Abstract
Background
The pectoralis major musculocutaneous flap (PMMF) is a pedicled flap often used as a reconstruction option in head and neck surgery, especially in cases with poor wound healing. However, applying PMMF after esophageal surgery is uncommon. We report here, the case of a successfully repaired refractory anastomotic fistula (RF) after total esophagectomy, by PMMF.
Case presentation
A 73-year-old man had a history of hypopharyngolaryngectomy, cervical esophagectomy, and reconstruction using a free jejunal graft for hypopharyngeal carcinosarcoma at the age of 54. He also received conservative treatment for pharyngo-jejunal anastomotic leakage (AL), then postoperative radiation therapy. This time, he was diagnosed with carcinosarcoma in the upper thoracic esophagus; cT3rN0M0, cStageII, according to the Japanese Classification of Esophageal Cancer 12th Edition. As a salvage surgery, thoracoscopic total resection of the esophageal remnant and reconstruction using gastric tube via posterior mediastinal route was performed. The distal side of the jejunal graft was cut and re-anastomosed with the top of the gastric tube. An AL was observed on the 6th postoperative day (POD), and after 2 months of conservative treatment was then diagnosed as RF. The 3/4 circumference of the anterior wall of the gastric tube was ruptured for 6 cm in length, and surgical repair using PMMF was performed on POD71. The edge of the defect was exposed and the PMMF (10 × 5 cm) fed by thoracoacromial vessels was prepared. Then, the skin of the flap and the wedge of the leakage were hand sutured via double layers with the skin of the flap facing the intestinal lumen. Although a minor AL was observed on POD19, it healed with conservative treatment. No complications, such as stenosis, reflux, re-leakage, were observed over 3 years of postoperative follow-up.
Conclusions
The PMMF is a useful option for repairing intractable AL after esophagectomy, especially in cases with large defect, as well as difficulties for microvascular anastomosis due to previous operation, radiation, or wound inflammation.
Similar content being viewed by others
Background
Anastomotic leakage (AL) after esophagectomy reportedly occurs in 3.0–30.0% of patients with an associated mortality rate of 7.2–35% [1, 2]. Although many ALs are treatable by conservative treatment, a small number of cervical ALs develop refractory anastomotic fistula (RF), a condition requiring surgical reconstruction [3]. The pectoralis major musculocutaneous flap (PMMF) is a well-established reconstruction technique for pharyngocutaneous fistula after pharyngolaryngectomy [4], but there are few reports of its use in treating RF after esophagectomy. We report a case in which the successful treatment of refractory gastric tube–cutaneous fistula after total esophagectomy was achieved by PMMF.
Case presentation
A 73-year-old man presented to our hospital with the chief complaint of dysphagia. He had a history of hypopharyngolaryngectomy, cervical esophagectomy, reconstruction using a free jejunal graft, and permanent tracheostomy for hypopharyngeal carcinosarcoma at the age of 54 (19 years ago). In addition, he received conservative treatment for pharyngo-jejunal AL followed by radiation therapy. He also had a history of paroxysmal atrial fibrillation, right retinal artery occlusion, right internal carotid artery occlusion, cerebral artery stenosis, and hypothyroidism.
His upper gastrointestinal endoscopy revealed a mass lesion with submucosal elevation at the upper thoracic esophagus, below the esophago-jejunal anastomosis (Fig. 1a). Contrast-enhanced computed tomography (CT) showed an irregular wall thickening at the esophageal side of the esophago-jejunal anastomosis with esophageal stenosis (Fig. 1b). No lymph node or distant metastasis was detected. The pathologic diagnosis of the endoscopic biopsy revealed malignant spindle cell tumor including components of squamous cell carcinoma. Although it could not be determined, the tumor was not considered a recurrence of hypopharyngeal carcinosarcoma because 10 years had passed since surgery for hypopharyngeal carcinosarcoma. In addition, this time the tumor located in the thoracic esophagus, whereas in the previous surgery, the hypopharynx and cervical esophagus were removed with a free jejunum in between. Therefore, he was diagnosed with a carcinosarcoma of the esophagus; Ut, entire circumference, 40 mm, type1, cT3r N0 M0 cStage, according to Japanese Classification of Esophageal Cancer 12th Edition.
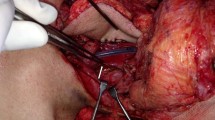
Then thoracoscopic total resection of the remaining esophagus followed by reconstruction using a gastric tube via the posterior mediastinal route was performed. In the cervical procedure, due to significant existing scar-tissue and adhesions due to previous surgeries and radiation therapy, careful dissection was required to avoid injury of the free jejunal pedicle. The distal side of the jejunal graft was cut at 1 cm proximal to the previous anastomosis (Fig. 2a) and re-anastomosed with the top of the pulled-up gastric tube by hand suture (Fig. 2b).
Operative findings of the total resection of the esophageal remnant. a The distal side of the jejunal graft was cut at 1 cm proximal to the esophago-jejunal anastomosis with preservation of the free jejunal graft. Double red line indicates the cutting line. b The cut end of the free jejunal graft was re-anastomosed with the top of the gastric tube which was pulled up via the posterior mediastinal route
A gastric tube–free jejunal anastomotic leakage was observed on the 6th postoperative day (POD6) with open drainage and daily wound irrigation continued. Although inflammation of the wound reduced after conservative treatment, the 3/4 circumference of the anterior wall of the gastric tube was ruptured for 6 cm in length (Fig. 3) and he was diagnosed as having a refractory gastric tube–cutaneous fistula that further conservative treatment was unlikely to heal.
On POD71, surgical repair of the refractory fistula was performed. The skin incision was designed, connecting the line for trimming the skin around the fistula in the neck and the line for harvesting PMMF in the left anterior thoracic region (Fig. 4a).
The operative findings of treatment using PMMF. a The skin incision was designed connecting the line for neck procedure and harvesting PMMF. b The skin of the PMMF and the wedge of the leakage were hand sutured via double layers with the skin inside. Scheme of the operative view is on the right. c The large defect of the anterior wall of the gastric tube was fully covered and repaired with PMMF. Scheme of the operative view is located on the right side. d The muscle side of the PMMF was covered with the mesh skin graft harvested from his left lateral thigh
The edge of the leakage was exposed by carefully peeling away the surrounding tissue. Then the PMMF (10 × 5 cm) fed by thoracoacromial vessels and 4th anterior intercostal branches of the internal thoracic vessels, were prepared by integrating with skin, fat tissue and feeding vessels. Intraoperative blood flow visualization using indocyanine green (ICG) fluorescence confirmed sufficient blood supply at the edge of the anastomotic sites. Then the skin of the flap and the wedge of the leakage were hand sutured via double layers with the skin of the flap facing the intestinal lumen (Fig. 4b, c). The thick fat tissue of the PMMF was in contact with the outer wall of the permanent tracheostomy, not the suture line between the gastric tube and the PMMF. In addition, the omentum of the gastric tube could not be used due to scarring. Therefore, no additional tissue was placed to protect the permanent tracheostomy.
The muscle side of the flap was covered with a mesh skin graft harvested from his left lateral thigh (Fig. 4d). The operation time was 6 h and 27 min, with a blood loss of 335 mL.
Although a minor anastomotic leakage was observed on POD19, it healed with conservative treatment (Fig. 5a). Postoperative fluoroscopy showed a smooth passage at the repaired site (Fig. 5b) with oral intake started on POD33. He was discharged with good physical condition on POD60. No complications, such as ischemia or peptic injury of the skin, stenosis, or reflux, were observed (Fig. 5c) over 3 years of postoperative follow-up.
Discussion
Many risk factors are associated with AL after esophagectomy, such as radiotherapy, diabetes mellitus, body mass index, age, congestive heart failure, atherosclerosis, smoking, that all exhibit insufficient blood supply at the anastomotic site [5,6,7]. In the present case, although the patient has several AL risk factors, a total esophagectomy was performed as it was the only curative treatment against the carcinosarcoma.
Most post-esophagectomy AL is successfully treated by conservative treatments, such as wound debridement, irrigation, and drainage [3]. However, a small number of ALs develop RF, defined as a non-curative anastomotic site–cutaneous fistula existing for longer than 2 months under conservative treatment [5].
In the present case, after debridement, irrigation, and antibiotic administration, the inflammation of the wound gradually healed, accompanied by expansion of the anastomotic wound defect. It takes about 2 months for the wound to stabilize without further changes, resulting in salvage surgery performed at POD71 (65 days after the onset of AL).
Surgical repair for RF after esophagectomy requires sufficient debridement of surrounding tissues, dead cavity filling, and patches larger than the leakage to prevent re-leakage and/or stricture. Autogenous tissues are used for the surgical repair of intractable AL. The free gastrointestinal graft, such as free jejunal graft, is favored for head and neck reconstructions, as the risks of stenosis or fistula are lower. However, this requires microvascular anastomosis [4]. A free flap, such as the anterolateral thigh flap, secures sufficient tissue volume, but also requires microvascular anastomosis [4]. On the other hand, a pedicle flap such as the PMMF, provides sufficient tissue volume yet doesn’t require microvascular anastomosis. Thus, it is used in situations where the free graft is not ideal [4, 8]. The PMMF is a readily available source of vascularized tissue, easily harvested for use in the head and neck. Especially in cases with poor wound healing, such as irradiated patients and those with postoperative salivary contamination, as the vascularized soft tissue coverage of this muscle flap is effective in preserving the major vessels [9]. Consequently, the use of a pedicled flap is broadly accepted as a reconstruction option in head and neck surgery [10]. On the other hand, reconstruction using PMMF after esophageal surgery is uncommon, accounting about 1% of AL reconstructions [4].
To date, 20 cases in total are reported have undergone reconstruction using PMMF for AL after esophagectomy (Table 1). Most of them were performed for RFs of esophago-gastric or esophago-colon anastomosis, at an average 68.5 days after initial surgery [4, 5, 8, 11,12,13,14,15]. In RFs following AL of anastomoses reconstructed by the subcutaneous route, the leakage defect was primary closed and then the PMMF was covered as reinforcement of the closure with the skin surface facing outside [8, 15].
In RFs following retrosternal or posterior mediastinal reconstruction in which the defect was too large to primary close, PMMF was inserted to the defect with the skin side of the flap placed on the lumen side, avoiding both overextension and stenosis [11,12,13,14].
The skin directed toward the lumen was thought to prevent damage of the flap from exposure to digestive fluids, as well [11]. Re-leakage after repair was seen in some cases, but all have healed by conservative treatment, conceivably owing to the volume of the PMMF to fill the tissue defect [8, 11, 13, 14]. Reports have also shown that majority of the patients were able to start oral intake 10–15 days after PMMF repair, unless re-leakage was observed [8, 13, 15], providing us with a clinical indicator of when to start oral intake after surgery.
In the present case, the defect extended to 3/4 circumference of the anastomosis and was contaminated with digestive fluid, so primary closure was difficult. In addition, due to the previous reconstruction using a free jejunal graft and radiation therapy, as well as history of carotid artery occlusion, securing a recipient vascular bed for microvascular anastomosis was also considered difficult. Taken together, the PMMF was selected for reconstruction with the skin surface inward to successfully repair.
In post-irradiation cases such as the present case, considerable technical tips for designing the PMMF are to obtain a larger PMMF with sufficient thickness of fat tissue so that the gap could be filled after removal of scar tissue around the fistula, and to confirm the maintenance of blood flow at the margins with intraoperative blood flow visualization using ICG fluorescence.
Conclusions
PMMF is a useful method to treat intractable anastomotic leakage after esophagectomy, especially in cases with large defect, as well as difficulties in microvascular anastomosis due to previous operation, radiation, and wound inflammation. This surgical option may enable surgeons to aggressively perform radical surgery for high-risk cases in which surgical resection is the only curative treatment.
Availability of data and materials
The datasets of this case report are available from the corresponding author upon reasonable request.
Abbreviations
- PMMF:
-
Pectoralis major musculocutaneous flap
- RF:
-
Refractory anastomotic fistula
- AL:
-
Anastomotic leakage
- POD:
-
Postoperative day
- CT:
-
Computed tomography
References
Verstegen MHP, Bouwense SAW, van Workum F, ten Broek R, Siersema PD, Rovers M, et al. Management of intrathoracic and cervical anastomotic leakage after esophagectomy for esophageal cancer: a systematic review. World J Emerg Surg. 2019;14:17.
Fabbi M, Hagens ERC, van Berge Henegouwen MI, Gisbertz SS. Anastomotic leakage after esophagectomy for esophageal cancer: definitions, diagnostics, and treatment. Dis Esophagus. 2021;34:doaa39.
Biere SS, Maas KW, Cuesta MA, van der Peet DL. Cervical or thoracic anastomosis after esophagectomy for cancer: a systematic review and meta-analysis. Dig Surg. 2011;28:29–35.
McLen JN, Carlson GW, Losken A. The pectoralis major myocutaneous pedicled flap revisited—a reliable technique for head and neck reconstruction. Ann Plast Surg. 2020;64:570–3.
Yamana I, Takeno S, Yamada T, Sato K, Hashimoto T, Yamashita Y. The risk factors for refractory fistula after esophagectomy with gastric tube reconstruction in patients with esophageal cancer. Dig Surg. 2017;34:18–24.
Li SJ, Wang ZQ, Li YJ, Fan J, Zhang WB, Che GW, et al. Diabetes mellitus and risk of anastomotic leakage after esophagectomy: a systematic review and meta-analysis. Dis Esophagus. 2017;30:1.
Goense L, van Rossum PS, Ruurda JP, van Vulpen M, Mook S, Meijer GJ, et al. Radiation to the gastric fundus increases the risk of anastomotic leakage after esophagectomy. Ann Thorac Surg. 2016;102:1798.
Morita M, Ikeda K, Sugiyama M, Saeki H, Egashira A, Yoshinaga K, et al. Repair using the pectoralis major muscle flap for anastomotic leakage after esophageal reconstruction via the subcutaneous. Surgery. 2010;147:212–8.
Zbar RIS, Funk GF, McCulloch TM, Graham SM, Hoffman SM. Pectoralis major myofascial flap: a valuable tool in contemporary head and neck reconstruction. Head Neck. 1997;19:412–8.
Wei W, Qiu Y, Fang Q, Jia Y. Pectoralis major myocutaneous flap in salvage reconstruction following free flap failure in head and neck cancer surgery. J Int Med Res. 2019;47:76–83.
Williams JK, Wood RJ, Hawes A, Mansour KA. The use of the pectoralis myocutaneous flap for repair of a retrosternal esophagocolonic anastomotic leak. Plast Reconstr Surg. 1998;101:802–5.
Yin K, Xu H, Cooke DT, Pu LL. Successful management of oesophageal conduit necrosis by a single-stage reconstruction with the pedicled pectoralis major myocutaneous flap. Interact Cardiovasc Thorac Surg. 2015;21:124–6.
Deng L, Li Y, Li W, Liu M, Xu S, Peng H. Management of refractory cervical anastomotic fistula after esophagectomy using the pectoralis major myocutaneous flap. Braz J Otorhinolaryngol. 2022;88:53–62.
Heitmiller RF, McQuone SJ, Eisele DW. The utility of the pectoralis myocutaneous flap in the management of select cervical esophageal anastomotic complications. J Thorac Cardiovasc Surg. 1998;115:1250–4.
Hirao M, Yoshitatsu S, Tsujinaka T, Nishiyama A, Fujitani K, Nakamori S, et al. Pectoralis myocutaneous flap with T-tube drainage for cervical anastomotic leakage after salvage operation. Esophagus. 2006;3:33–6.
Acknowledgements
Not applicable.
Funding
This work was partly supported by JSPS KAKENHI Grant Number 21K08729 and 22K07185.
Author information
Authors and Affiliations
Contributions
YO and TO produce the case report conception and design, and wrote the main manuscript body. TO, TM, TW and TS obtained informed consent, performed surgery. YO, TO, TM, YN, SM, HK, NK, MF, MN, KM, NT, KY, MI, YN, CT, TW, KH, TI, HT, IH, KS, SH, IY, HA, TS, and TF participated in the patients’ care and critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed consent was obtained from the patient for the publication of this case report and accompanying images.
Competing interests
The authors have no related competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oga, Y., Okumura, T., Miwa, T. et al. Repair using the pectoralis major musculocutaneous flap for refractory anastomotic leakage after total esophagectomy. surg case rep 9, 88 (2023). https://doi.org/10.1186/s40792-023-01659-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-023-01659-y