Abstract
Background
Leiomyosarcoma is classified as a soft tissue sarcoma. In adults, leiomyosarcoma is the most common malignancy affecting the vascular system; however, vascular leiomyosarcoma in children is extremely rare as most pediatric soft tissue tumors are rhabdomyosarcomas. The survival rate is very low, and incomplete resection is a poor prognostic factor. There is also a high rate of distant recurrence, with the lungs and liver being the most common sites of metastasis. There is no established effective chemotherapy, and complete surgical resection is the only potentially curative treatment for leiomyosarcoma.
Case presentation
A 15-year-old female patient with no significant medical history presented with severe upper abdominal pain and was admitted. Abdominal contrast-enhanced computed tomography and magnetic resonance imaging showed a large retroperitoneal tumor protruding into the lumen of the inferior vena cava behind the liver and multiple small nodules, and metastasis to the liver was suspected. The tumor was 6 × 4 × 5 cm in diameter, located just behind the hepatic hilar structures, and was suspected to infiltrate into the right portal vein. The tumor was diagnosed as a leiomyosarcoma through an open tumor biopsy. As the multiple liver metastases were located only in the right lobe of the liver on imaging, we performed tumor resection with right hepatectomy and replacement of the inferior vena cava (IVC). The postoperative course was uneventful; however, on postoperative day 51, distant metastatic recurrences were found in the remaining liver and right lung. The patient was immediately started on chemotherapy and trabectedin proved to be the most effective drug in the treatment regimen; however, severe side effects, such as hepatotoxicity, prevented timely administration, and the patient passed away 19 months after surgery.
Conclusions
IVC resection and reconstruction combined with right hepatectomy were able to be safely performed even in a pediatric case. To improve the prognosis of leiomyosarcoma with multiple metastases, an effective treatment strategy combining surgical treatment and chemotherapy, including molecularly targeted drugs, should be established as early as possible.
Similar content being viewed by others
Background
Leiomyosarcoma is classified as a soft tissue sarcoma. Sarcomas are malignant tumors that arise from primitive mesenchymal tissue. Pediatric soft tissue sarcomas account for 7% of all pediatric tumors, the majority of which are rhabdomyosarcomas, and leiomyosarcomas are very rare, accounting for 1.8% of all soft tissue sarcomas [1]. There are several reports of pediatric cases of mandibular, bronchopulmonary, ovarian, and brain primary tumors; however, to the best of our knowledge, there are only two previous reports describing a pediatric primary vascular tumor such as this one [2,3,4,5,6,7]. Therefore, data on treatment and prognosis are limited to adults.
In adults, however, leiomyosarcoma is the most common malignancy affecting the vascular system; it mostly occurs in veins rather than arteries and in more than 50% of cases, it originates in the inferior vena cava (IVC) [8, 9]. According to various small, single-center, retrospective studies published to date, survival rates are low, ranging from 31% to 62%, with incomplete resection being a poor prognostic factor [9,10,11,12,13,14,15,16,17,18]. In contrast to other sarcomas arising in the retroperitoneum, where local recurrence is common, vascular leiomyosarcoma has a high rate of distant recurrence, with the lungs and liver being the most common metastatic sites [9,10,11,12,13,14,15,16,17,18,19]. As there is no established effective chemotherapy at present, complete surgical resection remains the only potentially curative treatment for leiomyosarcoma.
Herein, we report a case of a 15-year-old girl with primary IVC leiomyosarcoma. Despite the fact that she had already developed multiple liver metastases at the time of diagnosis, we performed partial IVC resection along with a combined right hepatectomy and replaced the IVC with a vascular prosthesis. We present our experience along with a literature review.
Case presentation
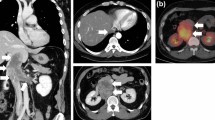
A 15-year-old previously healthy girl, of height 151.1 cm and weight 46.5 kg, reported severe upper abdominal pain and a retroperitoneal tumor was found on ultrasound examination. Subsequent abdominal contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) showed a large retroperitoneal tumor protruding into the lumen of the IVC behind the liver. The tumor was 6 × 4 × 5 cm in size, located just behind the hepatic hilar structures, and was suspected to infiltrate the right portal vein (Fig. 1). An open tumor biopsy was performed and the tumor was diagnosed as leiomyosarcoma. All liver nodules were detected on the hepatobiliary phase of contrast-enhanced MRI with Gd-EOB–DTPA as hypointense nodules, suggesting multiple liver metastases (Fig. 1) Lymph node metastases and distant metastases, except for the liver, were not observed on positron emission tomography (PET). Liver function was Child–Pugh score A, and even though the patient had multiple liver metastases (located in the right lobe of the liver on images), we thought that curative resection could be performed. Since there was no reported effective preoperative chemotherapy treatment for leiomyosarcoma, surgical treatment was selected as the preferred management option. This decision was determined through a shared decision-making process with a pediatric oncologist.
a–d Contrast-enhanced abdominal CT (a) and MRI (b–d) showed a 6 × 4 × 5 cm mass (solid arrow) protruding into the lumen of the IVC. The mass was located slightly superior to the renal vein confluence, ventrally compressing the hepatic hilum, and the right portal vein was stenotic. The MRI showed areas of decreased Gd-EOB–DTPA uptake at S1 (5 mm × 1), S5 (15 mm × 1, dotted arrow), and S6 (5 mm × 1) in the hepatocellular phase (c) e Three-dimensional reconstructed image. GB gall bladder, RPV right portal vein, LRV left renal vein, CT computed tomography, MRI magnetic resonance imaging
As a result, we performed combined tumor resection with a right hepatectomy and replacement of the IVC. The tumor was adherent to the liver, gallbladder, and duodenum, but no ascites or peritoneal dissemination was observed. The IVC on the superior and inferior side of the tumor and the right and left renal veins were taped circumferentially. The gallbladder was resected together with the tumor due to suspected tumor invasion but the duodenum and hilar region were easily detached from the tumor and invasion was negative. The IVC was dissected 2 cm from the tumor on the superior side and just above the bifurcation of the right and left renal veins on the inferior side, and the tumor was removed along with the IVC.
The tumor was 6 × 5 cm in size, elastic and hard. The defect of the IVC was reconstructed using a 6 cm Gore-Tex® expanded polytetrafluoroethylene (e-PTFE) graft (φ20 mm) (Fig. 2). Intraoperative ultrasonography revealed a 15 mm nodular lesion in the liver S5 and 5 mm nodular lesions in S6 and S1 (right side); there were no obvious nodules on the left side of the liver or in S1 (left side). Liver transection was performed, and the right lobe and part of S1 were removed. The operation time was 9 h 45 min, the total ischemic time was 35 min, and the blood loss was 700 ml.
The lumen of the removed IVC was occupied by solid white lesions. The excised liver also contained a round, white, solid mass, similar to the primary lesion, and a 5 mm lesion was also identified near the detached surface of S1. However, its boundaries were clear and the transection surface was not expressed (Fig. 3). Pathological examination revealed spindle-shaped atypical cells densely proliferated in a bundle-like structure, and polygonal and multinucleated atypical cells were also observed. On immunostaining, the atypical cells were desmin-positive and α-smooth muscle actin (αSMA)-positive and were diagnosed as leiomyosarcoma (Fig. 4). Microscopic margins of the liver transection section were confirmed (R0), but there were many microscopic nodules scattered in the liver that were not noted preoperatively.
a Lumen of the removed IVC was occupied by a white segmental solid tumor. b There are several round white enriched lesions at S1 (10 mm × 1, 5 mm × 2, 2 mm × 1), S5 (15 mm × 1), and S6 (7 mm × 1) of the resected liver. A tumor in S1 was observed near the resection surface but the surgical margin was 1 mm. IVC inferior vena cava
a, b Low-power (20×) image (a) and a high-power (100×) image (b) of the IVC lesion; spindle-shaped atypical cells are densely proliferated in a bundle-like structure. Polygonal and multinucleated atypical cells are also seen. Inside the white dotted line: tumor; arrow: IVC. c, d Immunohistochemistry: atypical cells were desmin-positive (c) and αSMA-positive (d). IVC inferior vena cava, αSMA α-smooth muscle actin
The patient’s postoperative course was uneventful; she was discharged on postoperative day 11. However, a CT and MRI performed on postoperative day 51 revealed distant metastatic recurrences in the remaining liver and right lung. The patient was immediately started on doxorubicin 60 mg/m2 and ifosfamide 2500 mg/m2 at a nearby pediatric specialty center. However, the metastatic lesion increased in size after 1 month. Pazopanib 550 mg/m2 was added to the then-current chemotherapy regimen but was ineffective. The patient was then switched to eribulin 1.4 mg/m2 and then to trabectedin 1.2 mg/m2. Trabectedin had the strongest antitumor effect, but also caused severe adverse effects. The patient passed away 19 months postoperatively due to uncontrollable hyperkalemia and intra-abdominal bleeding.
Discussion
Previous reports on pediatric leiomyosarcomas can be broadly divided into two categories: Epstein–Barr virus (EBV)-related leiomyosarcomas in immunocompromised patients and others[20]. There have been several reports of leiomyosarcoma in non-immunocompromised patients; however, the only reports of primary vascular leiomyosarcomas such as the one in this case are of a 3-year-old girl with a lesion in the femoral vein and a 2-year-old girl with a lesion in the greater saphenous vein [6, 7]. Therefore, the information is very limited, and we referred to evidence in adult cases to determine the treatment plan.
In pediatric patients, tumor resection with replacement of the IVC is uncommon, and there have been only a few reports on neuroblastoma and other diseases [21]. On the other hand, there have been reports on adult patients with primary leiomyosarcoma of the IVC, where radical tumor resection was associated with 5- and 10-year survival rates, and of patients who underwent IVC replacement for vena cava tumors, including leiomyosarcoma, having improved outcomes [18, 22]. Preoperative chemotherapy has also been attempted, but there is no consensus on its efficacy [23,24,25]. Unfortunately, our patient had multiple liver metastases at the time of surgery, but since the metastases were confined to the right lobe, we thought that R0 resection could be performed through resection of the right lobe of the liver and replacement of the IVC. The liver is the major metastatic site of leiomyosarcoma, but there are few reports of cases with multiple liver metastases at the time of initial diagnosis (Table 1).
There have been two cases in which surgery was performed for multiple liver metastases. In Case 1, tumor resection and right hepatectomy were performed as in the present case, but the patient had recurrence 9 months after surgery and passed away 33 months after surgery (the postoperative treatment after recurrence was unknown). In Case 3, the surgeons were unable to resect the entirety of the liver metastases, because numerous liver metastases were found during surgery. As a result, chemotherapy was administered postoperatively, and the patient was alive 10 months after the surgery. In our case, microscopic margins were secured; however, small tumors were scattered throughout the removed liver. Minute distant metastases that could not be seen on the preoperative CT and MRI scans could have been present in the liver and lungs at the time of surgery. This might have led to distant recurrence as early as 2 months after surgery. The benefit of adjuvant chemotherapy after R0 resection is controversial and it was not performed in Case 1. Since chemotherapy alone had a survival of 29 months (Case 4), postoperative chemotherapy may have been appropriate in our case, considering the possibility of residual lesions that could not be detected preoperatively.
Regarding chemotherapy for soft tissue tumors (including leiomyosarcoma), there is a meta-analysis showing a slight survival improvement with doxorubicin-based multidrug therapy; however, there are also reports suggesting that chemotherapy does not improve survival or recurrence risk [30,31,32]. In Japan when anthracycline chemotherapy fails, recently approved agents such as pazopanib, eribulin, and trabectedin are used as second-line or subsequent chemotherapy. Trabectedin was the most effective drug in this case but could not be administered on schedule due to hepatotoxicity. A study of the Japanese Musculoskeletal Oncology Group also showed that 96% of patients experienced adverse events and hepatotoxicity being the most frequent, at 37.9% of patients having grade 3 or higher hepatotoxicity. Additionally, the low acceptability of trabectedin is an issue [33].
It is a controversial question as to which molecularly targeted drug is the best option as second-line therapy. A randomized phase II trial of pazopanib, eribulin, and trabectedin in metastatic or unresectable advanced soft tissue sarcomas that have progressed after the initial chemotherapy including doxorubicin is currently underway in Japan, and the outcomes will be followed with interest.
Conclusions
We report a rare case of primary vascular leiomyosarcoma in a pediatric patient. IVC resection and reconstruction combined with right hepatectomy were able to be safely performed even in a pediatric case. Although pazopanib, eribulin, and trabectedin are expected to play a role in the treatment strategy of leiomyosarcoma in the future, surgical resection remains the only curative option. However, since a high rate of hematogenous distant metastasis occurs, effective (preoperative) chemotherapy, including molecularly targeted drugs, should be established early and effectively combined with surgical treatment.
Availability of data and materials
Not applicable.
Abbreviations
- IVC:
-
Inferior vena cava
- CT:
-
Computed tomography
- MRI:
-
Magnetic resonance imaging
- GB:
-
Gall bladder
- RPV:
-
Right portal vein
- LRV:
-
Left renal vein
- PET:
-
Positron emission tomography
- e-PTFE:
-
Expanded polytetrafluoroethylene
- αSMA:
-
α-Smooth muscle actin
- CK:
-
Creatinine kinase
- EBV:
-
Epstein–Barr virus
References
Board PDQPTE. Childhood soft tissue sarcoma treatment (PDQ®): health professional version PDQ cancer information summaries. Bethesda (MD): National Cancer Institute. 2002
Kenea TT, Kebede BA, Gozjuze FM, Kiros H, Wilde F. Primary leiomyosarcoma of the mandibular alveolar mucosa of a 12-year-old child from Ethiopia: a case report. Craniomaxillofac Trauma Reconstr. 2017;10:56–9. https://doi.org/10.1055/s-0036-1582459.
Takeda F, Yamagiwa I, Ohizumi H, Shiono S. Leiomyosarcoma of the main bronchus in a girl: a long-time survivor with multiple lung metastases. Pediatr Pulmonol. 2004;37:368–74. https://doi.org/10.1002/ppul.10456.
Eckhardt BP, Brandner S, Zollikofer CL, Wentz KU. Primary cerebral leiomyosarcoma in a child. Pediatr Radiol. 2004;34:495–8. https://doi.org/10.1007/s00247-003-1123-2.
O’Sullivan SG, Das Narla L, Ferraro E. Primary ovarian leiomyosarcoma in an adolescent following radiation for medulloblastoma. Pediatr Radiol. 1998;28:468–70. https://doi.org/10.1007/s002470050386.
Byard RW, Bourne AJ, Phillips GE, Rice MS, Davey RB. Leiomyosarcoma of the saphenous vein in a child with 12-year follow-up. J Pediatr Surg. 1993;28:271–4. https://doi.org/10.1016/s0022-3468(05)80294-5.
Allison MF. Leiomyosarcoma of the femoral vein: report of a case in a child. Clin Pediatr (Phila). 1965;4:28–31. https://doi.org/10.1177/000992286500400110.
Burke AP, Virmani R. Sarcomas of the great vessels. A clinicopathologic study. Cancer. 1993;71:1761–73. https://doi.org/10.1002/1097-0142(19930301)71:5%3c1761::aid-cncr2820710510%3e3.0.co;2-7.
Dzsinich C, Gloviczki P, van Heerden JA, Nagorney DM, Pairolero PC, Johnson CM, et al. Primary venous leiomyosarcoma: a rare but lethal disease. J Vasc Surg. 1992;15:595–603. https://doi.org/10.1016/0741-5214(92)90003-Q.
Roland CL, Boland GM, Demicco EG, Lusby K, Ingram D, May CD, et al. Clinical observations and molecular variables of primary vascular leiomyosarcoma. JAMA Surg. 2016;151:347–54. https://doi.org/10.1001/jamasurg.2015.4205.
Wachtel H, Jackson BM, Bartlett EK, Karakousis GC, Roses RE, Bavaria JE, et al. Resection of primary leiomyosarcoma of the inferior vena cava (IVC) with reconstruction: a case series and review of the literature. J Surg Oncol. 2015;111:328–33. https://doi.org/10.1002/jso.23798.
Dull BZ, Smith B, Tefera G, Weber S. Surgical management of retroperitoneal leiomyosarcoma arising from the inferior vena cava. J Gastrointest Surg. 2013;17:2166–71. https://doi.org/10.1007/s11605-013-2385-0.
Ito H, Hornick JL, Bertagnolli MM, George S, Morgan JA, Baldini EH, et al. Leiomyosarcoma of the inferior vena cava: survival after aggressive management. Ann Surg Oncol. 2007;14:3534–41. https://doi.org/10.1245/s10434-007-9552-z.
Kieffer E, Alaoui M, Piette JC, Cacoub P, Chiche L. Leiomyosarcoma of the inferior vena cava: experience in 22 cases. Ann Surg. 2006;244:289–95. https://doi.org/10.1097/01.sla.0000229964.71743.db.
Dew J, Hansen K, Hammon J, McCoy T, Levine EA, Shen P. Leiomyosarcoma of the inferior vena cava: surgical management and clinical results. Am Surg. 2005;71:497–501. https://doi.org/10.1177/000313480507100609.
Hollenbeck ST, Grobmyer SR, Kent KC, Brennan MF. Surgical treatment and outcomes of patients with primary inferior vena cava leiomyosarcoma. J Am Coll Surg. 2003;197:575–9. https://doi.org/10.1016/S1072-7515(03)00433-2.
Hines OJ, Nelson S, Quinones-Baldrich WJ, Eilber FR. Leiomyosarcoma of the inferior vena cava: prognosis and comparison with leiomyosarcoma of other anatomic sites. Cancer. 1999;85:1077–83. https://doi.org/10.1002/(SICI)1097-0142(19990301)85:5%3c1077::AID-CNCR10%3e3.0.CO;2-0.
Mingoli A, Cavallaro A, Sapienza P, Di Marzo L, Feldhaus RJ, Cavallari N. International registry of inferior vena cava leiomyosarcoma: analysis of a world series on 218 patients. Anticancer Res. 1996;16:3201–5.
Gronchi A, Miceli R, Allard MA, Callegaro D, Le Péchoux C, Fiore M, et al. Personalizing the approach to retroperitoneal soft tissue sarcoma: histology-specific patterns of failure and postrelapse outcome after primary extended resection. Ann Surg Oncol. 2015;22:1447–54. https://doi.org/10.1245/s10434-014-4130-7.
McClain KL, Leach CT, Jenson HB, Joshi VV, Pollock BH, Parmley RT, et al. Association of Epstein-Barr virus with leiomyosarcomas in young people with AIDS. N Engl J Med. 1995;332:12–8. https://doi.org/10.1056/NEJM199501053320103.
Grimaldi C, Bertocchini A, Crocoli A, de Ville de Goyet J, Castellano A, Serra A, et al. Caval replacement strategy in pediatric retroperitoneal tumors encasing the vena cava: a single-center experience and review of literature. J Pediatr Surg. 2019;54:557–61. https://doi.org/10.1016/j.jpedsurg.2018.06.008.
Bower TC, Nagorney DM, Cherry KJ Jr, Toomey BJ, Hallett JW, Panneton JM, et al. Replacement of the inferior vena cava for malignancy: an update. J Vasc Surg. 2000;31:270–81. https://doi.org/10.1016/s0741-5214(00)90158-7.
Ronellenfitsch U, Karampinis I, Dimitrakopoulou-Strauss A, Sachpekidis C, Jakob J, Kasper B, et al. Preoperative pazopanib in high-risk soft tissue sarcoma: Phase II window-of opportunity study of the German interdisciplinary sarcoma group (NOPASS/GISG-04). Ann Surg Oncol. 2019;26:1332–9. https://doi.org/10.1245/s10434-019-07183-4.
Saponara M, Stacchiotti S, Casali PG, Gronchi A. (Neo)adjuvant treatment in localised soft tissue sarcoma: the unsolved affair. Eur J Cancer. 2017;70:1–11. https://doi.org/10.1016/j.ejca.2016.09.030.
Gronchi A, Ferrari S, Quagliuolo V, Broto JM, Pousa AL, Grignani G, et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017;18:812–22. https://doi.org/10.1016/S1470-2045(17)30334-0.
Hardwigsen J, Balandraud P, Ananian P, Saïsse J, Le Treut YP. Leiomyosarcoma of the retrohepatic portion of the inferior vena cava: clinical presentation and surgical management in five patients. J Am Coll Surg. 2005;200:57–63. https://doi.org/10.1016/j.jamcollsurg.2004.09.035.
Motodaka H, Aryal B, Mizuta Y, Tada N, Yoshikawa K, Kaieda M, et al. Simultaneous surgery for inferior vena cava leiomyosarcoma with multiple hepatic metastases: justified challenge. Am J Case Rep. 2019;20:902–7. https://doi.org/10.12659/ajcr.915995.
Sephien A, Mousa MS, Bui MM, Kedar R, Thomas K. Leiomyosarcoma of the inferior vena cava with hepatic and pulmonary metastases: case report. J Radiol Case Rep. 2019;13:30–40. https://doi.org/10.3941/jrcr.v13i5.3641.
Devi Y, Pangarkar M, Pagey R, Juvekar SL. Inferior vena cava leiomyosarcoma with liver metastasis at presentation in a young male: a challenging diagnostic quandary. Indian J Pathol Microbiol. 2022;65:686–8. https://doi.org/10.4103/ijpm.ijpm_296_21.
Istl AC, Ruck JM, Morris CD, Levin AS, Meyer CF, Johnston FM. Call for improved design and reporting in soft tissue sarcoma studies: a systematic review and meta-analysis of chemotherapy and survival outcomes in resectable STS. J Surg Oncol. 2019;119:824–35. https://doi.org/10.1002/jso.25401.
Zer A, Prince RM, Amir E, Abdul Razak AR. Multi-agent chemotherapy in advanced soft tissue sarcoma (STS)—a systematic review and meta-analysis. Cancer Treat Rev. 2018;63:71–8. https://doi.org/10.1016/j.ctrv.2017.12.003.
Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573–81. https://doi.org/10.1002/cncr.23592.
Kobayashi H, Iwata S, Wakamatsu T, Hayakawa K, Yonemoto T, Wasa J, et al. Efficacy and safety of trabectedin for patients with unresectable and relapsed soft-tissue sarcoma in Japan: a Japanese Musculoskeletal Oncology Group study. Cancer. 2020;126:1253–63. https://doi.org/10.1002/cncr.32661.
Acknowledgements
We would like to thank Editage [http://www.editage.com] for editing and reviewing this manuscript for English language.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by KY, YT, YO, and TK. The first draft of the manuscript was written by KY, and all authors commented on the various versions of the manuscript. Conceptualization: KY. Formal analysis and investigation: KY and YO. Writing—original draft preparation: KY. Writing—review and editing: YO, YT, TH, TK, MT, and YS. Supervision: YS. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted retrospectively using data obtained for clinical purposes. Approval was obtained from the Ethics Committee of Shinshu University. The procedures used in this study adhered to the tenets of the 1964 Declaration of Helsinki and its later amendments.
Consent for publication
The patient and their relatives provided written informed consent regarding publishing the patient’s data and photographs.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoshizawa, K., Ohno, Y., Kurata, T. et al. Primary leiomyosarcoma of the inferior vena cava in a pediatric case: a case report and literature review. surg case rep 9, 52 (2023). https://doi.org/10.1186/s40792-023-01630-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-023-01630-x