Abstract
Background
Although metastatic spread of breast cancer to the gastrointestinal tract is very rare, it is more likely to occur in invasive lobular carcinoma (ILC) than in ductal carcinoma. Colonic metastasis is particularly rare, and the treatment strategies for these cases are not clearly defined. Herein, we report three cases of ILC with various abdominal symptoms associated with colonic metastasis.
Case presentation
Case 1 A 70-year-old female patient with vomiting and melena was referred to our hospital. Endoscopic examination revealed a Dieulafoy ulcer in the rectum and an elevated lesion in the descending colon. She also had two breast nodules, and was diagnosed as ILC with colonic metastasis. Considering her general condition, the best supportive care (BSC) was offered. The patient died 4 months after confirmation of the diagnosis. Case 2 An 80-year-old female patient presented with diarrhea and vomiting. She was diagnosed with ILC with colonic metastasis, and a coloscopy revealed stenosis of the transverse colon with a metastatic lesion. Ileosigmoid bypass surgery was performed for intestinal obstruction, and systemic treatment for breast cancer was initiated. The patient developed peritoneal carcinomatosis and died 1 year and 2 months after surgery. Case 3 A 56-year-old female patient underwent left total mastectomy for ILC, and laparoscopic transverse colectomy was conducted for a colonic lesion 9 years and 2 months after. The diagnosis as colonic metastasis was not confirmed at that time. Two years and 2 months later, torose lesions were detected in the hepatic flexural and descending colon, and histopathological findings indicated that all colon tumors, including the previously resected tumor, were metastatic spread of ILC. Systemic treatment was continued, but the transverse colonic lesion penetrated the abdominal wall, and an abscess formed 2 years and 11 months after the resection. The fistula improved by continuous suction drainage following ileostomy but recurred, and the patient died 3 years and 8 months after colectomy.
Conclusions
Colonic metastases from breast cancer can trigger various abdominal symptoms, and the prognosis in these cases is generally poor. In selected cases, surgical treatment for abdominal symptoms and subsequent systemic therapy can contribute to a prolonged prognosis.
Similar content being viewed by others
Background
Breast cancer is the most prevalent malignancy in women worldwide, with an age-adjusted mortality rate of 13.6% [1]. As it progresses, hematogenous and lymphatic metastases are often observed, and the most commonly involved organs are the liver, lungs, brain, and bones [2]. Spread to the gastrointestinal tract is relatively rare; McLemore et al. reported that it was found in 73 of 12,001 cases of primary breast cancer [3]. In contrast, a recent study reported that 11% of autopsy cases with distant metastases had colonic foci [4]. Invasive lobular carcinoma (ILC) accounts for 10–15% of breast cancers [5] and is more likely to metastasize to the gastrointestinal tract than invasive ductal carcinoma [6, 7]. Colorectal metastasis might be even rarer than involvement of the stomach and small intestine [3, 8]. The clinical presentation of gastrointestinal lesions varies, and the treatment strategies for these cases are not clearly defined. Here, we report three cases of ILC with various abdominal symptoms due to colonic metastases including one previously reported case [9].
Case presentation
Case 1
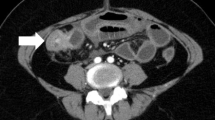
A 70-year-old female patient who underwent rehabilitation following a subdural hemorrhage was transferred to the hospital because of repeated vomiting and melena. Computed tomography (CT) scan revealed two nodules in the left mammary gland, multiple osteolytic changes, and fractures throughout the body (Fig. 1). Endoscopic examination suggested bleeding from a Dieulafoy ulcer in the rectum, and electrocoagulation was performed.
A repeat examination two days later revealed a torose lesion with redness in the descending colon, which was biopsied for diagnosis (Fig. 2). There was no evidence of tumor bleeding. Tumor marker tests indicated elevation of carcinoembryonic antigen (CEA) (43.7 ng/mL) and cancer antigen 15-3 (> 300 U/mL). Mammary gland ultrasonography revealed 19 mm and 16-mm-sized nodules in the left EAC and C segments, respectively. Histological examination of the left mammary nodule was negative for E-cadherin, leading to the diagnosis of ILC. The estrogen receptor (ER) and progesterone receptor (PgR) statuses were strongly positive, whereas HER2 was negative (Fig. 3). The colonic lesion had a histopathological appearance similar to that of signet ring cell carcinoma and was ER-positive, PgR-negative, and GATA-binding protein 3 (GATA3) -positive (Fig. 4). She was diagnosed with ILC with colonic and multiple bone metastases; however, considering her general condition, the best supportive care (BSC) was offered. The patient died 4 months after the diagnosis was confirmed.
Histopathologic findings of breast cancer in Case 1 (invasive lobular carcinoma). A Atypical cells with large mucin vacuoles, which mimicked signet ring cell carcinoma, were seen (hematoxylin and eosin [HE]. Bar: 50 μm). B Tumor cells were negative for E-cadherin expression. (E-cadherin stain. Bar: 50 μm)
Case 2
An 80-year-old female patient presented with diarrhea and vomiting. A CT scan suggested left breast cancer with ascending to transverse colon metastases and peritoneal dissemination (Fig. 5). Ultrasonography revealed a 14-mm nodule in the left C segment, which was diagnosed as ILC, characterized by positive ER and PgR expression and negative HER2 expression. A colonoscopy revealed stenosis of the right side of the transverse colon with tumor spread (Fig. 6). The histopathological diagnosis was ILC metastases, with tumor cells positive for ER and GATA3 and negative for E-cadherin. Ileosigmoid bypass surgery was performed for intestinal obstruction, and anastrozole was initiated on postoperative day six. After 2 months, the treatment for breast cancer was changed to fulvestrant and palbociclib because the peritoneal metastases had enlarged. Eight months after the operation, the tumor metastasized to the bone, and eribulin treatment was initiated. However, the patient developed peritoneal carcinomatosis and died 1 year and 2 months after the intestinal bypass.
Case 3
A 56-year-old female patient underwent a left total mastectomy and axillary dissection for ILC that was ER-positive, PgR-positive, HER2-negative, and gross cystic disease fluid protein (GCDFP) 15-positive. Bone metastasis was shown six years later despite postoperative hormonal therapy; the treatment was switched to toremifene and zoledronic acid, and the tumor was under control. Nine years and 2 months after the breast surgery, she had an elevated CEA level (8.5 ng/ml) and a large tumor was detected in the transverse colon. Therefore, laparoscopic transverse colectomy was performed for the colonic lesion. Pathologically, it was similar to previous breast cancers in that small tumor cells with high nuclear-to-cytoplasmic ratio infiltrated, however, immunostaining for PgR and GCDFP 15 was negative, which was different from them. At that time, it was diagnosed with poorly differentiated adenocarcinoma of the colon, and postoperative chemotherapy with FOLFIRI (5-fluorouracil, folinic acid and irinotecan) was performed in accordance with advanced colon cancer.
Two years and 2 months after the colectomy, torose lesions were detected in the hepatic flexure and descending colon (Fig. 7). The histological findings of these tumors mimicked those of previous transverse colon tumors and breast cancers, with positivity for ER and GATA3 and negativity for E-cadherin. All colon tumors, including previously resected tumors, were diagnosed as ILC metastases. Systemic treatment was continued; however, multiple metastases to the transverse colon, bilateral ovaries, and peritoneal metastases gradually progressed. Two years and 11 months after the abdominal surgery, the transverse colon lesion penetrated the abdominal wall, and an abscess formed. The patient underwent continuous suction drainage following ileostomy (Fig. 8). As the fistula improved 1 month later, vinorelbine treatment was started. Subsequently, colonic penetration recurred, and the patient died 3 years and 8 months after the colectomy.
Discussion
The time interval between the initial breast cancer and gastrointestinal metastasis varies considerably from synchronous presentation to > 20 years after the diagnosis of the primary lesion [10, 11]. Mutations in E-cadherin, which contributes to cell adhesion, may be related to the susceptibility of ILCs to spread to the gastrointestinal tract [12, 13]. In approximately 1% of cases, gastrointestinal involvement is found to be the first distant metastasis [14]. For an accurate diagnosis, it is necessary to evaluate the histopathological characteristics of primary and metastatic foci. In terms of immunohistochemical surveys, ER and GATA3 positivity and caudal type homeobox 2 (CDX2) negativity may support the diagnosis of gastrointestinal metastases from breast cancer. Additionally, the usefulness of mammaglobin and GCDFP15 molecular markers has been reported in several studies [6, 15]. However, the properties of hormone receptors are not always consistent with those of the initial lesions [16]. In the present study, the expression of PgR in Cases 1 and 3 differed between the foci.
Since the clinical manifestations associated with gastrointestinal metastases are nonspecific, including abdominal pain, diarrhea, nausea, and sometimes asymptomatic, breast cancer is rarely detected based on these symptoms [17, 18]. In this report, vomiting and melena were observed in Case 1, while Case 2 experienced diarrhea and vomiting. In Case 1, bleeding from a Dieulafoy ulcer was indicated, but this was not evident from the metastatic mass in the descending colon. Colonic metastases from breast cancer have been reported to show diffuse intestinal wall thickening and ulcerated or nodular lesions, and endoscopic findings often resemble primary colon cancer or inflammatory bowel disease [19, 20]. Although a biopsy of the hemorrhagic site was not performed in this case, tumor infiltration may have played a role in the colorectal bleeding.
In Case 2, as metastatic masses in the transverse colon were responsible for intestinal obstruction, an ileosigmoid colon bypass was performed. Rapid examination and diagnosis of primary and metastatic lesions enabled early hormonal treatment after the improvement of abdominal manifestations.
Although Case 3 had no gastrointestinal symptoms, colonic metastasis was found due to elevated CEA levels during the postoperative follow-up of ILC. However, once the metastatic focus was resected, the tumor recurred and penetrated the abdominal wall. Colonic perforation or infiltration of the abdominal wall, which is implicated in metastatic lesions, is extremely rare. This was possibly associated with rapid tumor growth and increased bowel pressure due to intestinal stenosis. Also, high tumor invasiveness due to loss of E-cadherin might be related to this phenomenon. In this case, the fistula gradually shrank with surgical drainage, and temporary tumor regression was observed with chemotherapy. It was an intractable disease, and the metastatic lesion re-enlarged, causing a recurrence of the abdominal wall perforation and an abscess 8 months later. Nevertheless, the surgical treatment for gastrointestinal manifestations followed by systemic therapy for primary breast cancer was effective similar to that in Case 2.
Survival after gastrointestinal metastases is generally poor, and few patients survive for more than 2 years [6]. In this report, Case 1, in which BSC was provided, died 4 months after diagnosis. In contrast, the survival time was 1 year and 2 months after the detection of gastrointestinal metastasis in Case 2 and 3 years and 8 months in Case 3, who underwent systemic treatment subsequent to invasive procedures.
Conclusions
When colonic foci are suspected in patients with breast cancer, especially ILC, the possibility of distant metastasis should be considered despite its rare nature. Metastasis to the gastrointestinal tract can trigger a wide range of abdominal symptoms and sometimes contribute to the detection of the primary lesion. Since the prognosis for these cases is poor, prompt examination of both foci is essential. Furthermore, in select cases, it is reasonable to consider surgical intervention for gastrointestinal metastasis to alleviate abdominal symptoms and lead to systemic therapy.
Availability of data and materials
Not applicable.
Abbreviations
- BSC:
-
Best supportive care
- CEA:
-
Carcinoembryonic antigen
- CT:
-
Computed tomography
- ER:
-
Estrogen receptor
- PgR:
-
Progesterone receptor
- GATA3:
-
GATA-binding protein 3
- GCDFP15:
-
Gross cystic disease fluid protein 15
- CDX2:
-
Caudal type homeobox 2
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Makita M, Sakai T, Ogiya A, Kitagawa D, Morizono H, Miyagi Y, et al. Optimal surveillance for postoperative metastasis in breast cancer patients. Breast Cancer. 2016;23:286–94. https://doi.org/10.1007/s12282-014-0571-x.
McLemore EC, Pockaj BA, Reynolds C, Gray RJ, Hernandez JL, Grant CS, et al. Breast cancer: presentation and intervention in women with gastrointestinal metastasis and carcinomatosis. Ann Surg Oncol. 2005;12:886–94. https://doi.org/10.1245/ASO.2005.03.030.
Cummings MC, Simpson PT, Reid LE, Jayanthan J, Skerman J, Song S, et al. Metastatic progression of breast cancer: Insights from 50 years of autopsies. J Pathol. 2014;232:23–31. https://doi.org/10.1002/path.4288.
Reed MEMC, Kutasovic JR, Lakhani SR, Simpson PT. Invasive lobular carcinoma of the breast: morphology, biomarkers and omics. Breast Cancer Res. 2015;17:1. https://doi.org/10.1186/s13058-015-0519-x.
Arrangoiz R, Papavasiliou P, Dushkin H, Farma JM. Case report and literature review: metastatic lobular carcinoma of the breast an unusual presentation. Int J Surg Case Rep. 2011;2:301–5. https://doi.org/10.1016/j.ijscr.2011.06.010.
Xu L, Liang S, Yan N, Zhang L, Gu H, Fei X, et al. Metastatic gastric cancer from breast carcinoma: a report of 78 cases. Oncol Lett. 2017;14:4069–77. https://doi.org/10.3892/ol.2017.6703.
Saranovic D, Kovac JD, Knezevic S, Susnjar S, Stefanovic AD, Saranovic DS, et al. Invasive lobular breast cancer presenting an unusual metastatic pattern in the form of peritoneal and rectal metastases: a case report. J Breast Cancer. 2011;14:247–50. https://doi.org/10.4048/jbc.2011.14.3.247.
Moriya Y, Suzuoki M, Takahashi R, Komuro K, Iwashiro N, Ohara M, Kimura N. A case of invasive lobular carcinoma with colonic metastasis 10 years after surgery and abdominal wall penetration during chemotherapy. Hokkaido J Surg. 2020;65:65–9.
Asmar N, Rey JF, Sattonnet C, Barriere J. Gastric metastasis mimicking linitis plastica 20 years after primary breast cancer. A case report. J Gastrointestin Liver Dis. 2018;27:469–71. https://doi.org/10.15403/jgld.2014.1121.274.gas.
Nikkar-Esfahani A, Kumar BG, Aitken D, Wilson RG. Metastatic breast carcinoma presenting as a sigmoid stricture: report of a case and review of the literature. Case Rep Gastroenterol. 2013;7:106–11. https://doi.org/10.1159/000348760.
Berx G, Cleton-Jansen AM, Strumane K, De Leeuw WJF, Nollet F, Van Roy F, et al. E-cadherin is inactivated in a majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene. 1996;13:1919–25.
Lehr HA, Folpe A, Yaziji H, Kommoss F, Gown AM. Cytokeratin 8 immunostaining pattern and E-cadherin expression distinguish lobular from ductal breast carcinoma. Am J Clin Pathol. 2000;114:190–6. https://doi.org/10.1309/CPUX-KWEH-7B26-YE19.
Zhang B, Copur-Dahi N, Kalmaz D, Boland BS. Gastrointestinal manifestations of breast cancer metastasis. Dig Dis Sci. 2014;59:2344–6. https://doi.org/10.1007/s10620-014-3155-x.
Zengel B, Çavdar D, Özdemir Ö, Taşli F, Karataş M, Şimşek C, et al. Gastrointestinal tract metastases of invasive lobular carcinoma of the breast: an immunohistochemical survey algorithm. Eur J Breast Health. 2022;18:375–80. https://doi.org/10.4274/ejbh.galenos.2022.2022-1-5.
Broom RJ, Tang PA, Simmons C, Bordeleau L, Mulligan AM, O’Malley FP, et al. Changes in estrogen receptor, progesterone receptor and Her-2/neu status with time: discordance rates between primary and metastatic breast cancer. Anticancer Res. 2009;29:1557–62.
Zhang LL, Rong XC, Yuan L, Cai LJ, Liu YP. Breast cancer with an initial gastrointestinal presentation: a case report and literature review. Am J Transl Res. 2021;13:13147–55.
Van Halteren HK, Peters H, Gerlag PGG. Large bowel mucosal metastases from breast cancer. J Clin Oncol. 1998;16:3711–3. https://doi.org/10.1200/JCO.1998.16.11.3711.
Matsuda I, Matsubara N, Aoyama N, Hamanaka M, Yamagishi D, Kuno T, et al. Metastatic lobular carcinoma of the breast masquerading as a primary rectal cancer. World J Surg Oncol. 2012;10:231. https://doi.org/10.1186/1477-7819-10-231.
Balakrishnan B, Shaik S, Burman-Solovyeva I. An unusual clinical presentation of gastrointestinal metastasis from invasive lobular carcinoma of breast. J Investig Med High Impact Case Rep. 2016;4:2324709616639723. https://doi.org/10.1177/2324709616639723.
Acknowledgements
We would like to thank Editage [http://www.editage.com] for English language editing.
Funding
The authors declare no funding for this study.
Author information
Authors and Affiliations
Contributions
SO and KK designed the study; SO collected the data and drafted the manuscript; SH, MT, and KK performed the endoscopic investigations; and KK, MS, SH, MT, KK, TU, YM, NK, and MO critically reviewed the manuscript. All the authors have read and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethical Committee of NHO Hakodate National Hospital (R5-0711001).
Consent for publication
Written informed consent was obtained from all three patient’s families for publication of this case report.
Competing interests
The authors declare that they do not have any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Otsuka, S., Komuro, K., Suzuoki, M. et al. Invasive lobular carcinoma of the breast with colonic metastasis: a case series of three patients. surg case rep 9, 181 (2023). https://doi.org/10.1186/s40792-023-01762-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-023-01762-0