Abstract
Background
The development of immunohistochemical staining has revealed that gastric adenocarcinoma with the gastric phenotype can be divided into the foveolar, fundic gland, and pyloric gland phenotypes. Gastric adenocarcinoma of the pyloric gland type is difficult to diagnose using biopsy because of its low atypia and rarity. Herein, we describe a case of gastric adenocarcinoma of the pyloric gland type that was diagnosed immunohistochemically after endoscopic resection.
Case presentation
A 67-year-old man was referred to our hospital for the diagnosis and treatment of a 30-mm elevated lesion on the lesser curvature side of the middle of the gastric body. Although four biopsies were performed, it was difficult to determine whether the lesion was benign or malignant. Therefore, endoscopic submucosal dissection was performed, and the presence of tumor cells infiltrating the submucosa with venous invasions was identified. Immunohistochemical staining revealed that the tumor cells were positive for MUC5AC and MUC6 and negative for Pepsinogen I and H + /K + -ATPase. From the above findings, he was diagnosed as having gastric adenocarcinoma with pyloric gland type. The patient underwent a laparoscopic distal gastrectomy and was discharged without any adverse events.
Conclusions
Gastric adenocarcinoma of the pyloric gland type is a rare disease, and endoscopic resection can serve as a viable diagnostic option for this condition when it is difficult to diagnose using biopsy. Immunohistochemical pathology images can aid in the diagnosis of gastric adenocarcinoma of the pyloric gland type.
Similar content being viewed by others
Background
Recently, the development of immunohistochemical staining has revealed that gastric adenocarcinoma differentiates into the gastric and intestinal phenotypes. The gastric phenotype is further divided into the foveolar, fundic gland, and pyloric gland phenotypes [1]. Gastric adenocarcinoma of the fundic gland type is a subtype of gastric adenocarcinoma that was proposed in 2010 [2] and newly included in the WHO classification in 2019 [3]. However, gastric adenocarcinomas with gastric phenotypes other than gastric adenocarcinoma with fundic gland type have not yet been widely recognized. Each subtype of gastric adenocarcinoma with gastric phenotype is characterized by low atypia and is often difficult to diagnose [4,5,6]. Therefore, as with other low-grade well-differentiated adenocarcinomas of the gastric phenotype (LG-WDA-G), gastric adenocarcinoma of the pyloric gland type is difficult to diagnose on biopsy specimens because of its low atypia and rarity. So far, only one case of gastric adenocarcinoma of the pyloric gland type has been reported. In this report, we describe a case of gastric adenocarcinoma of the pyloric gland type which was diagnosed immunohistochemically after endoscopic resection due to diagnostic difficulties on biopsy specimens. In this report, detailed immunohistochemical staining was performed, and the immunohistological pathology images in this report will be helpful in the diagnosis of gastric adenocarcinoma of the pyloric gland type.
Case presentation
A 67-year-old man visited a local hospital with abdominal pain. He underwent esophagogastroduodenoscopy (EGD), which revealed a 30 mm-sized elevated lesion with a concave surface and mucus production on the lesser curvature side of the middle of gastric body; however, the biopsy specimens obtained showed no malignant findings. The patient underwent another EGD the following month, and the biopsy specimens revealed pyloric adenoma (Group 3) with no malignant findings. The HE-stained specimen was positive for Helicobacter pylori infection, and he underwent the required eradication therapy.
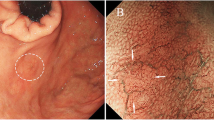
Three months later, an EGD was restudied and biopsies were performed again; however, no malignant findings were observed. Then, the patient was referred to our hospital for a thorough examination of the lesion. An EGD revealed a 30-mm 0–IIa + IIc lesion with a depressed surface on the lesser curvature side of the middle of the gastric body (Fig. 1a, b). Magnifying narrow band imaging (NBI) revealed that the center of the lesion was white in color and vascularized (Fig. 1c). Endoscopic findings showed the good wall extensivity of the tumor, but the wall thickening remained by air suppression. Biopsy specimens revealed the presence of atypical cells and proliferating glandular ducts with irregular branching and fusion, raising suspicion of pyloric gland adenoma or pyloric gland adenocarcinoma. Endoscopic ultrasonography (EUS) showed isoechoic tumor with an elongated anechoic area, suggesting an ectopic pancreas. In addition, EUS findings showed the deep third layer and forth layer were remained, suggesting that the isoechoic tumor did not invade the deep submucosa and muscularis propria. Since it was hard to determine whether the lesion was benign or malignant, endoscopic submucosal dissection was performed. The resected specimen measured 68 × 50 mm. A 39 × 32-mm submucosal tumor (SMT)-like elevation was found, forming an ulcer measuring 26 × 17-mm (Fig. 2). Histopathologically, the lobular proliferation of pyloric grand-like acinar structures was observed but there were neither chief cells nor parietal cells. These atypical glands infiltrated the submucosal tissue (Fig. 3a–d). Venous invasion was observed (Fig. 3e). Immunohistochemically, tumor cells were positive for both MUC5AC and MUC6 (Fig. 3f and g), while pepsinogen-I and H+/K+-ATPase were both negative (Fig. 3h, i). The tumor’s Ki-67 labeling index was approximately 5% within the tumor (Fig. 3j). Contrast-enhanced computed tomography revealed no obvious lymph node or distant metastases. Based on the above, the patient was diagnosed with gastric adenocarcinoma of the pyloric gland type, pType 0-IIa + IIc, 39 × 32 mm, pT1b2(SM2) (2200 µm), UL0, Ly0, V1, pHM0, pVMX, cN0M0 cStage I. Due to submucosal layer infiltration and venous invasion, a laparoscopic distal gastrectomy with D1 + lymph node dissection was performed three months after the endoscopic submucosal dissection. The pathological findings of the resected specimen showed the absence of a residual tumor and no lymph node metastasis in 42 lymph nodes that were dissected. On postoperative day 3, the patient started oral intake, and he was discharged on postoperative day 7.
0–IIa + IIc lesion measuring 30 mm in size with a depressed surface at the lesser curvature side of the middle of the gastric body (a and b). Magnifying NBI revealed the vascular structure at the center of the lesion (c). Endoscopic ultrasonography revealed an isoechoic tumor with an elongated anechoic area suggestive of an ectopic pancreas, and also showed that the deep third layer and forth layer were remained (d)
Histopathological findings of the tumor. a–c Lobular proliferation of pyloric gland-like glands was observed. d Tumor glands infiltrated submucosal tissues. e Venous invasion was observed in Elastica van Gierson staining. f–i Immunohistochemical staining of MUC5AC (f) and MUC6 (g), Pepsinogen-I (h), and H + /K + -ATPase. (j) The Ki-67 labeling index of the tumor is approximately 5%
Discussion
LG-WDA-G has three subgroups, which are gastric adenocarcinoma of the foveolar, fundic gland, and pyloric gland types [1]. The fundic gland type is further divided into gastric adenocarcinoma of the fundic gland type (GAFG) and gastric adenocarcinoma of the fundic gland mucosa type (GAFGM) [7]. In this case, in addition to mucin histochemistry, the diagnosis of gastric adenocarcinoma of the pyloric gland type was made using immunohistochemistry staining for H+/K+-ATPase, a staining marker of gastric parietal cells, and pepsinogen-I, a staining marker of chief cells and mucous neck cells. Immunohistochemistry is useful for distinguishing the three subgroups from each other, and the characteristic immunohistochemical staining findings are summarized in Table 1.
Pyloric metaplasia is a metaplastic change of mucous neck cells in fundic glands caused by H. pylori infection, and it is considered a regenerative change that serves to compensate for damage to the gastric mucosa [8]. Pyloric metaplasia occurs by replacing mucin-rich oxyntic glands secreted by mucous neck cells of pyloric-type glands, which is positive for MUC6 [9]. Pyloric adenomas, despite exhibiting the pyloric gland phenotype, mostly arise from the fundic gland area with chronic gastritis or autoimmune gastritis [10, 11]. Similarly, the fact that in both cases of the gastric adenocarcinoma of the pyloric gland type (including the present case), the tumor cells originate from the fundic gland area, and the presence of gastritis in the background mucosa suggests that pyloric gland metaplasia may play an important role in the histogenesis of gastric adenocarcinoma of the pyloric gland type. In this case, biopsies were performed four times; however, the diagnosis was not made because the tumor cells were well differentiated and had low atypia, which is one of the pathological characteristics of LG-WDA-G. It was reported that LG-WDA-G, even with repeated biopsies, could not differentiate LG-WDA-G from inflamed or regenerative changes with hematoxylin–eosin staining alone [12]. Thus, sufficiently deep tissue sampling (for example, endoscopic resection) is critical to making the correct diagnosis. In addition, it is known that LG-WDA-G tends to have an undermining growth pattern [13]. This indicates that LG-WDA-G tends to infiltrate structures deeper than muscularis mucosa, which makes it more likely for the patient to have distant and lymphatic metastases. As with the study reported by Mochizuki, which shows a patient with advanced gastric adenocarcinoma of the pyloric gland type (pT3N3a), our case showed submucosal infiltration and venous invasion [1]. Therefore, an accurate diagnosis is necessary.
Conclusions
Gastric adenocarcinoma of the pyloric gland type is a rare disease, and endoscopic resection could be a viable diagnostic option when it is difficult to make a biopsy-based diagnosis. Although its rate of atypia is low, cases of venous invasion and advanced carcinoma suggest the possibility of high malignancy. Further case series and investigations are needed to clarify the clinicopathological features of gastric adenocarcinoma of the pyloric gland type. Our case of gastric adenocarcinoma of the pyloric gland type demonstrated that this entity can be underrecognized or misdiagnosed as benign tumor if the clinicians are not aware of this entity.
Availability of data and materials
All data on which the conclusions of this case report are based are included in the present publication.
Abbreviations
- LG-WDA-G:
-
Low-grade well-differentiated adenocarcinoma of gastric phenotype
- EGD:
-
Esophagogastroduodenoscopy
- NBI:
-
Narrow band imaging
- EUS:
-
Endoscopic ultrasonography
- SMT:
-
Submucosal tumor
- GAFG:
-
Gastric adenocarcinoma of the fundic gland type
- GAFGM:
-
Gastric adenocarcinoma of the fundic gland mucosa type
References
Mochizuki K, Kondo T, Tahara I, Inoue T, Kasai K, Oishi N, et al. Gastric adenocarcinoma of pyloric gland type with high-grade malignancy. Pathol Int. 2015;65:148–50.
Ueyama H, Yao T, Nakashima Y, Hirakawa K, Oshiro Y, Hirahashi M, et al. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol. 2010;34:609–19.
Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182–8.
Ueyama H, Matsumoto K, Nagahara A, Hayashi T, Yao T, Watanabe S. Gastric adenocarcinoma of the fundic gland type (chief cell predominant type). Endoscopy. 2014;46:153–7.
Ushiku T, Kunita A, Kuroda R, Shinozaki-Ushiku A, Yamazawa S, Tsuji Y, et al. Oxyntic gland neoplasm of the stomach: expanding the spectrum and proposal of terminology. Mod Pathol. 2020;33:206–16.
Shibagaki K, Fukuyama C, Mikami H, Izumi D, Yamashita N, Mishiro T, et al. Gastric foveolar-type adenomas endoscopically showing a raspberry-like appearance in the Helicobacter pylori-uninfected stomach. Endosc Int Open. 2019;7:E784–91.
Imamura K, Yao K, Nimura S, Tanabe H, Kanemitsu T, Miyaoka M, et al. Characteristic endoscopic findings of gastric adenocarcinoma of fundic-gland mucosa type. Gastric Cancer. 2021;24:1307–19.
Hattori T, Helpap B, Gedigk P. The morphology and cell kinetics of pseudopyloric glands. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;39:31–40.
Wada Y, Kushima R, Kodama M, Fukuda M, Fukuda K, Okamoto K, et al. Histological changes associated with pyloric and pseudopyloric metaplasia after Helicobacter pylori eradication. Virchows Arch. 2020;477:489–96.
Kushima R, Sekine S, Matsubara A, Taniguchi H, Ikegami M, Tsuda H. Gastric adenocarcinoma of the fundic gland type shares common genetic and phenotypic features with pyloric gland adenoma. Pathol Int. 2013;63:318–25.
Vieth M, Kushima R, Mukaisho K, Sakai R, Kasami T, Hattori T. Immunohistochemical analysis of pyloric gland adenomas using a series of Mucin 2, Mucin 5AC, Mucin 6, CD10, Ki67 and p53. Virchows Arch. 2010;457:529–36.
Nokubi M, Kawanowa K, Kawata H, Hanatsuka K, Hosoya Y. Extremely well-differentiated adenocarcinoma of the gastric cardia: a unique case with columnar cells and laminated stones. Pathol Int. 2004;54:854–60.
Lee J, Lee IS, Ahn JY, Park YS, Kim J. Extremely well-differentiated adenocarcinoma of the stomach: diagnostic pitfalls in endoscopic biopsy. J Pathol Transl Med. 2022;56:63–72.
Acknowledgements
This work was partially supported by a grant-in-aid of the 106th Annual Congress of the JSS Memorial Surgical Research Fund, Tokyo, Japan.
Funding
The authors declare that this study was not funded externally.
Author information
Authors and Affiliations
Contributions
SY, KY, NE, and KN performed the surgery. HM and TI performed the pathological diagnosis. NA performed the endoscopic resection. KH prepared the manuscript under the supervision of SY. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
As this article is the case report, we did not need the ethics approval of our institutional review board.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Competing interests
All authors have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hirata, K., Yagi, S., Miyazaki, H. et al. A case of gastric adenocarcinoma with pyloric gland-type infiltrating submucosa. surg case rep 10, 78 (2024). https://doi.org/10.1186/s40792-024-01835-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-024-01835-8