex EPS (extracellular polymeric substances) for protection [123, 144,145,146,147,148]. At the time of colonization, readily available polymeric oligomers [149,150,151] or additive leachates [152], produced by weathering of plastic provide energy to pioneering microbial species. These preliminary energy sources are however exhausted within days, and a continuous supply of energy is sustained by photosynthate from other plastisphere phototrophs and organic matter obtained from the environment. As per reports [47, 92] cyanobacteria, green algae, and diatoms are pioneers of the marine plastisphere community. Studies have suggested that the plastic biodegradation process is likely to be delayed due to plastisphere community succession [153,154,155]. It has been reported that plastisphere colonization occurs through different succession stages: (I) Colonization or initial surface attachment of pioneering microbial community, where the copious organisms are proficient in effective surface adhesion; (II) selection phase, where polymer degrading organisms become embellished and diversified; and (III) succession phase, during which the plastic degraders are overhauled by cross-feeders, grazers and other microbes [153,154,155,156] (Fig. 2). Although microorganisms easily colonize on the MP surface, the formation of a stable biofilm takes more time [9]. Early settling members belonging to Alphaproteobacteria and Gammaproteobacteria are reported as pioneer colonizers [9]. According to reports, Gammaproteobacteria dominated in the early stages of biofilms [71, 89, 143]. Poseobacter, Alteromonas, Phodobacteriaceae, Thalassobius, and Neptuniibacter were also reported from the early stages of biofilm formation [157]. Secondary colonizers such as Flavobacteriaceae (Bacteroidetes) flourish utilizing the organic substrates released by pioneer colonizers [9, 157]. The formation of secondary biofilm requires several months [71, 158]. Tu et al. [82] reported Rhodobacteraceae (Alphaproteobacteria), Microtrichaceae (Acidimicrobiia), and Flavobacteriaceae (Bacteroidia) as the pioneers of the early biofilms (30 days). After 75 days, the biofilms are dominated by Moraxellaceae (Gammaproteobacteria) and Bacillaceae (Bacilli) groups. At the matured stage (135 days), the dominance is shifted again to the Flavobacteriaceae (Bacteroidia), with a significant increase of Microtrichaceae (Acidimicrobiia), Pirellulaceae (Planctomycetes), and Rhodobacteraceae (Alphaproteobacteria).
Depiction of plastic colonization in an oceanic environment in an abstract form; plastic degrading microbes (PDM) might belong to the pioneer community, especially if oligomeric and polymeric additives are available as carbon and energy sources. Once these sources are exhausted, the specialized microbes which can utilize the labile photosynthate produced by phototrophs will outcompete the PDM [158]
Biofilm formation on aged microplastic
Physical and chemical properties of aged MPs differ from those of unaged MPs. MPs are subjected to numerous aging processes in natural environments, including ultraviolet (UV) exposure, physical corrosion, oxidation, and biodegradation [159]. Plastics in the ocean emit dissolved organic carbon (DOC), promoting heterotrophic microbial activity [150]. Aging increases the hydrophilicity, charge, and polarity of MPs [160, 161]. UV absorption by unsaturated groups, oxidation, polymer radical generation, chain cleavage, and crosslinking are all considered key processes in the aging of hydrocarbon polymers [162,163,164]. However, little is known about microplastics aging behavior and the effects of aging on their adsorption of contaminants in the environment. As a result, it’s critical to investigate the aging properties of microplastics concerning their age. Microplastics may coexist in natural water with reactive oxygen species (ROS) produced by natural (e.g., biological [165] and photochemical [166, 167]) and photosensitization processes [164, 168]. However, little is known about the impacts of ROS on the aging behavior of microplastics to the best of our knowledge, which could be explained by the extended times necessary for natural processes due to the low oxidant concentration in aquatic environments. Due to their porous hydrophobic architecture, MPs in the environment have been proven to be a carrier for adsorbing several sorts of heavy metals, hydrophobic organic pollutants, and pathogenic microbes. After various aging processes, MPs' surface qualities such as specific surface area (SSA) and hydrophilicity change, impacting their adsorption behavior [169, 170]. After aging, the morphology of MPs changes with increased surface roughness and specific surface area (SSA) [171, 172]. Because bacteria are prone to attach to the coarse surface of MPs, these alterations may be helpful to their adhesion on the surface [173]. UV-induced photodegradation of MPs destroys the polymer's chemical bonds, forming new functional groups [172]. They could convert PE's hydrophobic surface to hydrophilic, allowing some hydrophilic bacteria to inhabit it [174]. Aging can stimulate the release of chemicals in MPs. According to research, several chemicals can be utilized as nutrient sources to help bacteria proliferate [175]. Through hydrophobic dispersion and the formation of extracellular polymeric molecules, the biofilm boosts the adsorption capacity of contaminants in the environment [176]. Changes in weathered MPs' physicochemical properties, such as decreased surface hydrophobicity and increased heterogeneity, can, on the other hand, impact their adsorption behavior [177]. The presence of biofilms may also help MPs age more slowly due to reduced photodegradation (e.g., UV irradiation) facilitated by biofilm shields [178]. Due to a rougher surface and poorer hydrophobicity, the adsorption of nutrients, surface area for bacterial colonization, and development of biofilms are improved [47, 179].
Plastisphere distribution
The distribution of MPs is variable in the aquatic environment, with PET, PVC, and PA dominating in the bottom sediments, while, PP and PE are mostly detected in the upper water columns [19]. This is also dependent on the surface area, volume, and density of MP fragments. The activity of the microbes is in accordance with the state of MP substrate. Reports suggest that the properties of the MPs, including roughness, surface area, hydrophobicity, surface charge, and density would be affected after biofilm formation [176, 180, 181]. Smaller MP particles are more likely to be dispersed in the deeper ocean and are relatively less studied [182]. The MPs deposited on the seafloor are transported via slower benthic currents throughout the seafloor [183]. Studies have shown that Proteobacteria and Bacteroidetes dominated the plastisphere even in deep water [89, 184]. Settling of MPs in the ocean can lead to more environmental stresses. MPs are reported to be transferred to higher organisms along the food chain [185, 186].
Physico-chemical, microbial, and phyto remediation of plastic
According to several studies [187,188,189,190,191] on previously treated polyethylene (priorly exposed to thermal treatment, UV light, or containing additives) (Additional file 1: Fig. S1) erstwhile measuring degradations, it is difficult to predict whether the microbes acted on high molecular weight polymers or its smaller fragments produced by abiotic factors (Fig. 3A). Small molecules of dissolved organic matter (low molecular weight) are leached from plastic and contribute to the microbial attachment on the plastic surface and metabolic activity in the aquatic environment. The molecular weight of the polymers must be low enough so they can pass through the microbial cell membrane and undergo intracellular oxidation [118] (Fig. 3B). Reports [192] have suggested that hydrolyzable polymers (polyethylene terephthalate, polycarbonate, polyurethane) in the photic zone act as plastisphere substrates, whereas non-hydrolyzable polymers (polypropylene, polyethylene, expanded polystyrene) frequently encountered in the pelagic zone of the marine environment (Additional file 1: Figs. S2, S3).
A Several physicochemical and biological processes interact to degrade conventional plastics. Most of the perceptions regarding biological processes are laboratory-cultured strains and consortia based, and many of these strains are present in terrestrial habitats. This diagram depicted a hypothetical representation of the processes that lead to plastic deterioration in aquatic environments like the open ocean. Floating plastic waste undergoes different types of degradation in the presence of sunlight. The visible spectrum facilitates thermal degradation, whereas the infrared radiation leads to the thermal oxidation of polymer chains, and UV is responsible for the photodegradation through the bond scission mechanism. B Biological pathways for polymer degradation involve the action of microorganisms growing on its surface and enzymatic processes leading to polymer hydrolysis into oligomers and eventually monomers. Hydroxyquinone, alkB, laccase, oxygenase, peroxide, etc., are reported enzymes that break down highly stable backbones of non-hydrolyzable polymers. Hydrolyzable polymers are comparatively susceptible to enzyme-mediated (PET hydrolase, esterase, cutinase, etc.) catalysis [3]
A group of alkane degrading enzymes such as hexadecane was reported as a potential degrader of recalcitrant polymers (polyethylene) [118]. A study [118] on mesophilic Pseudomonas sp. derived from marine beach sediment, incubated with a low molecular weight polyethylene (LMWPE) as a solitary carbon source has shown its potential for polyethylene biodegradation. Although hydrolyzable plastics are susceptible to microbial extracellular hydrolases (present in pre-existing degradation pathways) involved in cellulose and protein degradation, the other environmental conditions restrict the plastics from undergoing complete biodegradation. The recovery of PET hydrolytic enzyme PETase from the bacterium Ideonella sakaiensis [193] and the other subsequent enzymes from marine and terrestrial metagenomes included in public databases [194] specify the omnipresence of PET degrading capacity of plastisphere in those environments. Nevertheless, the extreme oxidation via microbial biotransformation or incomplete hydrolysis of polymers leads to the generation of nano plastic which might hold the risk of ingestion through the food chain. Although the consequence of nano plastic on human health is less explored, reports [195] have suggested the possibility of translocation of microplastics (less than 150 μm) across the human gut epithelium into the lymphatic system, triggering systemic exposure and leaving an eventual effect on health after ingestion. Basiomycote Trametes versicolor (white-rot fungus) and Phanerochaete chrysosporium, Ascomycete Humicola insolens (soft-rot fungus), and Engyodontium album are the reported terrestrial fungal representatives of plastic degrader [192]. Numerous microbial species reported as potential plastic degraders are listed in (Additional file 1: Table S2). Most of the conducted plastic biodegradation experiments are in vitro, and the rate of the field degradation study has shown biodegradation rate in both marine (1.6–1.9% weight loss in 1 year) [196] and terrestrial (0.1–0.4% weight loss in 800 days) [197] environments. Various additional factors such as suboptimal degradation temperature, preference of microbes for palatable carbon sources, and polymer crystallinity play a significant role in the observed differences in the field versus laboratory-based studies. Another group of fungi, Homobasidiomycetes consisting of esterase and other polymer degrading enzymes, have been reported to degrade nylon and polyethylene in a laboratory setting [198, 199]. Though these are rare inhabitants of the marine environment, they exist in mangrove ecosystems; yet their metabolic potential is an under-explored area.
Any remediation procedure tries to eliminate the contaminant from the environment while maintaining its natural balance. Phytoremediation is an environmentally favorable approach that is commonly used [200]. Similarly, many researchers' recent discoveries [201] verified that plants accumulate microplastics. Depending on the type and level of microplastic contamination at a given site, however, several phytoremediation procedures such as phytofilteration, phytoextraction, and phytostabilization can be used. The initial point of interaction for microplastics in contaminated soil is the root zone of the plants. Li et al. (2019) [201] discovered polystyrene microbeads (0.2 m) in the root cap of lettuce plants, proving that the rhizosphere is the initial point of interaction in any phytoremediation strategy. Tolerance for MPs, accumulation proficiency, biomass output, bioaccumulation factor, root system, and other factors should all be considered when choosing plants for phytoremediation. Aside from the numerous benefits, the main downside is the potential of microplastics entering the human food chain. As a result, consideration should be given to plant selection based on MP mobilization or immobilization in the soil [202]. Plants can also collect MP pollutants from soil, according to recent results [200, 201], making them a potential phytoremediator for MP in terrestrial ecosystems. Phytoextraction (phytoaccumulation), phytostabilization, and phytofiltration are all potentially helpful phytoremediation strategies for MP-contaminated soils or water. Plant roots acquire MP pollutants and transport them to their above-ground tissues through phytoextraction or phytoaccumulation, a popular (green) remediation technique. The utilization of specific plants to immobilize MPs in the soil is the foundation of phytostabilization. The process of sorption and precipitation can help to stabilize plants. MP tends to seep into the soil's deeper layers, potentially contaminating groundwater [203]. MP are absorbed and deposited onto roots or precipitated in the rhizosphere of plants as a result of phytostabilization. This decreases or even prohibits MP mobility, reducing migration into groundwater or the atmosphere, as well as decreasing MP bioavailability, inhibiting MP distribution through the food chain. Phytofiltration is a process that uses plant roots (rhizofiltration) or seedlings (blastofiltration) to collect or adsorb MP from groundwater and aqueous waste streams rather than soil. It is comparable to phytoextraction. The rhizosphere is the soil area immediately surrounding the root surface of a plant, usually up to a few millimeters. Contaminants are absorbed or adsorbed into the root surface [204, 205]. The roots are collected and carefully disposed of after they have been wet. The site’s contamination can be reduced to an acceptable level with repeated treatments [205]
Characterization technologies
Microplastic serves as an excellent substratum for biofilm attachment as they adsorb essential nutrients and organic matter from the surrounding environment to support microbial growth. Usually, the microbes colonize the plastics within 24 h [179] depending on several environmental factors, which significantly influence the microbial composition of the plastisphere [74]. The fluorescence microscopy (FM) and scanning electron microscopy (SEM) recognize the microbial association in the biofilm [47, 89, 92, 206, 207] (Table 3). The fluorescent self-transmissible plasmids detect the gene exchange rate between planktonic bacteria and the plastisphere community [206]. Confocal laser scanning microscopy (CLSM) properly examines the extracellular polymeric substances (EPS) of the biofilm matrix [207]. CLSM can obtain high-resolution images in various depths of a sample, generally 50–100 mm in the case of biological samples [208]. Although the previously mentioned techniques have contributed to this area of research, proper analysis and modeling of microbial colonization on plastics in different environmental settings are still needed. Variable region sequencing from the conserved 16S rRNA can detect changes in species diversity [32, 209,210,211,212]. Whole-genome sequencing (WGS) is an ideal method for observing the effect of heavy metals on resistance-related bacterial genes [213]. A combination of metagenomics and metatranscriptomics can analyze the regulation of metal resistance genes (MRGs) and antibiotic resistance genes (ARGs) at the functional/mRNA level of the whole plastisphere [32, 214]. Functional metagenomics is another convenient method to screen several resistance genes expressed in a specific environment, allowing the detection of novel functioning genes [215, 216]. Beneficial and bioremediation microorganisms were found through metagenomic analysis of sediment samples from the Ganga and Yamuna rivers [217, 218]. Furthermore, Antibiotic Resistance Genes (AMR) were discovered in the sediment metagenome of the Yamuna River [219]. The other methods of investigation are PCR [209, 210, 220], high-throughput qPCR chip technologies [212, 221], and WGS [211], although the genes investigated by the PCR technique are limited in number [64]. Metatranscriptomics for the whole transcriptome [214] and reverse transcription PCR for specific genes [222] can determine the alterations in microbial gene expression levels caused by heavy metal and plastic exposure. Although MRGs and ARGs are under investigation to study different micro-ecosystems, a comprehensive overview of the prevalence of resistance genes still needs to be done. The effect of plastics on antimicrobial resistance (AMR) at the single-cell bacterium level is possible to detect with the help of advanced technologies for the extraction and analysis of genetic elements [223]. Long-term culturing of resistant mutants exposed to subtoxic levels, followed by whole-genome sequencing (WGS), can identify the mutations during the prolonged microbial growth in a heavy metal enriched environment [224,225,226]. In the matter of evolution of resistant mutants accelerated before sequencing, Genome Replication Engineering Assisted Continuous Evolution is an alternative approach [226]. Two-dimensional gel electrophoresis [227], Western blotting [222], and liquid chromatography-tandem mass spectrometry (LC–MS/MS) [152] are proteomics methods frequently used in microbial monocultures. Gas chromatography mass spectrometry (GCMS) can explore the metabolites [222]. The possible impact of plastics on the proteomics and metabolomics level of the plastisphere community requires further investigation. Future next-generation sequencing (NGS) should consider the resistant gene expression levels in non-culturable bacteria present in the plastisphere. DeepARG, a tool used to explore the existing and novel ARGs and MRGs, integrated with metatranscriptomics and metagenomics, might be considered a suitable approach for this purpose [228, 229]. Although the investigation of heterogeneous modulation of gene expression of a single bacterium is possible, reports on single-cell RNA sequencing (scRNA-seq) are still scarce due to their differences from eukaryotic cells, e.g., lack of polyadenylation and low mRNA content. Microbial Split-Pool Ligation Transcriptomics (a scRNA sequencing platform), an approach adapted for Bacillus subtilis and Escherichia coli, reported several advantages, viz., (i) no requirement for single-cell physical isolation, (ii) adaptability with a broad scale of cell size and shapes, (iii) utilization of un-encapsulated and fixed cells [223, 230]. A study [231] has suggested that online separation by 1-Dimensional-LC is the most cost-effective method to amplify the number of identified proteins by MS. 1-dimensional and 2-dimensional-LCMS/MS spectrometry and mass spectrometry imaging (MSI) are promising tools to investigate the impact of microplastics on microbial proteomics and metabolomics activity levels. There are three commercially available MSI methods, which distinguish chemical compounds via their mass to charge ratio and provide chemical and spatial analysis for specific environmental samples. The chief limiting factor of a single MSI experiment is that it only yields a fraction of the metabolites present in samples [232]. Matrix-assisted laser desorption/ionization combined with FISH microscopy has linked metabolomes in complex specimens (group of 50–100 microbial cells) under investigation [233]. Microfluidics has the potential to study microbial colonization and formation of complex biofilm (Table 3) [234, 235], the impact of flow rate, and motility of bacteria on attachment surface [236]. A diverse range of resistance genes is present in both iso and heterogenic bacterial cells [237]. Droplet microfluidics might be one of the most promising technology for such investigation allowing high-throughput culturing of bacteria in a broad spectrum of isolated conditions [238, 239].
Significance and knowledge gaps
Considering all of these factors, variations in reported biodegradation rates are difficult to quantify, especially when the process is affected by multiple biotic and abiotic components. Biodegradation testing in laboratory settings does not adequately reflect ecological conditions, which may promote an overestimation of biodegradation levels of biodegradable plastics. As a result, biodegradation test standards should specify the limit of representativeness, and/or new particular standards should provide more alternatives for bridging the gap between natural and laboratory biodegradation. For example, depending on the geographical region, the temperature range should be adjusted. To mimic synergistic responses of biotic and abiotic variables, access to sunshine and water should also be considered. Many research has looked at the interactions between MPs and biofilms, but none have looked at how MPs physicochemical properties change as they age. More research should be done in diverse aging settings and real-world environments, taking into account the impacts of UV light, microbes, mechanical abrasion, and temperature changes. Exploring the interactions between MPs and biofilms generated by microbial colonization under ambient circumstances is extremely important and practical. These researches will give a rigorous risk assessment of the environmental impact of MPs. The amount of MP accumulated by plants from soil is still vague and unclear. As a result, more research is needed to gain a better understanding of Plant-MP-Soil interaction. Because MPs are known to absorb harmful compounds from the ambient environment, might plant collect dangerous metals alongside MP during soil uptake? This serves as the foundation for an accurate risk assessment. Studies on the potential for employing plants as phytoremediators of MP pollution in the environment are needed.
Conclusion and future directions
As anthropogenic actions are continuously modifying our planet, the impact assessment of these activities is necessary. Plastic materials have become an integral part of our daily life and made our lives convenient. Maybe it is time to reflect on the esteem we put on such comforts. Researchers have so far established that the plastisphere microenvironment does not starkly contrast the microbial communities forming on other similarly inert substrata, primarily because biofilms forming on matured assemblages may not directly interact with the surface material. Future explorations should focus on the purpose of the plastisphere in the physical alteration and chemical biotransformation of plastic wastes, including the modification of density, size, and oxidation state of polymers. Multi-omics approaches play an indispensable part in decoding the microbial-mediated biochemical changes in plastics. Future investigations are necessary to address the many unanswered questions regarding the plastisphere research, such as the effect of chemoattractant of plastic molecules (if released) on the formation of early plastisphere. A detailed physical assessment of plastisphere (biomass, biofilm thickness, and density), generating critical data to predict the potential impact of biofilms on microplastics. The following future directions were depicted from this review work:
-
(1)
We need a profound understanding of fundamental mechanisms involved in biofilm formation, with a focus on biofilm MP interactions as every submerged surface is susceptible to microbial colonization. Experimental designs should be focused on essential parameters that influence the properties of MP. It should be determined whether these parameters vary by MP material and whether they are equivalent to naturally occurring particles of similar size. Experiments should also account for variations in physicochemical qualities as a result of weathering. These tests should be carried out in a variety of weathering environments, including UV, temperature, and mechanical wear.
-
(2)
To specify predictive models of the transport of MP particles and their associated contaminants in the aquatic environment, we need to widen the perception of biofilm-plastic hydrodynamics including vertical movement. Simulation studies on sinking and flocculation using realistic biofilm-MP complexes on micro and mesocosm sizes are required.
-
(3)
The sorption of hydrophobic organic contaminants (HOCs) to MP has gotten a lot of recognition lately. However, a logical paradigm attributing to the influence of biofilm establishment and its implications for chemical partitioning kinetics is yet to be found, which makes experimental assessments difficult. Experimental research should be supplemented by modeling studies in water-plastic-biofilm, a three-phase system.
-
(4)
Majority of experimental data on the effects of MP on biota published to date lacks the accurate composition of the test MP fragments to imitate the natural biofilm layer. MP covered by biofilms should be included from preculture incubations, and the influence of algae, fungi, and bacterial strains should be investigated. Particle controls must also mimic natural particles in terms of density, size, and biofilm formation.
-
(5)
When calculating feeding uptake and exposure effects under actual settings, the importance of biofilms for the manner and MP uptake rate by consumers should not be overlooked. The existing (ecotoxicological) research on MP intake in artificial food chains should be supplemented by differential MP uptake due to biofilm formation.
-
(6)
To measure the resilience of aquatic systems to MP contamination, we should focus on the complexity of microbial assemblages in aquatic systems and their ability to form biofilms on diverse polymer substrates (“plastisphere”). Biofilms on plastic trash should be investigated and analyzed so that we may get a functional understanding of their diversity and productivity, along with their role as vectors in disseminating microorganisms for meaningful risk evaluation.
Availability of data and materials
Data sharing is not applicable to this review article as no datasets were generated or analyzed during manuscript preparation.
Code availability
Code availability is not applicable to this review article.
References
Huang D, Xu Y, Lei F, Yu X, Ouyang Z, Chen Y, Jia H, Guo X. Degradation of polyethylene plastic in soil and effects on microbial community composition. J Hazard Mater. 2021;416:126173.
Li J, Zhang K, Zhang H. Adsorption of antibiotics on microplastics. Environ Pollut. 2018;237:460–7.
Amaral-Zettler LA, Zettler ER, Mincer TJ. Ecology of the plastisphere. Nat Rev Microbiol. 2020;18:139–51.
Gross M. Our planet wrapped in plastic. Curr Biol. 2017;27:785–8.
Corradini F, Meza P, Eguiluz R, Casado F, Huerta-Lwanga E, Geissen V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci Total Environ. 2019;671:411–20.
Schwabl P, Köppel S, Königshofer P, Bucsics T, Trauner M, Reiberger T, Liebmann B. Detection of various microplastics in human stool: a prospective case series. Ann Intern Med. 2019;171:45–7.
Horton AA, Walton A, Spurgeon DJ, Lahive E, Svendsen C. Microplastics in freshwater and terrestrial environments: evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci Total Environ. 2017;586:127–41.
Cau A, Alvito A, Moccia D, Canese S, Pusceddu A, Rita C, Angiolillo M, Follesa MC. Submarine canyons along the upper Sardinian slope (Central Western Mediterranean) as repositories for derelict fishing gears. Mar Pollut Bull. 2017;123:357–64.
Quero GM, Luna GM. Surfing and dining on the “plastisphere”: microbial life on plastic marine debris. Adv Oceanogr Limnol. 2017;8:199–207.
van Sebille E, Wilcox C, Lebreton L, Maximenko N, Hardesty BD, van Franeker JA, Eriksen M, Siegel D, Galgani F, Law KL. A global inventory of small floating plastic debris. Environ Res Lett. 2015;10:124006.
Lebreton L, Andrady A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019;5:1–11.
Isobe A, Azuma T, Cordova MR, Cózar A, Galgani F, Hagita R, Kanhai LD, Imai K, Iwasaki S, Kako S, Kozlovskii N, Lusher AL, Mason SA, Michida Y, Mituhasi T, Morii Y, Mukai T, Popova A, Shimizu K, Tokai T, Uchida K, Yagi M, Zhang W. A multilevel dataset of microplastic abundance in the world’s upper ocean and the Laurentian great lakes. Microplast Nanoplast. 2021;1:1–14.
Frias J, Nash R. Microplastics: finding a consensus on the definition. Mar Pollut Bull. 2019;138:145–7.
Park SY, Kim CG. Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere. 2019;222:527–33.
Boucher J, Friot D. Primary microplastics in the oceans: a global evaluation of sources. Gland, Switzerland: IUCN; 2017.
Wang W, Gao H, Jin S, Li R, Na G. The ecotoxicological effects of microplastics on aquatic food web, from primary producer to human: a review. Ecotoxicol Environ Saf. 2019;173:110–7.
Wang J, Liu X, Liu G, Zhang Z, Wu H, Cui B, Bai J, Zhang W. Size effect of polystyrene microplastics on sorption of phenanthrene and nitrobenzene. Ecotoxicol Environ Saf. 2019;173:331–8.
Andrady AL. The plastic in microplastics: a review. Mar Pollut Bull. 2017;119:12–22.
Andrady AL. Microplastics in the marine environment. Mar Pollut Bull. 2011;62(8):1596–605.
Cook S, Chan H-L, Abolfathi S, Bending GD, Schäfer H, Pearson JM. Longitudinal dispersion of microplastics in aquatic flows using fluorometric techniques. Water Res. 2020;170:115337.
Woodall LC, Sanchez-Vidal A, Canals M, Paterson GLJ, Coppock R, Sleight V, Calafat A, Rogers AD, Narayanaswamy BE, Thompson RC. The deep sea is a major sink for microplastic debris. R Soc Open Sci. 2014;1:140317.
Koelmans AA, Nor NHM, Hermsen E, Kooi M, Mintenig SM, de France J. Microplastics in freshwaters and drinking water: critical review and assessment of data quality. Water Res. 2019;155:410–22.
Engler RE. The complex interaction between marine debris and toxic chemicals in the ocean. Environ Sci Technol. 2012;46:12302–15.
Li H, Wang F, Li J, Deng S, Zhang S. Adsorption of three pesticides on polyethylene microplastics in aqueous solutions: kinetics, isotherms, thermodynamics, and molecular dynamics simulation. Chemosphere. 2021;264:128556.
Wright SL, Thompson RC, Galloway TS. The physical impacts of microplastics on marine organisms: a review. Environ Pollut. 2013;178:483–92.
Ho YS, Ng JCY, McKay G. Kinetics of pollutant sorption by biosorbents: review. Sep Purif Methods. 2000;29:189–232.
Zhang W, Zhang S, Wang J, Wang Y, Mu J, Wang P, Lin X, Ma D. Microplastic pollution in the surface waters of the Bohai Sea. China Environ Pollut. 2017;231:541–8.
Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M. Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ Sci Technol. 2012;46:3060–75.
Andrady AL, Pegram JE, Song Y. Studies on enhanced degradable plastics II. Weathering of enhanced photodegradable polyethylenes under marine and freshwater floating exposure. J Environ Polym Degrad. 1993;1:117–26.
Turner A, Holmes L. Occurrence, distribution and characteristics of beached plastic production pellets on the island of Malta (central Mediterranean). Mar Pollut Bull. 2011;62:377–81.
Frias JPGL, Sobral P, Ferreira AM. Organic pollutants in microplastics from two beaches of the Portuguese coast. Mar Poll Bull. 2010;60:1988–92.
Meier MJ, Dodge AE, Samarajeewa AD, Beaudette LA. Soil exposed to silver nanoparticles reveals significant changes in community structure and altered microbial transcriptional profiles. Environ Pollut. 2020;258:113816.
Rouillon C, Bussiere P, Desnoux E, Collin S, Vial C, Therias S, Gardette JL. Is carbonyl index a quantitative probe to monitor polypropylene photodegradation? Polym Degrad Stab. 2016;128:200–8.
Stark NM, Matuana LM. Surface chemistry changes of weathered HDPE/wood-flour composites studied by XPS and FTIR spectroscopy. Polym Degrad Stab. 2004;86:1–9.
O’Connor IA, Golsteijn L, Hendriks AJ. Review of the partitioning of chemicals into different plastics: consequences for the risk assessment of marine plastic debris. Mar Poll Bull. 2016;113:17–24.
Silva PPG, Nobre CR, Resaffe P, Pereira CDS, Gusmao F. Leachate from microplastics impairs larval development in brown mussels. Water Res. 2016;106:364–70.
Hermabessiere L, Dehaut A, Paul-Pont I, Lacroix C, Jezequel R, Soudant P, Duflos G. Occurrence and effects of plastic additives on marine environments and organisms: a review. Chemosphere. 2017;182:781–93.
Hahladakis JN, Velis CA, Weber R, Iacovidou E, Purnell P. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater. 2018;344:179–99.
Teuten EL, Rowland SJ, Galloway TS, Thompson RC. Potential for plastics to transport hydrophobic contaminants. Environ Sci Technol. 2007;41:7759–64.
Fu L, Li J, Wang G, Luan Y, Dai W. Adsorption behavior of organic pollutants on microplastics. Ecotoxicol Environ Saf. 2021;217:112207.
Bowley J, Baker-Austin C, Porter A, Hartnell R, Lewis C. Oceanic hitchhikers–assessing pathogen risks from marine microplastic. Trends Microbiol. 2021;29:107–16.
Li H-X, Orihuela B, Zhu M, Rittschof D. Recyclable plastics as substrata for settlement and growth of bryozoans Bugula neritina and barnacles Amphibalanus amphitrite. Environ Pollut. 2016;218:973–80.
Foulon V, le Roux F, Lambert C, Huvet A, Soudant P, Paul-Pont I. Colonization of polystyrene microparticles by Vibrio crassostreae: light and electron microscopic investigation. Environ Sci Technol. 2016;50:10988–96.
Cai L, Wu D, Xia J, Shi H, Kim H. Influence of physicochemical surface properties on the adhesion of bacteria onto four types of plastics. Sci Total Environ. 2019;671:1101–7.
Mincer TJ, Zettler ER, Amaral-Zettler LA. Biofilms on plastic debris and their influence on marine nutrient cycling, productivity, and hazardous chemical mobility. In: Takada H, Karapanagioti H, editors. Hazardous chemicals associated with plastics in the marine environment. The handbook of environmental chemistry. Cham: Springer; 2016. p. 221–33.
Keswani A, Oliver DM, Gutierrez T, Quilliam RS. Microbial hitchhikers on marine plastic debris: human exposure risks at bathing waters and beach environments. Mar Environ Res. 2016;118:10–9.
Zettler ER, Mincer TJ, Amaral-Zettler LA. Life in the “plastisphere”: microbial communities on plastic marine debris. Environ Sci Technol. 2013;47:7137–46.
Sjollema SB, Redondo-Hasselerharm P, Leslie HA, Kraak MHS, Vethaak AD. Do plastic particles affect microalgal photosynthesis and growth? Aquat Toxicol. 2016;170:259–61.
Lim XZ. Microplastics are everywhere-but are they harmful? Nature. 2021;593:22–5.
Wu Y, Tam W-S, Chau H-F, Kaur S, Thor W, Aik WS, Chan W-L, Zweckstetter M, Wong K-L. Solid-phase fluorescent BODIPY–peptide synthesis via in situ dipyrrin construction. Chem Sci. 2020;11:11266–73.
Ramasamy EV, Sruthy S, Harit AK, Mohan M, Binish MB. Microplastic pollution in the surface sediment of Kongsfjorden, Svalbard. Arctic Marine Pollut Bull. 2021;173:112986.
Collard F, Husum K, Eppe G, Malherbe C, Hallanger IG, Divine DV, Gabrielsen GW. Anthropogenic particles in sediment from an Arctic fjord. Sci Total Environ. 2021;772:145575.
Hipfner JM, Galbraith M, Tucker S, Studholme KR, Domalik AD, Pearson SF, Good TP, Ross PS, Hodum P. Two forage fishes as potential conduits for the vertical transfer of microfibres in Northeastern Pacific Ocean food webs. Environ Pollut. 2018;239:215–22.
Savinelli B, Fernández TV, Galasso NM, D’Anna G, Pipitone C, Prada F, Zenone A, Badalamenti F, Musco L. Microplastics impair the feeding performance of a mediterranean habitat-forming coral. Mar Environ Res. 2020;155:104887.
Gonçalves C, Martins M, Sobral P, Costa PM, Costa MH. An assessment of the ability to ingest and excrete microplastics by filter-feeders: a case study with the mediterranean mussel. Environ Pollut. 2019;245:600–6.
Dawson A, Huston W, Kawaguchi S, King C, Cropp R, Wild S, Eisenmann P, Townsend K, Nash SB. Uptake and depuration kinetics influence microplastic bioaccumulation and toxicity in Antarctic krill (Euphausia superba). Environ Sci Technol. 2018;52:3195–201.
Li S, Wang P, Zhang C, Zhou X, Yin Z, Hu T, Hu D, Liu C, Zhu L. Influence of polystyrene microplastics on the growth, photosynthetic efficiency and aggregation of freshwater microalgae Chlamydomonas reinhardtii. Sci Total Environ. 2020;714:136767.
Sussarellu R, Suquet M, Thomas Y, Lambert C, Fabioux C, Pernet MEJ, Goïc NL, Quillien V, Mingant C, Epelboin Y, Corporeau C, Guyomarch J, Robbens J, Paul-Pont I, Soudant P, Huvet A. Oyster reproduction is affected by exposure to polystyrene microplastics. Proc Natl Acad Sci. 2016;113:2430–5.
Seidensticker S, Grathwohl P, Lamprecht J, Zarfl C. A combined experimental and modeling study to evaluate pH-dependent sorption of polar and non-polar compounds to polyethylene and polystyrene microplastics. Environ Sci Eur. 2018;30:1–12.
Khalid N, Aqeel M, Noman A, Hashem M, Mostafa YS, Alhaithloul HAS, Alghanem SM. Linking effects of microplastics to ecological impacts in marine environments. Chemosphere. 2021;264:128541.
Hodson ME, Duffus-Hodson CA, Clark A, Prendergast-Miller MT, Thorpe KL. Plastic bag derived-microplastics as a vector for metal exposure in terrestrial invertebrates. Environ Sci Technol. 2017;51:4714–21.
Wang W, Wang J. Comparative evaluation of sorption kinetics and isotherms of pyrene onto microplastics. Chemosphere. 2018;193:567–73.
Wang W, Wang J. Different partition of polycyclic aromatic hydrocarbon on environmental particulates in freshwater: microplastics in comparison to natural sediment. Ecotoxicol Environ Saf. 2018;147:648–55.
Zhang Y, Lu J, Wu J, Wang J, Luo Y. Potential risks of microplastics combined with superbugs: enrichment of antibiotic resistant bacteria on the surface of microplastics in mariculture system. Ecotoxicol Environ Saf. 2020;187:109852.
Haward M. Plastic pollution of the world’s seas and oceans as a contemporary challenge in ocean governance. Nat Commun. 2018;9:1–3.
Galloway TS, Cole M, Lewis C. Interactions of microplastic debris throughout the marine ecosystem. Nat Ecol Evol. 2017;1(5):1–8.
McCormick JM, Es TV, Cooper KR, White LA, Häggblom MM. Microbially mediated O-methylation of bisphenol a results in metabolites with increased toxicity to the developing zebrafish (Danio rerio) embryo. Environ Sci Technol. 2011;45:6567–74.
Seeley ME, Song B, Passie R, Hale RC. Microplastics affect sedimentary microbial communities and nitrogen cycling. Nat Commun. 2020;11:1–10.
Santos A, van Aerle R, Barrientos L, Martinez-Urtaza J. Computational methods for 16S metabarcoding studies using Nanopore sequencing data. Comput Struct Biotechnol J. 2020;18:296–305.
Davidov K, Iankelevich-Kounio E, Yakovenko I, Koucherov Y, Rubin-Blum M, Oren M. Identification of plastic-associated species in the Mediterranean Sea using DNA metabarcoding with Nanopore MinION. Sci Rep. 2020;10:1–11.
de Tender C, Devriese LI, Haegeman A, Maes S, Vangeyte J, Cattrijsse A, Dawyndt P, Ruttink T. Temporal dynamics of bacterial and fungal colonization on plastic debris in the North Sea. Environ Sci Technol. 2017;51:7350–60.
Chen L, Li J, Tang Y, Wang S, Lu X, Cheng Z, et al. Typhoon-induced turbulence redistributed microplastics in coastal areas and reformed plastisphere community. Water Res. 2021;204:117580.
Scales BS, Cable RN, Duhaime MB, Gerdts G, Fischer F, Fischer D, Mothes S, Hintzki L, Moldaenke L, Ruwe M, Kalinowski J, Kreikemeyer B, Pedrotti M-L, Gorsky G, Elineau A, Labrenz M, Oberbeckmann S. Cross-hemisphere study reveals geographically ubiquitous, plastic-specific bacteria emerging from the rare and unexplored biosphere. MSphere. 2021;6:e00851-e920.
Wright RJ, Bosch R, Langille MGI, Gibson MI, Christie-Oleza JA. A multi-OMIC characterisation of biodegradation and microbial community succession within the PET plastisphere. Microbiome. 2021;9:1–22.
Catão CPE, Pollet T, Garnier C, Barry-Martinet R, Rehel K, Linossier I, Tunin-Ley A, Turquet J, Biand J-F. Temperate and tropical coastal waters share relatively similar microbial biofilm communities while free-living or particle-attached communities are distinct. Mol Ecol. 2021;30:2891–904.
Ye G, Zhang X, Yan C, Lin Y, Huang Q. Polystyrene microplastics induce microbial dysbiosis and dysfunction in surrounding seawater. Environ Int. 2021;156:106724.
Gao R, Sun C. A marine bacterial community capable of degrading poly (ethylene terephthalate) and polyethylene. J Hazard Mater. 2021;416:125928.
Agostini L, Moreira JCF, Bendia AG, Kmit MCP, Waters LG, Santana MFM, Sumida PYG, Turra A, Pellizari VH. Deep-sea plastisphere: long-term colonization by plastic-associated bacterial and archaeal communities in the Southwest Atlantic Ocean. Sci Total Environ. 2021;793:148335.
Vaksmaa A, Knittel K, Abdala Asbun A, Goudriaan M, Ellrott A, Witte HJ, Vollmer I, Meirer F, Lott C, Weber M, Engelmann JC, Niemann H. Microbial communities on plastic polymers in the Mediterranean Sea. Front Microbiol. 2021;12:1021.
Dudek KL, Cruz BN, Polidoro B, Neuer S. Microbial colonization of microplastics in the Caribbean Sea. Limnol Oceanogr Lett. 2020;5:5–17.
Harvey BP, Kerfahi D, Jung Y, Shin J-H, Adams JM, Hall-Spencer JM. Ocean acidification alters bacterial communities on marine plastic debris. Mar Pollut Bull. 2020;161:111749.
Tu C, Chen T, Zhou Q, Liu Y, Wei J, Waniek JJ, et al. Biofilm formation and its influences on the properties of microplastics as affected by exposure time and depth in the seawater. Sci Total Environ. 2020;734:139237.
Delacuvellerie A, Cyriaque V, Gobert S, Benali S, Wattiez R. The plastisphere in marine ecosystem hosts potential specific microbial degraders including Alcanivorax borkumensis as a key player for the low-density polyethylene degradation. J Hazard Mater. 2019;380:120899.
Wu W, Lu H-P, Sastri A, Yeh Y-C, Gong G-C, Chou W-C, Hsieh C-H. Contrasting the relative importance of species sorting and dispersal limitation in shaping marine bacterial versus protist communities. ISME J. 2018;12:485–94.
Jiang P, Zhao S, Zhu L, Li D. Microplastic-associated bacterial assemblages in the intertidal zone of the Yangtze Estuary. Sci Total Environ. 2018;624:48–54.
Muthukrishnan T, Al Khaburi M, Abed RMM. Fouling microbial communities on plastics compared with wood and steel: are they substrate-or location-specific? Microb Ecol. 2019;78:361–74.
Kirstein IV, Wichels A, Krohne G, Gerdts G. Mature biofilm communities on synthetic polymers in seawater-specific or general? Mar Environ Res. 2018;142:147–54.
Dussud C, Meistertzheim AL, Conan P, Pujo-Pay M, George M, Fabre P, Coudane J, Higgs P, Elineau A, Pedrotti ML, Gorsky G, Ghiglione JF. Evidence of niche partitioning among bacteria living on plastics, organic particles and surrounding seawaters. Environ Pollut. 2018;236:807–16.
Dussud C, Hudec C, George M, Fabre P, Higgs P, Bruzaud S, Delort A-M, Eyheraguibel B, Meistertzheim A-L, Jacquin J, Cheng J, Callac N, Odobel C, Rabouille S, Ghiglione J-F. Colonization of non-biodegradable and biodegradable plastics by marine microorganisms. Front Microbiol. 2018;9:1–13.
Debroas D, Mone A, ter Halle A. Plastics in the North Atlantic garbage patch: a boat-microbe for hitchhikers and plastic degraders. Sci Total Environ. 2017;599–600:1222–32.
Oberbeckmann S, Osborn AM, Duhaime MB. Microbes on a bottle: substrate, season and geography influence community composition of microbes colonizing marine plastic debris. PLoS ONE. 2016;11:e0159289.
Bryant JA, Clemente TM, Viviani DA, Fong AA, Thomas KA, Kemp P, Karl DM, White AE, Long EF. Diversity and activity of communities inhabiting plastic debris in the North Pacific Gyre. mSystems. 2016;1:e00024-e116.
Lacerda ALDF, Proietti MC, Secchi ER, Taylor JD. Diverse groups of fungi are associated with plastics in the surface waters of the Western South Atlantic and the Antarctic Peninsula. Mol Ecol. 2020;29:1903–18.
Kettner MT, Oberbeckmann S, Labrenz M, Grossart H-P. The eukaryotic life on microplastics in brackish ecosystems. Front Microbiol. 2019;10:538.
Kettner MT, Rojas-Jimenez K, Oberbeckmann S, Labrenz M, Grossart H. Microplastics alter composition of fungal communities in aquatic ecosystems. Environ Microbiol. 2017;19:4447–59.
Stanier RY, Sieburth JM. Microbial seascapes, a pictorial essay on marine microorganisms and their environments. Limnol Oceanogr. 1975;1975(20):1059–60.
Flemming H-C, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14:563–75.
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott H. 1079 M. Microb Biofilms Annu Rev Microbiol. 1995;49:711–45.
Carson HS, Nerheim MS, Carroll KA, Eriksen M. The plastic-associated microorganisms of the North Pacific Gyre. Mar Pollut Bull. 2013;75:126–32.
Oberbeckmann S, Loeder MGJ, Gerdts G, Osborn AM. Spatial and seasonal variation in diversity and structure of microbial biofilms on marine plastics in Northern European waters. FEMS Microbiol Ecol. 2014;90:478–92.
Eich A, Mildenberger T, Laforsch C, Weber M. Biofilm and diatom succession on polyethylene (PE) and biodegradable plastic bags in two marine habitats: early signs of degradation in the Pelagic and Benthic Zone? PLoS ONE. 2015;10:e0137201.
Masó M, Fortuño JM, de Juan S, Demestre M. Microfouling communities from pelagic and benthic marine plastic debris sampled across Mediterranean coastal waters. Sci Mar. 2016;80:117–27.
Michels J, Stippkugel A, Lenz M, Wirtz K, Engel A. Rapid aggregation of biofilm-covered microplastics with marine biogenic particles. Proc R Soc B. 2018;285:20181203.
Mayali X. Metabolic interactions between bacteria and phytoplankton. Front Microbiol. 2018;9:727.
Carpenter EJ, Smith KL. Plastics on the Sargasso sea surface. Science. 1979;1972(175):1240–1.
Decelle J, Romac S, Stern RF, Bendif EM, Zingone A, Audic S, et al. PhytoREF: a reference database of the plastidial 16S rRNA gene of photosynthetic eukaryotes with curated taxonomy. Mol Ecol Resour. 2015;15:1435–45.
Amaral-Zettler LA, Zettler ER, Slikas B, Boyd GD, Melvin DW, Morrall CE, Proskurowski G, Mincer TJ. The biogeography of the Plastisphere: implications for policy. Front Ecol Environ. 2015;13:541–6.
de Tender C, Schlundt C, Devriese LI, Mincer TJ, Zettler ER, Amaral-Zettler LA. A review of microscopy and comparative molecular-based methods to characterize “Plastisphere” communities. Anal Methods. 2017;9:2132–43.
Levy I, Gantt E. Development of photosynthetic activity in Porphyridium purpureum (Rhodophyta) following nitrogen starvation. J Phycol. 1990;26:62–8.
Luo H, Moran MA. Evolutionary ecology of the marine Roseobacter clade. Microbiol Mol Biol Rev. 2014;78:573–87.
Ehrlich HL. Inorganic energy sources for chemolithotrophic and mixotrophic bacteria. Geomicrobiol J. 1978;1:65–83.
Eiler A. Evidence for the ubiquity of Mixotrophic bacteria in the upper ocean: implications and consequences. Appl Environ Microbiol. 2006;72:7431–7.
Rittenberg SC. The roles of exogenous organic matter in the physiology of chemolithotrophic bacteria. Adv Microb Physiol. 1969;3:159–96.
Matin A. Organic nutrition of chemolithotrophic bacteria. Ann Rev Microbiol. 1978;32:433–68.
Harshvardhan K, Jha B. Biodegradation of low-density polyethylene by marine bacteria from pelagic waters, Arabian Sea India. Mar Pollut Bull. 2013;77:100–6.
Gilan I, Sivan A. Effect of proteases on biofilm formation of the plastic-degrading actinomycete Rhodococcus ruber C208. FEMS Microbiol Lett. 2013;342:18–23.
Nakamiya K, Sakasita G, Ooi T, Kinoshita S. Enzymatic degradation of polystyrene by hydroquinone peroxidase of Azotobacter beijerinckii HM121. J Ferment Bioeng. 1997;84:480–2.
Gyung Yoon M, Jeong Jeon H, Nam KM. Biodegradation of polyethylene by a soil bacterium and AlkB cloned recombinant cell. J Biorem Biodegrad. 2012;3:145.
Kobayashi T, Nakano N, Muto T, Endo Y. Growth characteristics of Ephelota gigantea: a pest to seaweed culture along the northeastern coast of Japan. Acta Protozool. 2011;50:339–43.
Derraik JGB. The pollution of the marine environment by plastic debris: a review. Mar Pollut Bull. 2002;44:842–52.
Curren E, Leong SCY. Profiles of bacterial assemblages from microplastics of tropical coastal environments. Sci Total Environ. 2019;655:313–20.
de Tender CA, Devriese LI, Haegeman A, Maes S, Ruttink T, Dawyndt P. Bacterial community profiling of plastic litter in the Belgian part of the North Sea. Environ Sci Technol. 2015;49:9629–38.
Kirstein IV, Kirmizi S, Wichels A, Garin-Fernandez A, Erler R, Löder M, Gerdts G. Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar Environ Res. 2016;120:1–8.
Frère L, Maignien L, Chalopin M, Huvet A, Rinnert E, Morrison H, Kerninonf S, Cassonea A-L, Lamberta C, Reveillaudg J, Paul-Ponta I. Microplastic bacterial communities in the Bay of Brest: influence of polymer type and size. Environ Pollut. 2018;242:614–25.
McCormick A, Hoellein TJ, Mason SA, Schluep J, Kelly JJ. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ Sci Technol. 2014;48:11863–71.
Harrison JP, Hoellein TJ, Sapp M, Tagg AS, Ju-Nam Y, Ojeda JJ. Microplastic-associated biofilms: a comparison of freshwater and marine environments. In: Wagner M, Lambert S, editors. Freshwater microplastics, the handbook of environmental chemistry. Cham: Springer; 2018. p. 181–201.
Lafferty KD, Harvell CD, Conrad JM, Friedman CS, Kent ML, Kuris AM, Powell EN, Rondeau D, Saksida SM. Infectious diseases affect marine fisheries and aquaculture economics. Ann Rev Mar Sci. 2015;7:471–96.
Chatterjee S, Haldar S. Vibrio related diseases in aquaculture and development of rapid and accurate identification methods. J Mar Sci Res Dev. 2012;1:1–7.
Masó M, Garcés E, Pagès F, Camp J. Drifting plastic debris as a potential vector for dispersing harmful algal bloom (HAB) species. Sci Mar. 2003;67:107–11.
Casabianca S, Capellacci S, Giacobbe MG, Dell’Aversano C, Tartaglione L, Varriale F, et al. Plastic-associated harmful microalgal assemblages in marine environment. Environ Pollut. 2019;244:617–26.
Goldstein MC, Carson HS, Eriksen M. Relationship of diversity and habitat area in North Pacific plastic-associated rafting communities. Mar Biol. 2014;161:1441–53.
Viršek MK, Lovšin MN, Koren Š, Kržan A, Peterlin M. Microplastics as a vector for the transport of the bacterial fish pathogen species Aeromonas salmonicida. Mar Pollut Bull. 2017;125:301–9.
Zhu F, Massana R, Not F, Marie D, Vaulot D. Mapping of picoeucaryotes in marine ecosystems with quantitative PCR of the 18S rRNA gene. FEMS Microbiol Ecol. 2005;52:79–92.
Rummel CD, Jahnke A, Gorokhova E, Kühnel D, Schmitt-Jansen M. Impacts of biofilm formation on the fate and potential effects of microplastic in the aquatic environment. Environ Sci Technol Lett. 2017;4:258–67.
Oder M, Fink R, Bohinc K, Torkar KG. The influence of shear stress on the adhesion capacity of Legionella pneumophila. Arh Hig Rada Toksikol. 2017;68:109–15.
Pagán R, García-Gonzalo D. Influence of environmental factors on bacterial biofilm formation in the food industry: a review. Postdoc J Res. 2015;3:3–13.
Prakash B, Veeregowda BM, Krishnappa G. Biofilms: a survival strategy of bacteria. Curr Sci. 2003;10:1299–307.
O’Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304.
O’Toole GA, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol. 1998;28:449–61.
Petrova OE, Sauer K. Sticky situations: key components that control bacterial surface attachment. J Bacteriol. 2012;194:2413–25.
McDonough KA, Rodriguez A. The myriad roles of cyclic AMP in microbial pathogens: from signal to sword. Nat Rev Microbiol. 2012;10:27–38.
Obana N, Nakamura K, Nomura N. A sporulation factor is involved in the morphological change of Clostridium perfringens biofilms in response to temperature. J Bacteriol. 2014;196:1540–50.
Harrison JP, Schratzberger M, Sapp M, Osborn AM. Rapid bacterial colonization of low-density polyethylene microplastics in coastal sediment microcosms. BMC Microbiol. 2014;14:1–15.
Davey ME, O’Toole GA. Microbial biofilms: from ecology to molecular genetics. Microbiol Mol Biol Rev. 2000;64:847–67.
Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108.
Nadell CD, Foster KR, Xavier JB. Emergence of spatial structure in cell groups and the evolution of cooperation. PLoS Comput Biol. 2010;6:e1000716.
Nadell CD, Drescher K, Foster KR. Spatial structure, cooperation and competition in biofilms. Nat Rev Microbiol. 2016;14:589–600.
Dang H, Lovell CR. Microbial surface colonization and biofilm development in marine environments. Microbiol Mol Biol Rev. 2016;80:91–138.
Gewert B, Plassmann M, Sandblom O, Macleod M. Identification of chain scission products released to water by plastic exposed to ultraviolet light. Environ Sci Technol Lett. 2018;5:272–6.
Romera-Castillo C, Pinto M, Langer TM, Álvarez-Salgado XA, Herndl GJ. Dissolved organic carbon leaching from plastics stimulates microbial activity in the ocean. Nat Commun. 2018;9:1–7.
Erni-Cassola G, Wright RJ, Gibson MI, Christie-Oleza JA. Early colonization of weathered polyethylene by distinct bacteria in Marine Coastal seawater. Microb Ecol. 2020;79:517–26.
Li W, Wang G, Zhang S, Fu Y, Jiang Y, Yang X, Lin X. An integrated quantitative proteomic and metabolomics approach to reveal the negative regulation mechanism of LamB in antibiotics resistance. J Proteomics. 2019;194:148–59.
Datta MS, Sliwerska E, Gore J, Polz MF, Cordero OX. Microbial interactions lead to rapid micro-scale successions on model marine particles. Nat Commun. 2016;7:1–7.
Enke TN, Leventhal GE, Metzger M, Saavedra JT, Cordero OX. Microscale ecology regulates particulate organic matter turnover in model marine microbial communities. Nat Commun. 2018;9:1–8.
Wright RJ, Gibson MI, Christie-Oleza JA. Understanding microbial community dynamics to improve optimal microbiome selection. Microbiome. 2019;7:1–4.
Enke TN, Datta MS, Schwartzman J, Cermak N, Schmitz D, Barrere J, Pascual-García A, Cordero OX. Modular assembly of polysaccharide-degrading marine microbial communities. Curr Biol. 2019;29:1528–35.
Zhang S-J, Zeng Y-H, Zhu J-M, Cai Z-H, Zhou J. The structure and assembly mechanisms of plastisphere microbial community in natural marine environment. J Hazard Mater. 2022;421:126780.
Wright RJ, Erni-Cassola G, Zadjelovic V, Latva M, Christie-Oleza JA. Marine plastic debris: a new surface for microbial colonization. Environ Sci Technol. 2020;54:11657–72.
Liu P, Zhan X, Wu X, Li J, Wang H, Gao S. Effect of weathering on environmental behavior of microplastics: properties, sorption and potential risks. Chemosphere. 2020;242:125193.
Liu G, Zhu Z, Yang Y, Sun Y, Yu F, Ma J. Sorption behavior and mechanism of hydrophilic organic chemicals to virgin and aged microplastics in freshwater and seawater. Environ Pollut. 2019;246:26–33.
Liu P, Lu K, Li J, Wu X, Qian L, Wang M, et al. Effect of aging on adsorption behavior of polystyrene microplastics for pharmaceuticals: adsorption mechanism and role of aging intermediates. J Hazard Mater. 2020;384:121193.
Gewert B, Plassmann MM, MacLeod M. Pathways for degradation of plastic polymers floating in the marine environment. Environ Sci Process Impacts. 2015;17:1513–21.
Andrady AL. Persistence of plastic litter in the oceans. In: Bergmann M, Gutow L, Klages M, editors. In Marine anthropogenic litter. Cham: Springer; 2015. p. 57–72.
Singh B, Sharma N. Mechanistic implications of plastic degradation. Polym Degrad Stab. 2008;93:561–84.
Avery GB, Cooper WJ, Kieber RJ, Willey JD. Hydrogen peroxide at the Bermuda Atlantic time series station: temporal variability of seawater hydrogen peroxide. Mar Chem. 2005;97:236–44.
Fang G, Liu C, Wang Y, Dionysiou DD, Zhou D. Photogeneration of reactive oxygen species from biochar suspension for diethyl phthalate degradation. Appl Catal B. 2017;214:34–45.
Xu H, Cooper WJ, Jung J, Song W. Photosensitized degradation of amoxicillin in natural organic matter isolate solutions. Water Res. 2011;45:632–8.
Agboola O, Sadiku R, Mokrani T, Amer I, Imoru O. Polyolefins and the environment. In: Ugbolue SCO, editor. Polyolefin Fibres. Structure, Properties and Industrial Applications (Second Edition). USA: The Textile Institute Book Series; 2017. p. 89-133.
Arp HPH, Kühnel D, Rummel C, Macleod M, Potthoff A, Reichelt S, et al. Weathering plastics as a planetary boundary threat: exposure, fate, and hazards. Environ Sci Technol. 2021;55:7246–55.
Liu P, Qian L, Wang H, Zhan X, Lu K, Gu C, Gao S. New insights into the aging behavior of microplastics accelerated by advanced oxidation processes. Environ Sci Technol. 2019;53:3579–88.
Feldman D. Polymer weathering: photo-oxidation. J Polym Environ. 2002;10:163–73.
Fotopoulou KN, Karapanagioti HK. Surface properties of beached plastics. Environ Sci Pollut Res. 2015;22:11022–32.
Sun Y, Yuan J, Zhou T, Zhao Y, Yu F, Ma J. Laboratory simulation of microplastics weathering and its adsorption behaviors in an aqueous environment: a systematic review. Environ Pollut. 2020;265:114864.
Hadad D, Geresh S, Sivan A. Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis. J Appl Microbiol. 2005;98:1093–100.
Wen G, Kötzsch S, Vital M, Egli T, Ma J. BioMig-a method to evaluate the potential release of compounds from and the formation of biofilms on polymeric materials in contact with drinking water. Environ Sci Technol. 2015;49:11659–69.
Richard H, Carpenter EJ, Komada T, Palmer PT, Rochman CM. Biofilm facilitates metal accumulation onto microplastics in estuarine waters. Sci Total Environ. 2019;683:600–8.
Johansen MP, Prentice E, Cresswell T, Howell N. Initial data on adsorption of Cs and Sr to the surfaces of microplastics with biofilm. J Environ Radioact. 2018;190–191:130–3.
Weinstein JE, Crocker BK, Gray AD. From macroplastic to microplastic: degradation of high-density polyethylene, polypropylene, and polystyrene in a salt marsh habitat. Environ Toxicol Chem. 2016;35:1632–40.
Oberbeckmann S, Löder MGJ, Labrenz M. Marine microplastic-associated biofilms–a review. Environ Chem. 2015;12:551–62.
Gong M, Yang G, Zhuang L, Zeng EY. Microbial biofilm formation and community structure on low-density polyethylene microparticles in lake water microcosms. Environ Pollut. 2019;252:94–102.
Jacquin J, Cheng J, Odobel C, Pandin C, Conan P, Pujo-Pay M, Barbe V, Meistertzheim AL, Ghiglione JF. Microbial ecotoxicology of marine plastic debris: a review on colonization and biodegradation by the “Plastisphere.” Front Microbiol. 2019;10:865.
Reisser J, Slat B, Noble K, Du Plessis K, Epp M, Proietti M, de Sonneville J, Becker T, Pattiaratchi C. The vertical distribution of buoyant plastics at sea: an observational study in the North Atlantic Gyre. Biogeosciences. 2015;12:1249–56.
Kane IA, Clare MA, Miramontes E, Wogelius R, Rothwell JJ, Garreau P, Pohl F. Seafloor microplastic hotspots controlled by deep-sea circulation. Science. 2020;368:1140–5.
Emadian SM, Onay TT, Demirel B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017;59:526–36.
Feng Z, Zhang T, Shi H, Gao K, Huang W, Xu J, Wang J, Wang R, Li J, Gao G. Microplastics in bloom-forming macroalgae: distribution, characteristics and impacts. J Hazard Mater. 2020;397:122752.
Gao G, Clare AS, Chatzidimitriou E, Rose C, Caldwell G. Effects of ocean warming and acidification, combined with nutrient enrichment, on chemical composition and functional properties of Ulva rigida. Food Chem. 2018;258:71–8.
Bonhomme S, Cuer A, Delort AM, Lemaire J, Sancelme M, Scott G. Environmental biodegradation of polyethylene. Polym Degrad Stab. 2003;81:441–52.
Orr IG, Hadar Y, Sivan A. Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl Microbiol Biotechnol. 2004;65:97–104.
Sudhakar M, Trishul A, Doble M, Kumar KS, Jahan SS, Inbakandan D, Viduthalai RR, Umadevi VR, Murthy PS, Venkatesan R. Biofouling and biodegradation of polyolefins in ocean waters. Polym Degrad Stab. 2007;92:1743–52.
Balasubramanian V, Natarajan K, Hemambika B, Ramesh N, Sumathi CS, Kottaimuthu R, Rajesh KV. High-density polyethylene (HDPE)-degrading potential bacteria from marine ecosystem of Gulf of Mannar. India Lett Appl Microbiol. 2010;51:205–11.
Syranidou E, Karkanorachaki K, Amorotti F, Repouskou E, Kroll K, Kolvenbach B, Corvini PF, Fava F, Kalogerakis N. Development of tailored indigenous marine consortia for the degradation of naturally weathered polyethylene films. PLoS ONE. 2017;12:e0183984.
Krueger MC, Harms H, Schlosser D. Prospects for microbiological solutions to environmental pollution with plastics. Appl Microbiol Biotechnol. 2015;99:8857–74.
Yoshida S, Hiraga K, Takehana T, Taniguchi I, Yamaji H, Maeda Y, Toyohara K, Miyamoto K, Kimura Y, Oda K. A bacterium that degrades and assimilates poly (ethylene terephthalate). Science. 2016;351:1196–9.
Danso D, Schmeisser C, Chow J, Zimmermann W, Wei R, Leggewie C, Li X, Hazen T, Streit WR. New insights into the function and global distribution of polyethylene terephthalate (PET)-degrading bacteria and enzymes in marine and terrestrial metagenomes. Appl Environ Microbiol. 2018;84:e02773-e2817.
Hussain N, Jaitley V, Florence AT. Recent advances in the understanding of uptake of. Adv Drug Deliv Rev. 2001;50:107–42.
Artham T, Sudhakar M, Venkatesan R, Madhavan Nair C, Murty KVGK, Doble M. Biofouling and stability of synthetic polymers in sea water. Int Biodeterior Biodegrad. 2009;63:884–90.
Albertsson AC. The shape of the biodegradation curve for low and high density polyethenes in prolonged series of experiments. Eur Polym J. 1980;16:623–30.
Hibbett DS, Binder M. Evolution of marine mushrooms. Biol Bull. 2001;201:319–22.
Shimao M. Biodegradation of plastics. Curr Opin Biotechnol. 2001;12:242–7.
Qi Y, Yang X, Pelaez AM, Lwanga EH, Beriot N, Gertsen H, Garbeva P, Geissen V. Macro-and micro-plastics in soil-plant system: effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci Total Environ. 2018;645:1048–56.
Li L, Zhou Q, Yin N, Tu C, Luo Y. Uptake and accumulation of microplastics in an edible plant. Chin Sci Bull. 2019;64:928–34.
Ebere EC, Wirnkor VA, Ngozi VE. Uptake of microplastics by plant: a reason to worry or to be happy? World Sci News. 2019;131:256–67.
Fisner M, Taniguchi S, Moreira F, Bícego MC, Turra A. Polycyclic aromatic hydrocarbons (PAHs) in plastic pellets: variability in the concentration and composition at different sediment depths in a sandy beach. Mar Pollut Bull. 2013;70:219–26.
Fawzy EM. Soil remediation using in situ immobilisation techniques. Chem Ecol. 2008;24:147–56.
Gardea-Torresdey JL, de La Rosa G, Peralta-Videa JR. Use of phytofiltration technologies in the removal of heavy metals: a review. Pure Appl Chem. 2004;76:801–13.
Arias-Andres M, Klümper U, Rojas-Jimenez K, Grossart H-P. Microplastic pollution increases gene exchange in aquatic ecosystems. Environ Pollut. 2018;237:253–61.
Hossain MR, Jiang M, Wei Q, Leff LG. Microplastic surface properties affect bacterial colonization in freshwater. J Basic Microbiol. 2019;59:54–61.
Jonkman J, Brown CM, Wright GD, Anderson KI, North AJ. Tutorial: guidance for quantitative confocal microscopy. Nat Protoc. 2020;15:1585–611.
Knapp CW, Callan AC, Aitken B, Shearn R, Koenders A, Hinwood A. Relationship between antibiotic resistance genes and metals in residential soil samples from Western Australia. Environ Sci Pollut Res. 2017;24:2484–94.
Chen J, Li J, Zhang H, Shi W, Liu Y. Bacterial heavy-metal and antibiotic resistance genes in a copper tailing dam area in northern China. Front Microbiol. 2019;10:1916.
Learman DR, Ahmad Z, Brookshier A, Henson MW, Hewitt V, Lis A, et al. Comparative genomics of 16 microbacterium spp that tolerate multiple heavy metals and antibiotics. Peer J. 2019;6:e6258.
Zhao Y, Cocerva T, Cox S, Tardif S, Su J-Q, Zhu Y-G, et al. Evidence for co-selection of antibiotic resistance genes and mobile genetic elements in metal polluted urban soils. Sci Total Environ. 2019;656:512–20.
Pathak A, Jaswal R, Chauhan A. Genomic characterization of a mercury resistant Arthrobacter sp. H-02–3 reveals the presence of heavy metal and antibiotic resistance determinants. Front Microbiol. 2020;10:3039.
Cabral L, Júnior GVL, de Sousa STP, Dias ACF, Cadete LL, Andreote FD, et al. Anthropogenic impact on mangrove sediments triggers differential responses in the heavy metals and antibiotic resistomes of microbial communities. Environ Pollut. 2016;216:460–9.
Cheng G, Hu Y, Yin Y, Yang X, Xiang C, Wang B, et al. Functional screening of antibiotic resistance genes from human gut microbiota reveals a novel gene fusion. FEMS Microbiol Lett. 2012;336:11–6.
Staley C, Johnson D, Gould TJ, Wang P, Phillips J, Cotner JB, et al. Frequencies of heavy metal resistance are associated with land cover type in the upper Mississippi River. Sci Total Environ. 2015;511:461–8.
Behera BK, Patra B, Chakraborty HJ, Sahu P, Rout AK, Sarkar DJ, et al. Metagenome analysis from the sediment of river Ganga and Yamuna: in search of beneficial microbiome. PLoS ONE. 2020;15:e0239594.
Behera BK, Chakraborty HJ, Patra B, Rout AK, Dehury B, Das BK, et al. Metagenomic analysis reveals bacterial and fungal diversity and their bioremediation potential from sediments of river Ganga and Yamuna in India. Front Microbiol. 2020;11:556136.
Das BK, Behera BK, Chakraborty HJ, Paria P, Gangopadhyay A, Rout AK, et al. Metagenomic study focusing on antibiotic resistance genes from the sediments of River Yamuna. Gene. 2020;758:144951.
Medardus JJ, Molla BZ, Nicol M, Morrow WM, Rajala-Schultz PJ, Kazwala R, Gebreyes AW. In-feed use of heavy metal micronutrients in US swine production systems and its role in persistence of multidrug-resistant salmonellae. Appl Environ Microbiol. 2014;80:2317–25.
Zhao Y, Su J-Q, An X-L, Huang F-Y, Rensing C, Brandt KK, Zhu Y-G. Feed additives shift gut microbiota and enrich antibiotic resistance in swine gut. Sci Total Environ. 2018;621:1224–32.
Leng F, Li Y, Luo W, Wei Q, Jing Y, Wang X, Yang M, Wang Y. Cloning, expression, and bioinformatics analysis of heavy metal resistance gene afe_1862 from Acidithiobacillus ferrooxidans L1 in Escherichia coli. Biol Trace Elem Res. 2019;189:291–300.
Kuchina A, Brettner LM, Paleologu L, Roco CM, Rosenberg AB, Carignano A, Kibler R, Hirano M, Depaolo RW, Seelig G. Microbial single-cell RNA sequencing by split-pool barcoding. Science. 2020;371:eaba5257.
Chi L, Bian X, Gao B, Tu P, Ru H, Lu K. The effects of an environmentally relevant level of arsenic on the gut microbiome and its functional metagenome. Toxicol Sci. 2017;160:193–204.
Li X, Gu AZ, Zhang Y, Xie B, Li D, Chen J. Sub-lethal concentrations of heavy metals induce antibiotic resistance via mutagenesis. J Hazard Mater. 2019;369:9–16.
Qin W, Zhao J, Yu X, Liu X, Chu X, Tian J, Wu N. Improving cadmium resistance in Escherichia coli through continuous genome evolution. Front Microbiol. 2019;10:278.
Bar C, Patil R, Doshi J, Kulkarni MJ, Gade WN. Characterization of the proteins of bacterial strain isolated from contaminated site involved in heavy metal resistance—a proteomic approach. J Biotechnol. 2007;128:444–51.
Arango-Argoty G, Garner E, Pruden A, Heath LS, Vikesland P, Zhang L. DeepARG: a deep learning approach for predicting antibiotic resistance genes from metagenomic data. Microbiome. 2018;6:1–15.
Cuadrat RRC, Sorokina M, Andrade BG, Goris T, Dávila AMR. Global ocean resistome revealed: exploring antibiotic resistance gene abundance and distribution in TARA Oceans samples. Gigascience. 2020;9:1–12.
Ma Q, Bücking H, Gonzalez Hernandez JL, Subramanian S. Single-cell RNA sequencing of plant-associated bacterial communities. Front Microbiol. 2019;10:2452.
Hinzke T, Kouris A, Hughes R-A, Strous M, Kleiner M. More is not always better: evaluation of 1D and 2D-LC-MS/MS methods for metaproteomics. Front Microbiol. 2019;10:238.
Dunham SJB, Ellis JF, Li B, Sweedler JV. Mass spectrometry imaging of complex microbial communities. Acc Chem Res. 2017;50:96–104.
Geier B, Sogin EM, Michellod D, Janda M, Kompauer M, Spengler B, Dubilier N, Liebeke M. Spatial metabolomics of in situ host–microbe interactions at the micrometre scale. Nat Microbiol. 2020;5:498–510.
Yawata Y, Nguyen J, Stocker R, Rusconi R. Microfluidic studies of biofilm formation in dynamic environments. J Bacteriol. 2016;198:2589–95.
Pousti M, Zarabadi MP, Amirdehi MA, Paquet-Mercier F, Greener J. Microfluidic bioanalytical flow cells for biofilm studies: a review. Analyst. 2019;144:68–86.
Secchi E, Vitale A, Miño GL, Kantsler V, Eberl L, Rusconi R, Stocker R. The effect of flow on swimming bacteria controls the initial colonization of curved surfaces. Nat Commun. 2020;11:1–12.
El-Halfawy OM, Valvano MA. Antimicrobial heteroresistance: an emerging field in need of clarity. Clin Microbiol Rev. 2015;28:191–207.
Kaminski TS, Scheler O, Garstecki P. Droplet microfluidics for microbiology: techniques, applications and challenges. Lab Chip. 2016;16:2168–87.
Scheler O, Makuch K, Debski PR, Horka M, Ruszczak A, Pacocha N, Sozański K, Smolander O-P, Postek W, Garstecki P. Droplet-based digital antibiotic susceptibility screen reveals single-cell clonal heteroresistance in an isogenic bacterial population. Sci Rep. 2020;10:1–8.
Imhof HK, Laforsch C, Wiesheu AC, Schmid J, Anger PM, Niessner R, Ivleva NP. Pigments and plastic in limnetic ecosystems: a qualitative and quantitative study on microparticles of different size classes. Water Res. 2016;98:64–74.
Cho H, Felts JR, Yu MF, Bergman LA, Vakakis AF, King WP. Improved atomic force microscope infrared spectroscopy for rapid nanometer-scale chemical identification. Nanotechnology. 2013;24:444007.
Sullivan GL, Gallardo JD, Jones EW, Hollliman PJ, Watson TM, Sarp S. Detection of trace sub-micron (nano) plastics in water samples using pyrolysis-gas chromatography time of flight mass spectrometry (PY-GCToF). Chemosphere. 2020;249:126179.
Gimiliani GT, Fornari M, Redígolo MM, Bustillos JOWV, de Souza Abessa DM, Pires MAF. Simple and cost-effective method for microplastic quantification in estuarine sediment: a case study of the Santos and São Vicente Estuarine system. Case Stud Chem Environ Eng. 2020;2:100020.
Kaile N, Lindivat M, Elio J, Thuestad G, Crowley QG, Hoell IA. Preliminary results from detection of microplastics in liquid samples using flow cytometry. Front Mar Sci. 2020;7:856.
Acknowledgements
The authors are highly gratified toward the CSIR-Council of Scientific & Industrial Research for providing the support and inspiration for this work. We also acknowledge Mr. Asim Kumar Jana, Senior Technical Assistant, Aquatic Environmental Biotechnology and Nanotechnology Division, ICAR-Central Inland Fisheries Research Institute, Kolkata 700120, West Bengal, India.
Funding
The authors declare that no funds were received during the preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
All authors equally contributed to the conception and design of this review article. SD: Draft review manuscript preparation, writing. AKR: Writing, Data curation. BKB: Conceptualization and supervision. KG: Conceptualization and critical revision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Ethical approval is not applicable to this review article. Participation consent is not applicable to this review article.
Consent for publication
Consent for publication is not applicable to this review article.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
: Table S1. Global scenario of microplastic abundance on major aquatic bodies. Table S2. Potential microbial consortia associated with plastic biodegradation. Fig. S1. Common radical reactions in non-hydrolyzable polymers [269]. A The auto-oxidation process involves initiation by light and heat, followed by propagation and termination of a reaction which is influenced by the physical properties of the polymer. B Intramolecular and C Intermolecular hydrogen transfer reaction in polymer occurs through the abstraction and exchange of hydrogen atoms. Fig. S2. Polyethylene (PE), polypropylene (PP), and polyvinylchloride (PVC) are known to consist of similar carbon-carbon backbone chains. Pyrolysis (in the absence of air) is an effective depolymerization method to convert them to the respective low molecular weight aliphatic hydrocarbons [270]. According to the reports [118, 271, 272], the pyrolytic hydrocarbon products of PE are degraded through a terminal oxidation mechanism which is analogous to the n-alkane degradation pathway facilitated by microbes. Fig. S3. Polystyrene (PS) is broken down to its aromatic monomer styrene through pyrolysis [273] and according to O’Leary et al., (2002) [274] several microbes utilize it as a carbon source with the help of two different catabolic pathways. In the first one, which is the direct aromatic ring cleavage pathway, styrene dioxygenase (SDO) hydroxylates the aromatic ring of styrene to styrene cis-glycol. Ultimately it will generate β-D-Hydroxybutyryl-CoA by PhaB (an acetoacetyl-CoA reductase) or can be converted to PHA by PhaC (known as a PHA synthase) [275]. Another styrene metabolism pathway encompasses oxidation of its vinyl side-chain forming polyhydroxyalkanoate (PHA) as an end product.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dey, S., Rout, A.K., Behera, B.K. et al. Plastisphere community assemblage of aquatic environment: plastic-microbe interaction, role in degradation and characterization technologies. Environmental Microbiome 17, 32 (2022). https://doi.org/10.1186/s40793-022-00430-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40793-022-00430-4