Abstract
Background
Mixed acinar-neuroendocrine carcinoma (MANEC) of the pancreas is a rare tumor. We report a case of successful surgical resection of expansively growing MANEC of the pancreas with microsatellite instability (MSI)-high.
Case presentation
The patient was an asymptomatic 65-year-old male. A computed tomography (CT) scan for a follow-up after treatment of pneumonia incidentally revealed a hypoenhancing 12-cm expansively growing tumor in the pancreatic body. An endoscopic ultrasound-guided fine-needle aspiration of the tumor suggested the diagnosis of MANEC. We performed distal pancreatectomy with combined resection of the spleen, left adrenal gland, transverse colon, small bowel, and stomach. The intraoperative findings showed that the tumor was capsular and was in contact with the SMA, SMV, and CA; however, obvious infiltration of these vessels was not observed..Pathological findings indicated MANEC with MSI-high. Among mismatch repair (MMR) gene proteins, PMS2 was lost and MLH1, MSH2, and MSH6 were retained. The tumor recurred 5 months after surgery. The patient was treated with gemcitabine plus nab-paclitaxel followed by pembrolizumab, which did not show objective response.
Discussion
This is the first report investigating MSI and MMR in MANEC. Standard chemotherapy has not been established for MANEC. Detection of MSI-high is essential since PD-1 monoclonal antibodies for MSI-high cases might be one of the good treatment options. Herein, we discuss the various cytomorphologic and clinical features of MANEC and present a brief review of the literatures.
Conclusions
The accumulation of data from additional cases is necessary to further evaluate this type of carcinoma and provide a standardized optimal therapy for MANEC.
Similar content being viewed by others
Introduction
A mixed acinar-neuroendocrine carcinoma (MANEC) is a rare pancreatic neoplasm, with less than fifty reported cases [1]. MANEC is a variant of acinar cell carcinoma (ACC) exhibiting neuroendocrine differentiation only immunohistochemically, and is distinguished from mixed-neuroendocrine-nonendocrine neoplasm (MiNEN) which is a mixture of acinar and neuroendocrine tumors confirmed by solely morphological features. The key diagnostic feature of MANEC is that MANEC expresses both neuroendocrine antigens (eg, synaptophysin and chromogranin) and pancreatic exocrine antigens (eg, trypsin and lipase). The behavior of MANEC may be similar to that of acinar cell carcinoma (ACC) [2], and surgical resection is the first choice, if the tumor is localized and resectable [1, 3]. However, with the limited number of reported cases of MANEC, its appropriate treatment modalities and overall prognosis remain unclear.
Recently, pembrolizumab, an anti-programmed cell death-1 (PD-1) monoclonal antibody, has been used in malignant solid tumors with microsatellite instability (MSI)-high. However, there have been no reports investigating MSI in MANEC. Herein, we present a case who underwent a successful surgical resection of an expansively growing MANEC of the pancreas with MSI-high and brief literature review.
Case presentation
The patient was an asymptomatic 65-year-old man with a recent history of pneumonia. A follow-up computed tomography (CT) scan incidentally revealed a large tumor on his left upper abdomen. The patient did not have a reported family history of pancreatic cancer. Laboratory examinations showed elevated serum C-reactive protein (CRP) levels (2.76 mg/dl). His serum albumin and hemoglobin levels slightly decreased to 3.9 g/dl (normal range 4.1–5.1 g/dl) and 13.5 g/dl (normal range 13.7–16.8), respectively. His serum transaminase, pancreatic enzymes (amylase and lipase), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), total bilirubin, blood glucose, and HbA1c levels were within normal limits. His serum elastase-1 level elevated to 3250 (normal range 0–300), while his carcinoembryonic antigen (CEA), cancer antigen 19–9 (CA19-9), duke pancreatic monoclonal antigen type 2 (DUPAN-2), and s-pancreas antigen-1 (Span-1) levels were within their normal ranges. A contrast-enhanced CT revealed a hypoenhancing 12-cm tumor in the pancreas body and tail, suggesting a primary pancreatic cancer. The tumor was close to the celiac artery (CA), superior mesenteric artery (SMA) and vein (SMV), and common hepatic artery (CHA, Fig. 1A–D). Notably, the splenic artery was patent despite circumferential involvement by the tumor (Fig. 1 A–C), which indicated the expansive-growth pattern of the tumor. The magnetic resonance imaging (MRI) showed that the tumor had a low intensity on the T1-weighted imaging, a high intensity on the T2-weighted imaging, and a marked restricted diffusion on the diffusion-weighted imaging. Neither CT nor MRI scans indicated any distant metastases. The patient underwent an endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) of the mass. This revealed the presence of tumor cells with round nuclei and eosinophilic to amphophilic granular cytoplasm. An immunohistochemical examinations showed that the tumor was positive for BCL-10, trypsin, chymotrypsin, chromogranin A (80% positive), and synaptophysin (20% positive). We diagnosed the tumor as a pancreatic ACC or MANEC. The patient underwent a complete surgical resection by distal pancreatectomy with combined resection of the spleen, left adrenal gland, transverse colon, small bowel, and stomach (Fig. 2). The intraoperative findings showed that the tumor was capsular and was in contact with the SMA, SMV, and CA; however, obvious infiltration of these vessels was not observed. The pathological examination indicated an MANEC with positivity for BCL-10, trypsin, chymotrypsin, and chromogranin A (Fig. 3A–E). The MIB-1 as per the Ki-67 expression was 80% positive in the immunochemical staining (UICC TNM classification 8th edition: pT3, pN2, pMX StageIII) (Fig. 3F). The tumor tissue DNA was analyzed for genomic abnormalities, and the resected specimen indicated that the tumor was MSI-high and negative for RAS/BRAF mutation. Immunohistochemically, The mismatch repair (MMR) gene protein PMS2 was lost and MLH1, MSH2, and MSH6 were retained (Fig. 4). The patient did not receive any adjuvant therapy because of fatigue and loss of appetite. The progress after the surgery is shown in Fig. 5. Three months after the surgery, there was no recurrence of the tumor. However, five months after surgery, a CT scan revealed multiple liver, lung, and lymph node metastases and peritoneal dissemination. Chemotherapy with gemcitabine plus nab-paclitaxel was administered as the first-line treatment. Due to the side effects (leukopenia and severe malaise) and poor efficacy (tumors enlarged < 20%), the patient stopped receiving the treatment after two courses. Considering MSI-high of the tumor, pembrolizumab was administered as the second-line treatment. Although the patient tolerated this regimen, a CT scan revealed the tumor´s progression. The patient's general condition gradually deteriorated, and he died 8 months following the surgery.
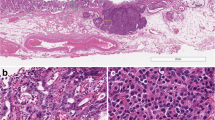
Histopathological findings. Hematoxylin and eosin staining at a magnification of A × 40 and B × 400; C BCL-10 staining, magnification, × 400; D trypsin staining, magnification, × 400; E chromogranin A staining, magnification, × 400; F MIB-1 staining, magnification, × 400). A, B Hematoxylin and eosin staining revealed an acinar growth of tumor cells with round nuclei and eosinophilic vesicles. Immunohistochemistry revealed positive BCL-10 (C), trypsin (D), and chromogranin A staining (E). The MIB-1 as Ki-67 expression was 80% positive (F)
Summary of the treatments. Five months after surgery, a CT scan revealed the presence of multiple liver, lung, and lymph node metastases and peritoneal dissemination. Chemotherapy with gemcitabine (GEM) plus nab-paclitaxel (nabPTX) was administered. Subsequently, pembrolizumab was administered as the second-line treatment, however, did not show a good response
Discussion
MANEC of the pancreas is extremely rare, and very little is known about its clinical and radiological features and pathogenesis. Therefore, it is difficult to differentiate MANEC from ACC or endocrine neoplasms on clinical and radiological evaluations. EUS-FNA is the most common technique used for the diagnosis of pancreatic neoplasms, including MANEC, and it also plays a crucial role in determining the treatment and triage. However, there are only a few reports on the effectiveness of perioperative chemotherapy for MANEC [4,5,6,7], and a standardized management protocol for a pancreatic MANEC has not yet been established. Hence, it has been agreed that generally surgery is the sole curative therapy for resectable MANEC [8, 9].
The list of reports describing cases of MANEC is shown in Table 1. [1, 2, 4,5,6, 9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34] MANEC is common in males, and the susceptible age is 50–70 years old. Despite the presence of endocrine components, most patients do not have specific hormonal symptoms. Moreover, there are no useful tumor markers related to MANEC. Hence, MANEC is usually diagnosed at advanced stages [median size of 7.9 cm with synchronous distant metastases being present in 40.5% (17/42) of the cases]. These findings are based only on small case series or case reports with very short follow-up periods (median 12 months), and they do not thoroughly discuss the treatment strategies and their effects.
Concerning neoadjuvant therapy, the usefulness of FOLFIRINOX has only been described in two case reports [6, 35]. Yu et al. performed chemoradiation therapy with 5-fluorouracil; however, the effectiveness of the treatment was not evaluated [5].
The malignant potential of MANEC is considered to be less than that of pancreatic ductal adenocarcinoma. MANEC has the characteristics of expansive growth, a well-defined margin with a capsule, and a lack of or relatively mild vascular and bile duct encasement [36]. In our case, even though the tumor appeared to be in contact with major vessels such as CA and SMA on CT imaging, it did not actually infiltrate the vessels, and margin-negative curative resection was achieved through aggressive surgical procedure. Meanwhile, tumor recurrence was observed in approximately 50% of patients who underwent curative resection, indicating that micrometastases were already present, even in a clinically resectable MANEC. Therefore, adjuvant therapy may be considered to reduce recurrence risk and improve outcomes, even after a curative resection. However, there have been no reports with respect to adjuvant chemotherapy following surgery for MANEC. For unresectable or recurrent MANEC, there are only a few case reports of treatment with FOLFIRINOX, FOLFOX, and S-1 and their effectiveness is not clear. [4,5,6,7] Currently, the efficacy of anti-PD-1 monoclonal antibodies for MSI-high solid tumors has attracted considerable attentions. This is the first report investigating MSI in MANEC. In MiNEN, several reports have examined the presence of MSI. Sahnane et al. reported a MSI-high rate of 12.4% in gastroenteropancreatic neuroendocrine carcinoma and MiNEN tumors [37]. Additionally, Ishida et al. studied MiNEN tumors of the stomach and reported MSI- high rate of 7.7% [38]. The tumor was MSI-high and out of the four mismatch repair genes, only PMS2 was inactivated. In a previous report, PMS2 was required for the cisplatin-induced activation of p53, which is a member of the p53 family of transcription factors with proapoptotic activity in ovarian cancer [39]. Furthermore, Jia et al. reported that PMS2 expression was regulated post-translationally by Akt and was essential for the platinum-induced apoptosis in ovarian cancer [40]. Thus, the efficacy of chemotherapy may differ depending on the type of MMR deficiency. A PMS2 deficiency may be associated with the efficacy of chemotherapy. Although the recurrent tumors in our case did not show a good response to pembrolizumab despite the MSI-high status, pembrolizumab may be one of the good treatment options for MSI-high MANEC, given the lack of robust evidence of chemotherapies for MANEC. In our case, a mutation in the RAS/BRAF gene mutation was not identified. In previous reports, only two reports have investigated genetic mutation in MANEC [30, 34]. Therefore, further investigations are needed to expand our understanding of genetic mutations in MANEC.
Conclusion
While surgical resection remains the first choice for the treatment of MANEC without distant metastasis, new modalities such as anti-PD-1 monoclonal antibodies may be considered for advanced MANEC with MSI-high. The accumulation of more data from additional cases is necessary to further evaluate this type of carcinoma and provide a standardized optimal therapy for MANEC.
Availability of data and materials
The data that support the findings of this manuscript are available from the corresponding author, Kenji Yoshino, upon reasonable request.
Abbreviations
- ACC:
-
Acinar cell carcinoma
- ALP:
-
Alkaline phosphatase
- CA:
-
Celiac artery
- CA19-9:
-
Cancer antigen 19–9
- CEA:
-
Carcinoembryonic antigen
- CHA:
-
Common hepatic artery
- CRP:
-
C-reactive protein
- CT:
-
Computed tomography
- DUPAN-2:
-
Duke pancreatic monoclonal antigen type 2
- EUS-FNA:
-
Endoscopic ultrasound-guided fine-needle aspiration
- LDH:
-
Lactate dehydrogenase
- MANEC:
-
Mixed acinar-neuroendocrine carcinoma
- MiNEN:
-
Mixed-neuroendocrine-nonendocrine neoplasm
- MMR:
-
Mismatch repair
- MRI:
-
Magnetic resonance imaging
- MSI:
-
Microsatellite instability
- PD-1:
-
Anti-programmed cell death-1
- SMA:
-
Superior mesenteric artery
- SMV:
-
Superior mesenteric vein
- Span-1:
-
S-pancreas antigen-1
References
Liu Z, Dong C, Wang C, Liu Q, Sun D, Wang L. Mixed acinar-endocrine carcinoma of pancreas: a case report and brief review of the literature. Onco Targets Ther. 2015;8:1633–42.
Klimstra DS, Rosai J, Heffess CS. Mixed acinar-endocrine carcinomas of the pancreas. Am J Surg Pathol. 1994;18(8):765–78. https://doi.org/10.1097/00000478-199408000-00002.
Holen KD, Klimstra DS, Hummer A, et al. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol. 2002;20(24):4673–8. https://doi.org/10.1200/jco.2002.02.005.
Kanemasa Y, Kamisawa T, Tabata T, et al. Mixed acinar-endocrine carcinoma of the pancreas treated with S-1. Clin J Gastroenterol. 2013;6(6):459–64. https://doi.org/10.1007/s12328-013-0416-8.
Yu R, Jih L, Zhai J, et al. Mixed acinar-endocrine carcinoma of the pancreas: new clinical and pathological features in a contemporary series. Pancreas. 2013;42(3):429–35. https://doi.org/10.1097/MPA.0b013e318264d073.
Strait AM, Sharma N, Tsapakos MJ, Vaickus LJ, Liu X. Pancreatic mixed acinar-neuroendocrine carcinoma, a unique diagnostic challenge on FNA cytology: a small series of two cases with literature review. Diagn Cytopathol. 2018;46(11):971–6. https://doi.org/10.1002/dc.23981.
Yokode M, Itai R, Yamashita Y, Zen Y. A case report of mixed acinar-endocrine carcinoma of the pancreas treated with S-1 chemotherapy: does it work or induce endocrine differentiation? Medicine (Baltimore). 2017;96(45): e8534. https://doi.org/10.1097/md.0000000000008534.
Zhou W, Han X, Fang Y, et al. Clinical analysis of acinar cell carcinoma of the pancreas: a single-center experience of 45 consecutive cases. Cancer Control Jan-Dec. 2020;27(1):1073274820969447. https://doi.org/10.1177/1073274820969447.
Kyriazi MA, Arkadopoulos N, Stafyla VK, et al. Mixed acinar-endocrine carcinoma of the pancreas: a case report and review of the literature. Cases J. 2009;2:6481. https://doi.org/10.1186/1757-1626-0002-0000006481.
Ulich T, Cheng L, Lewin KJ. Acinar-endocrine cell tumor of the pancreas. Report of a pancreatic tumor containing both zymogen and neuroendocrine granules. Cancer. 1982;50(10):2099–105. https://doi.org/10.1002/1097-0142(19821115)50:10%3c2099::AID-CNCR2820501021%3e3.0.CO;2-1.
Ichijima K, Akaishi K, Toyoda N, et al. Carcinoma of the pancreas with endocrine component in childhood. A case report. Am J Clin Pathol. 1985;83(1):95–100. https://doi.org/10.1093/ajcp/83.1.95.
Hassan MO, Gogate PA. Malignant mixed exocrine-endocrine tumor of the pancreas with unusual intracytoplasmic inclusions. Ultrastruct Pathol. 1993;17(5):483–93. https://doi.org/10.3109/01913129309041300.
Cho KJ, Kim JY, Lee SS, Khang SK, Kim CW. Mixed acinar-endocrine carcinoma of the pancreas: a case report. J Korean Med Sci. 1996;11(2):188–92. https://doi.org/10.3346/jkms.1996.11.2.188.
Shimoike T, Goto M, Nakano I, et al. Acinar-islet cell carcinoma presenting as insulinoma. J Gastroenterol. 1997;32(6):830–5. https://doi.org/10.1007/bf02936964.
Frank M, Bittinger A, Rothmund M, Arnold R. Immunohistochemical analysis and clinical course of high-malignant composite endocrine-acinar cell carcinoma: a case report. Pancreas. 1998;17(2):210–2. https://doi.org/10.1097/00006676-199808000-00017.
Muramatsu T, Kijima H, Tsuchida T, et al. Acinar-islet cell tumor of the pancreas: report of a malignant pancreatic composite tumor. J Clin Gastroenterol. 2000;31(2):175–8. https://doi.org/10.1097/00004836-200009000-00020.
Ogawa T, Isaji S, Yabana T. A case of mixed acinar-endocrine carcinoma of the pancreas discovered in an asymptomatic subject. Int J Pancreatol. 2000;27(3):249–57. https://doi.org/10.1385/ijgc:27:3:249.
Skacel M, Ormsby AH, Petras RE, McMahon JT, Henricks WH. Immunohistochemistry in the differential diagnosis of acinar and endocrine pancreatic neoplasms. Appl Immunohistochem Mol Morphol. 2000;8(3):203–9. https://doi.org/10.1097/00129039-200009000-00006.
Mizuno N, Naruse S, Kitagawa M, et al. Insulinoma with subsequent association of Zollinger-Ellison syndrome. Intern Med. 2001;40(5):386–90. https://doi.org/10.2169/internalmedicine.40.386.
Ohike N, Kosmahl M, Klöppel G. Mixed acinar-endocrine carcinoma of the pancreas. A clinicopathological study and comparison with acinar-cell carcinoma. Virchows Arch. 2004;445(3):231–5. https://doi.org/10.1007/s00428-004-1037-x.
Imaoka H, Amano Y, Moriyama I, Itoh S, Yanagisawa A, Kinoshita Y. Endoscopic ultrasound-guided fine-needle aspiration of a mixed acinar-endocrine carcinoma: a case report. Am J Gastroenterol. 2008;10:2659–60.
Chung WJ, Byun JH, Lee SS, Lee MG. Imaging findings in a case of mixed acinar-endocrine carcinoma of the pancreas. Korean J Radiol. 2010;11(3):378–81. https://doi.org/10.3348/kjr.2010.11.3.378.
Kobayashi S, Asakura T, Ohike N, et al. Mixed acinar-endocrine carcinoma of the pancreas with intraductal growth into the main pancreatic duct: report of a case. Surg Today. 2010;40(4):380–4. https://doi.org/10.1007/s00595-009-4083-9.
Soubra A, Faraj W, Saab J, Shamseddine A. Peri-ampullary mixed acinar-endocrine carcinoma. Rare Tumors. 2011;2: e15.
Lee L, Bajor-Dattilo EB, Das K. Metastatic mixed acinar-neuroendocrine carcinoma of the pancreas to the liver: a cytopathology case report with review of the literature. Diagn Cytopathol. 2013;41(2):164–70. https://doi.org/10.1002/dc.21799.
Sullivan PS, Clebanoff JL, Hirschowitz SL. Hints to the diagnosis of mixed acinar-endocrine carcinoma on pancreatic fine-needle aspiration: avoiding a potential diagnostic pitfall. Acta Cytol. 2013;57(3):296–302. https://doi.org/10.1159/000343683.
Ogbonna OH, Garcon MC, Syrigos KN, Saif MW. Mixed acinar-neuroendocrine carcinoma of the pancreas with neuroendocrine predominance. Case Rep Med. 2013;2013:705092. https://doi.org/10.1155/2013/705092.
Yusuke K, Takashi K, Hiroshi T, Hiroshi K, Ryo N, Masahiko W. Liver metastasis from mixed acinar-endocrine carcinoma of the pancreas: a case report and review of the literature. J Liver Clin Res. 2015;2(2):1015.
Sugimoto M, Hines OJ, Dawson DW, Muthusamy VR, Reber HA, Donahue TR. Preoperative treatment with FOLFIRINOX and successful resection for a patient with mixed acinar-endocrine carcinoma of the pancreas. Pancreas. 2017;4:e32–4.
Takano A, Hirotsu Y, Amemiya K, et al. Genetic basis of a common tumor origin in the development of pancreatic mixed acinar-neuroendocrine-ductal carcinoma: a case report. Oncol Lett. 2017;14(4):4428–32. https://doi.org/10.3892/ol.2017.6786.
Hara T, Fujiwara Y, Takahashi H, et al. Metastatic mixed acinar-endocrine carcinoma of the pancreas treated with a multidisciplinary approach: a case report. Surg Case Rep. 2017;3(1):51. https://doi.org/10.1186/s40792-017-0326-y.
Tang XJ, Fang XF, Zhu LZ, et al. Metastatic mixed acinar-neuroendocrine carcinoma of the pancreas treated by a multidisciplinary team: a case report and brief review of the literature. J Dig Dis. 2019;20(6):318–22. https://doi.org/10.1111/1751-2980.12752.
Niiya F, Takano Y, Azami T, et al. A case of pancreatic mixed acinar-neuroendocrine carcinoma successfully diagnosed with endoscopic ultrasound-guided fine needle aspiration. Clin J Gastroenterol. 2020;13(5):951–8. https://doi.org/10.1007/s12328-020-01136-1.
Akki AS, Liu X, Clapp WL, et al. Mixed acinar-neuroendocrine carcinoma with amphicrine features of the pancreas: Two rare cases with diffuse co-expression of acinar and neuroendocrine markers. Pathol Int. 2021;71(7):485–7. https://doi.org/10.1111/pin.13105.
Sugimoto H, Okochi O, Hirota M, et al. Early detection of liver failure after hepatectomy by indocyanine green elimination rate measured by pulse dye-densitometry. J Hepatobiliary Pancreat Surg. 2006;13(6):543–8. https://doi.org/10.1007/s00534-006-1114-4.
Wei YY, Li Y, Shi YJ, Li XT, Sun YS. Primary extra-pancreatic pancreatic-type acinar cell carcinoma in the right perinephric space: a case report and review of literature. World J Clin Cases. 2021;9(20):5637–46. https://doi.org/10.12998/wjcc.v9.i20.5637.
Sahnane N, Furlan D, Monti M, et al. Microsatellite unstable gastrointestinal neuroendocrine carcinomas: a new clinicopathologic entity. Endocr Relat Cancer. 2015;22(1):35–45. https://doi.org/10.1530/erc-14-0410.
Ishida S, Akita M, Fujikura K, et al. Neuroendocrine carcinoma and mixed neuroendocrine-non-neuroendocrine neoplasm of the stomach: a clinicopathological and exome sequencing study. Hum Pathol. 2021;110:1–10. https://doi.org/10.1016/j.humpath.2020.12.008.
Marinovic-Terzic I, Yoshioka-Yamashita A, Shimodaira H, et al. Apoptotic function of human PMS2 compromised by the nonsynonymous single-nucleotide polymorphic variant R20Q. Proc Natl Acad Sci USA. 2008;105(37):13993–8. https://doi.org/10.1073/pnas.0806435105.
Jia J, Wang Z, Cai J, Zhang Y. PMS2 expression in epithelial ovarian cancer is posttranslationally regulated by Akt and essential for platinum-induced apoptosis. Tumour Biol. 2016;37(3):3059–69. https://doi.org/10.1007/s13277-015-4143-2.
Acknowledgements
Not applicable.
Funding
Nothing.
Author information
Authors and Affiliations
Contributions
KY, YK, AI, and KT analyzed and interpreted the data. KY collected and assembled the data. KY and MK drafted the article. All authors participated in critical revision of article for important intellectual content.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The consent for publication was obtained from the patient.
Competing interests
All authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoshino, K., Kasai, Y., Kurosawa, M. et al. Mixed acinar-neuroendocrine carcinoma of the pancreas with positive for microsatellite instability: a case report and review of the literature. surg case rep 9, 122 (2023). https://doi.org/10.1186/s40792-023-01709-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-023-01709-5