Abstract
Pontibacillus yanchengensis Y32T is an aerobic, motile, Gram-positive, endospore-forming, and moderately halophilic bacterium isolated from a salt field. In this study, we describe the features of P. yanchengensis strain Y32T together with a comparison with other four Pontibacillus genomes. The 4,281,464 bp high-quality-draft genome of strain Y32T is arranged into 153 contigs containing 3,965 protein-coding genes and 77 RNA encoding genes. The genome of strain Y32T possesses many genes related to its halophilic character, flagellar assembly and chemotaxis to support its survival in a salt-rich environment.
Similar content being viewed by others
Introduction
Pontibacillus yanchengensis Y32T (= CGMCC 1.10680T = CCTCC AB209311T = NRRL B-59408T ) was isolated from a salt field in Yancheng, China [1], and affiliated to the family Bacillaceae , order Bacillales , phylum Firmicutes [2, 3]. The genus Pontibacillus means “ Bacillus pertaining to the sea” and was first identified by Lim et al. in 2005 [4]. To date, the genus contains six species, including Pontibacillus yanchengensis [1], Pontibacillus chungwhensis [4], Pontibacillus marinus [5], Pontibacillus halophilus [6], Pontibacillus litoralis [7], and Pontibacillus salicampi [8], which are isolated from a salt field, a solar saltern, a solar saltern, a sea urchin, a sea anemone, and a saltern soil, respectively.
The Pontibacillus members are characterized as moderately halophilic, Gram-positive, aerobic, endospore-forming and rod-shaped bacteria. They are motile by peritrichous flagella and their DNA has a low G + C content. They are able to survive in salt-rich environments and grow optimally at 5-20 % NaCl (w/v) [9]. To adapt to saline environments, halophilic microorganisms have developed various biochemical strategies to maintain cell function, such as induction of Na+/H+ antiporter systems and the production of compatible solutes. The compatible solutes are gaining increasing interest since they can be used as stabilizers, salt antagonists, or stress-protective agents [10–13]. In addition, a Pontibacillus strain could produce biosurfacants which is useful in degradation of paraffinic mixture or saline organic contamination [11].
In this study, we sequenced five Pontibacillus type strains, including P. yanchengensis Y32T , P. chungwhensis BH030062T, P. marinus BH030004T, P. halophilus JSM076056T and P. litoralis JSM072002T(The GenBank accession summary of the strains is shown in Additional file 2). Here we present the draft genome sequence of P. yanchengensis Y32T and compare it to the genomes of four other type strains. To the best of our knowledge, this is the first description of the Pontibacillus genome.
Organism information
Classification and features
P. yanchengensis Y32T was isolated from a salt field in Yancheng prefecture, on the east Yellow Sea in China. A taxonomic analysis was conducted based on the 16S rRNA gene sequence. The representative 16S rRNA gene sequences of the most closely related strains were downloaded from NCBI and multi-aligned by CLUSTAL W [14]. Phylogenetic consensus trees were constructed based on the aligned gene sequences using the neighbor-joining method with 1,000 bootstraps by using MEGA 6.0 [15]. The phylogenetic tree based on the 16S rRNA gene sequences indicated that strain Y32T was clustered within a branch containing other species in the genus Pontibacillus (Fig. 1a).
Phylogenetic analysis. a The 16S rRNA gene-based phylogenetic tree showing the position of P. yanchengensis Y32T. b The NJ phylogenetic tree of P. yanchengensis Y32T relative to 16 genome-sequenced strains from the Bacillaceae family was built based on the core protein sequences. All genome FASTA files were downloaded from NCBI except for the Pontibacillus genus. A total of 602 conserved proteins were identified using the cluster algorithm tool OrthoMCL [16, 17]. The phylogenetic trees were constructed using the neighbor-joining method by MEGA 6.0 software [15] with a bootstrap value of 1,000
Seventeen related strains of Bacillaceae [2] with complete genome sequences were chosen for further phylogenetic analysis, including the four draft-genome sequences of Pontibacillus that were sequenced by us. In total, 602 core protein sequences were extracted using the cluster algorithm tool OrthoMCL [16, 17] with default parameters. The neighbor-joining (NJ) phylogenetic tree showed that the five Pontibacillus species clustered into the same branch (Fig. 1b), which was in accordance with the 16S rRNA gene-based phylogeny (Fig. 1a).
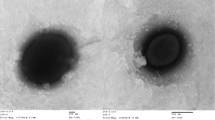
P. yanchengensis Y32T is Gram-positive, rod-shaped (0.5–0.9 × 1.9–2.5 μm), motile with flagella (Fig. 2) and endospore-forming. It can grow on Bacto marine broth 2216 (Difco) agar medium containing 3–20 % (w/v) NaCl and does not grow in the absence of NaCl [1]. The optimal growth temperature for Y32T is 35–40 °C (Table 1). The strain is oxidase- and catalase-positive and negative for the production of H2S or indole. It has been reported to reduce nitrate to nitrite [1]. P. yanchengensis Y32T can use a few kinds of sole carbon sources, including D-glucose, D-fructose, D-mannitol, D-maltose and D-trehalose [1]. Compared to the other Pontibacillus genus type strains, only P. yanchengensis Y32T can utilize D-mannitol as sole carbon source [1]. KEGG pathway analysis of the five Pontibacillus genomes (see below) revealed that only strain Y32T had the key enzyme mannitol-1-phosphate 5-dehydrogenase (gene ID: N782_14920) which could potentially catalyze D-mannitol 1-phosphate to D-fructose 6-phosphate. This result was consistent with the phenotype. As one of the most abundant polyols in nature, mannitol metabolism provides an important physiologic contribution in microbial stress responses [18].
Chemotaxonomic data
When grown on Bacto marine broth 2216 (Difco) agar medium plus 3 % (w/v) NaCl, P. yanchengensis Y32T contained anteiso-C15:0, iso-C15:0, and iso-C14:0 as the major fatty acids and menaquinone (MK-7) as the predominant respiratory quinone. The cell wall peptidoglycan type was meso-diaminopimelic [1]. The classification and general features of P. yanchengensis Y32T are shown in Table 1.
Genome sequencing information
Genome project history
P. yanchengensis Y32T was selected for sequencing on the basis of its taxonomic representativeness, halophilic features and potential industrial applications. Genome sequencing was performed by Majorbio Bio-pharm Technology Co., Ltd., Shanghai, China. The draft genome sequence was deposited in NCBI with contigs larger than 200 bp. The GenBank accession number is AVBF00000000. A summary of the genome sequencing project information is shown in Table 2.
Growth conditions and DNA isolation
P. yanchengensis Y32T was grown aerobically in 50 mL Bacto marine broth 2216 (Difco) plus 5 % NaCl (w/v) at 37 °C for 2 d with 150 rpm shaking. Cells were harvested by centrifugation and a pellet with an approximate wet weight of 20 mg was obtained. The genomic DNA was extracted using the QIAamp DNA kit according to the manufacturer’s instructions (Qiagen, Germany). The quality and quantity of total DNA was determined using a NanoDrop Spectrophotometer 2000. Five micrograms of genomic DNA was sent to Majorbio (Shanghai, China) for sequencing on a Hiseq2000 (Illumina, CA) sequencer.
Genome sequencing and assembly
The Illumina Hiseq2000 technology of Paired-End (PE) library with an average insert size of 300 bp was used to determine the sequence of P. yanchengensis Y32T. A total of 4,083,912 × 2 high quality reads totaling 824,950,224 bp of data with an average coverage of 186.5 x was generated. Raw reads were filtered using a FastQC toolkit followed by assembly with SOAP denovo v1.05 and optimizing through local gap filling and base correction with Gap Closer.
Genome annotation
The draft genome sequence was deposited at NCBI and was annotated through the Prokaryotic Genome Annotation Pipeline, which combined the Best-Placed reference protein set and the gene caller GeneMarkS+. The WebMGA server was used to identify the Clusters of Ortholog Groups [19]. Transmembrane helices and signal peptides were predicted by the online bioinformatic tools TMHMM 2.0 [20, 21] and SignalP 4.1 [22], respectively.
Genome properties
The final whole genome of P. yanchengensis Y32T was 4,283,464 bp long, distributed in 153 contigs, and had an average GC content of 39.11 %. Of the total 4,080 predicted genes, 3,965 were protein-coding genes (CDSs), and 77 were RNA genes. A total of 2,615 CDSs (65.95 %) were assigned putative functions, and the remaining proteins were annotated as hypothetical proteins. The genome properties and statistics are summarized in Table 3. The distribution of genes into COGs functional categories is shown in Table 4.
Insights from the genome sequence
In this study, we compared the genome sequence of P. yanchengensis Y32T with the genomes of P. chungwhensis BH030062T, P. halophilus JSM076056T, P. marinus BH030004T and P. litoralis JSM072002T. The general features of the five genomic sequences are summarized in Table 5. The results of the core genome analysis suggested that the five Pontibacillus species share 2,160 core genes, and P. yanchengensis Y32T possesses 1,651 unique genes (Fig. 3a). Among the 1,651 unique genes for strain Y32T, 1,154 unique genes were classified into 20 COG functional categories, which mainly belonged to the general function prediction group, the carbohydrate transport, the metabolism group and the function unknown group. The remaining 590 unique genes were not classified into any COG categories (Additional file 1: Table S1). The CG View Comparison Tool [23] was used to draw a comparison graphical circular map of the five Pontibacillus strains (Fig. 3b).
Comparative genomic analysis of the genus Pontibacillus. a The flower plot shows the numbers of species-specific genes found in each genome of each species (in the petals) and the core orthologous gene number (in the center) of Pontibacillus. b Comparison map of strain P. yanchengensis Y32T and the other four sequenced Pontibacillus strains. From outside to inside: rings 1, 4 show protein-coding genes colored by COG categories on the forward/reverse strand, respectively; rings 2, 3 represent genes on the forward/reverse strand, respectively; rings 5, 6, 7, 8 denote the CDS vs CDS BLAST results of P. marinus BH030004T, P. chungwhensis BH030062T, P. halophilus JSM076056T, and P. litoralis JSM072002T, respectively; ring 9 shows the GC skew
All the Pontibacillus species were isolated from salty environments. They were characterized as moderately halophilic and cannot grow in the absence of NaCl. As moderate halophiles, effective establishment of ionic and osmotic equilibrium was important for survival in a saline environment. The genome comparison analysis showed that the five Pontibacillus strains possessed genes encoding cation/proton antiporter (e.g., Na+/H+ antiporter, Na+/Ca2+ antiporter), which played a role in tolerance to high concentrations of Na+, K+, Li+ and/or alkali (Additional file 1: Table S2). Numerous studies showed that Na+/H+ antiporters play important roles in the pH and Na+ homeostasis of cells [24, 25]. Meanwhile, the prediction of the membrane helices of the P. yanchengensis Y32T genome suggested that nearly 30% of the genes had transmembrane helix structures (Table 3), which may be involved in ion transport.
Other than ion transport, the synthesis of compatible solutes (e.g., betaine, ectoine, amino acids) was beneficial for survival under extreme osmotic stress. Many compatible solute synthesis-related genes were identified in the genomes of the five Pontibacillus species (Additional file 1: Table S2). The Kyoto Encyclopedia of Genes and Genomes was used to reconstruct the glycine, serine and threonine metabolic pathways (Fig. 4). The metabolic pathways suggested that the five Pontibacillus strains could synthesize glycine as the main compatible solute. In addition, P. yanchengensis Y32T, P. chungwhensis BH030062T and P. marinus BH030004T could synthesize betaine through the precursor choline. P. marinus BH030004T also possessed the pathway of ectoine synthesis. These results indicated that the five Pontibacillus species use different strategies to cope with osmotic stress.
The glycine, serine and threonine metabolic pathways of the five Pontibacillus strains (including P. yanchengensis Y32T, P. marinus BH030004T, P. chungwhensis BH030062T, P. halophilus JSM076056T, and P. litoralis JSM072002T) reconstructed by KEGG. The green box represents the enzyme shared by all five strains to synthesize glycine. The blue boxes denote the enzymes involved in betain synthesis, which were found in P. yanchengensis Y32T, P. chungwhensis BH030062T and P. marinus BH030004T. The pathway with pink boxes is found only by P. marinus BH030004T and is related to ectoine synthesis
Many flagella-related genes were identified in the genomes of the five Pontibacillus species. Reconstruction of a multi-organism KEGG map suggested that the five Pontibacillus strains had intact chemotaxis systems (Fig. 5a) and flagella assembly-related genes (flg, fli and flh) (Fig. 5b). The moderately halophilic Pontibacillus strains were unable to grow with NaCl as the sole salt unless artificial seawater was added [1, 4–8]. Flagella and chemotaxis may play important roles in response to environmental salts.
Reconstructed bacterial chemotaxis (a) and flagellar assembly (b) KEGG map of the five Pontibacillus strains. Green boxes represent the chemotaxis- and flagellar-related protein-coding genes identified in all five Pontibacillus genomes (P. yanchengensis Y32T, P. marinus BH030004T, P. chungwhensis BH030062T, P. halophilus JSM076056T, and P. litoralis JSM072002T)
Conclusions
This study provided genomic information for P. yanchengensis strain Y32T and the comparison of five Pontibacillus genomes. Strain Y32T has functional genes encoding cation/proton antiporters and proteins for biosynthesis of compatible solutes such as glycine and ectoine. Compatible solutes could be of use in the cosmetic and food industries [13]. The comparative genomic analysis suggested that the five Pontibacillus strains possess different synthetic pathways for compatible solutes which provided diverse applications of the strains.
References
Yang Y, Zou Z, He M, Wang G. Pontibacillus yanchengensis sp. nov., a moderately halophilic bacterium isolated from salt field soil. Int J Syst Evol Microbiol. 2011;61:1906–11. doi:10.1099/ijs.0.023911-0.
Skerman VBD, McGowan V, Sneath PHA. Approved lists of bacterial names. Int J Syst Bacteriol. 1980;30:225–420.
Fischer A. Untersuchungen über bakterien. Jahrbüch für Wissenschaftliche Botanik. 1895;27:1–163.
Lim JM, Jeon CO, Song SM, Kim CJ. Pontibacillus chungwhensis gen. nov., sp. nov., a moderately halophilic Gram-positive bacterium from a solar saltern in Korea. Int J Syst Evol Microbiol. 2005;55:165–70. doi:10.1099/ijs.0.63315-0.
Lim JM, Jeon CO, Park DJ, Kim HR, Yoon BJ, Kim CJ. Pontibacillus marinus sp. nov., a moderately halophilic bacterium from a solar saltern, and emended description of the genus Pontibacillus. Int J Syst Evol Microbiol. 2005;55:1027–31. doi:10.1099/ijs.0.63489-0.
Chen YG, Zhang YQ, Xiao HD, Liu ZX, Yi LB, Shi JX, et al. Pontibacillus halophilus sp. nov., a moderately halophilic bacterium isolated from a sea urchin. Int J Syst Evol Microbiol. 2009;59:1635–9. doi:10.1099/ijs.0.002469-0.
Chen YG, Zhang YQ, Yi LB, Li ZY, Wang YX, Xiao HD, et al. Pontibacillus litoralis sp. nov., a facultatively anaerobic bacterium isolated from a sea anemone, and emended description of the genus Pontibacillus. Int J Syst Evol Microbiol. 2010;60:560–5. doi:10.1099/ijs.0.009910-0.
Lee JC, Kim YS, Yun BS, Whang KS. Pontibacillus salicampi sp. nov., a moderate halophilic bacterium isolated from a saltern soil. Int J Syst Evol Microbiol. 2014. Available at: doi:10.1099/ijs.0.066423-0.
DasSarma S, DasSarma P. Halophiles. In: eLS. John Wiley & Sons, Ltd; 2006. doi:10.1002/9780470015902.a0000394.pub3
Margesin R, Schinner F. Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles. 2001;5:73–83. doi:10.1007/s007920100184.
Kheiralla ZH, Ashour SM, Rushdy AA, Ahmed HA. Characterization of biosurfactants produced by Halobacillus dabanensis and Pontibacillus chungwhensis isolated from oil-contaminated mangrove ecosystem in Egypt. Appl Biochem Microbiol. 2013;49:263–9. doi:10.1134/S0003683813030186.
Roberts MF. Organic compatible solutes of halotolerant and halophilic microorganisms. Saline systems. 2005;1:1–30. doi:10.1186/1746-1448-1-5.
Sorokin DY, Janssen AJH, Muyzer G. Biodegradation potential of palo(alkali)philic prokaryotes. Crit Rev Env Sci Tec. 2011;42:811–56. doi:10.1080/10643389.2010.534037.
Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80.
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary geneticsanalysis version 6.0. Mole Biol Evol. 2013;30:2725–9. doi:10.1093/molbev/mst197.
Li L, Stoeckert CJ, Roos DS. OrthoMCL: Identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13:2178–89. doi:10.1101/gr.1224503.
Fischer S, Brunk BP, Chen F, Gao X, Harb OS, Iodice JB, et al. Using OrthoMCL toassign proteins to OrthoMCL-DB groups or to clusterproteomes into new orthologgroups: John Wiley & Sons, Inc.; 2011. doi:10.1002/0471250953.bi0612s35
Iwamoto K, Shiraiwa Y. Salt-Regulated Mannitol Metabolism in Algae. Mar Biotechnol. 2005;7:407–15. doi:10.1007/s10126-005-0029-4.
Wu S, Zhu Z, Fu L, Niu B, Li W. WebMGA: a customizable web server for fast metagenomic sequence analysis. BMC Genomics. 2011;12:444. doi:10.1186/1471-2164-12-444.
Sonnhammer EL, Von Heijne G, Krogh A. A hidden Markov model for predicting transmembrane helices in protein sequences. Proc Int Conf Intell Syst Mol Biol. 1998;6:175–82.
Krogh A, Larsson B, von Heijne G, Sonnhammer ELL. Predicting transmembrane protein topology with a hidden markov model: application to complete genomes. J Mol Biol. 2001;305:567–80. doi:10.1006/jmbi.2000.4315.
Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Meth. 2011;8:785–6. doi:10.1038/nmeth.1701.
Grant J, Arantes A, Stothard P. Comparing thousands of circular genomes using the CGView comparison tool. BMC Genomics. 2012;13:202. doi:10.1186/1471-2164-13-202.
Padan E, Venturi M, Gerchman Y, Dover N. Na(+)/H(+) antiporters. Biochim Biophys Acta. 2001;1505:144–57. doi:10.1016/S0005-2728(00)00284-X.
Hunte C, Screpanti E, Venturi M, Rimon A, Padan E, Michel H. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature. 2005;435:1197–202. doi:10.1038/nature03692.
Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, et al. The minimum information about a genome sequence (MIGS) specification. Nat Biotechnol. 2008;26:541–7. doi:10.1038/nbt1360.
Gibbons NE, Murray RGE. Proposals concerning the higher taxa of bacteria. Int J Syst Bacteriol. 1978;28:1–6. doi:10.1099/00207713-28-1-1.
Murray R. The higher taxa, or, a place for everything. Bergey’s Manual Syst Bacteriol. 1984;1:31–4.
Editor L. List of new names and new combinations previously effectively, but not validly, published. List no. 132. Int J Syst Evol Microbiol. 2010;60:469–72.
Ludwig W, Schleifer KH, Whitman WB. Class I. Bacilli class nov. Bergey’s Manual of Systematic Bacteriology. 2009; 3:19–20
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene Ontology: tool for the unification of biology. Nat Genet. 2000;25:25–9. doi:10.1038/75556.
Acknowledgment
This work was supported by the China Postdoctoral Science Foundation (2014 M562037) and the National Natural Science Foundation of China (31470227).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JH performed the sequence annotation and genomic analysis. ZXQ and JWT helped performing the comparative genomic analysis. JH wrote the draft manuscript. GJW organized the study and revised the manuscript. All authors read and approved the final manuscript.
Additional files
Additional file 1: Table S1.
COG functional categories of the 1651 genes unique to P.yanchengensis Y32T. Table S2 Species distribution analysis of osmotic stress related gene families. (DOCX 15 kb)
Additional file 2:
GenBank Accession Summary, Strain ID Summary, Reference Search Summary. (DOC 33 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Huang, J., Qiao, Z., Tang, J. et al. High quality draft genome sequence of the moderately halophilic bacterium Pontibacillus yanchengensis Y32T and comparison among Pontibacillus genomes. Stand in Genomic Sci 10, 93 (2015). https://doi.org/10.1186/s40793-015-0085-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40793-015-0085-y