Abstract
Background
Intraductal papillary neoplasm of the bile duct (IPNB) is a subtype of biliary tumor. The 5-year survival rate of patients with IPNB who underwent curative resection is 81%. However, IPNB is known to often recur in other parts of the bile duct. Nevertheless, its mechanism remains poorly understood. Herein, we report the case of a patient with recurrent IPNB, which was considered to be attributed to intraductal dissemination in the common bile duct at 12 months after curative resection. We also made a review of the existing literature.
Case presentation
A 69-year-old man was referred to our hospital for the evaluation and dilation of an intrahepatic bile duct (IHBD) mass. Computed tomography (CT) findings confirmed a mass in the left hepatic duct. Left trisectionectomy, extrahepatic bile duct resection with biliary reconstruction, and regional lymph node dissection were performed. Intraoperative examination of the resection margin at the common bile duct and posterior segmental branch of the hepatic duct was negative for the presence of malignant cells. Histologically, the tumor showed intraductal papillary growth of the mucinous epithelium and was diagnosed as non-invasive IPNB. It had a papillary structure with atypical epithelial cells lined up along the neoplastic fibrovascular stalks. Immunohistochemically, this was as a gastric-type lesion. At 12 postoperative months, CT revealed a 1.5-cm mass in the lower remnant common bile duct. We performed subtotal stomach-preserving pancreaticoduodenectomy. The tumor exhibited papillary growth and was microscopically and immunohistochemically similar to the first tumor. At approximately 16 months after the patient’s second discharge, CT showed an abdominal mass at the superior mesenteric plexus, which was diagnosed as recurrent IPNB. Chemotherapy is ongoing, and the patient is still alive. In this case, as described in many previous reports, IPNB recurred below the primary lesion in the bile duct.
Conclusion
Based on our review of previous reports on IPNB recurrence, intraductal dissemination was considered one of the mechanisms underlying recurrence after multicentric development. Considering the high frequency and oncological conversion of recurrence in IPNB, regular follow-up examination is essential to achieve better prognosis in patients with recurrent IPNB.
Similar content being viewed by others
Background
Intraductal papillary neoplasm of the bile duct (IPNB) is classified as a biliary tumor subtype, according to the World Health Organization [1]. IPNB is an exophytic biliary epithelial tumor that historically includes various diseases, both benign and malignant [2]. It is considered as the biliary counterpart of intraductal papillary mucinous neoplasm of the pancreas (IPMN) [3]. According to the immunohistochemical profiles of mucin core proteins, IPNBs can be classified into four types: pancreaticobiliary, intestinal, gastric, and oncocytic types. The pancreaticobiliary type is the most common, whereas the oncocytic and gastric types are the rarest [4]. Lee et al. reported that patients with IPNB had good prognosis and that the 5-year survival rate of patients with IPNB who underwent curative resection was 81% [5]. However, IPNB often recurs in other parts of the bile duct. Rocha et al. [6] reported recurrence in 20 (51%) out of 39 patients with IPNB. Although several of these articles have reported that IPNB recurrence was multicentric, none provided pathological details regarding the recurrence pattern.
Herein, we report the case of a patient with recurrent IPNB because of intraductal dissemination in the common bile duct at 12 months after curative resection. We also review previous case reports on IPNB recurrence and discuss the mechanism of recurrence.
Case presentation
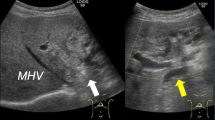
A 69-year-old man was referred to our hospital for the evaluation and dilation of an intrahepatic bile duct (IHBD) mass. His initial laboratory values were as follows: total bilirubin, 1.0 mg/dL; direct bilirubin, 0.2 mg/dL; aspartate aminotransferase, 36 mg/dL; alanine aminotransferase, 50 mg/dL; alkaline phosphatase, 358 U/L; and γ-glutamyl transferase, 260 U/L. The tumor marker levels (including carcinoembryonic antigen and carbohydrate antigen 19–9) were within the normal ranges. Contrast-enhanced computed tomography (CT) revealed IHBD dilation in the left hemiliver (Fig. 1A) and a mass in the left hepatic duct (LHD) (Fig. 1B). The posterior segmental branch of the hepatic duct (PHD) draining into the LHD was also dilated. There was no evidence of distant metastasis. Magnetic resonance cholangiopancreatography (MRCP) revealed a 2-cm intraductal mass located between the LHD and PHD. Endoscopic retrograde cholangiography findings indicated the presence of a defect in the LHD and dilated LHD (Fig. 2), and bile duct brushing cytology was performed. Cytology showed atypical cells in the specimen from the mass, but the diagnosis was not clear.
Brush cytology did not indicate that the tumor was an adenocarcinoma, but atypical cells were detected and the presence of adenocarcinoma could not be excluded. We performed left trisectionectomy, extrahepatic bile duct (EHBD) resection with biliary reconstruction, and regional lymph node dissection for curative resection. An elastic, hard papillary mass was found in the LHD. The patient had hepatic duct variation (PHD draining to the LHD), and the tumor was located close to the PHD. Concerning curative resection, excision of the PHD bifurcation was necessary. Intraoperative examination of the common bile duct and PHD resection margin was negative for the presence of malignant cells. By gross appearance, a papillary tumor was observed in the LHD (Fig. 3). Microscopically, the tumor showed intraductal papillary growth of the atypical columnar epithelium with thin fibrovascular cores. The cytological atypia was moderate to severe, but no invasion was detected. The patient was diagnosed as having IPNB. The atypical epithelium contained abundant mucin and resembled crypt cells of the stomach. Immunohistochemically, mucin 1 cell surface associated protein (MUC1) and MUC2 were negative, while MUC5AC and MUC6 were positive. Based on these findings, this lesion was regarded as of the gastric type (Fig. 4). There was no lymphovascular permeation or lymph node metastasis. The surgical margin in the permanent specimen was negative. The postoperative course was uneventful, and the patient was discharged at 21 days after surgery.
Histopathological findings of the primary lesion. A Hematoxylin and eosin staining findings. The tumor showed intraductal papillary growth of the atypical columnar epithelium with thin fibrovascular cores. The tumor cells contained abundant mucin and resembled crypt cells of the stomach. B–D Immunohistochemically, the tumor was MUC1- and MUC2-negative and MUC5AC-positive. These findings indicated that the tumor was of the gastric type
After surgery, annual assessments involving blood tests and abdominal CT scans were planned. At 12 postoperative months, the patient visited our outpatient department with fever. Laboratory tests revealed elevated total bilirubin levels (1.6 mg/dL), but normal carcinoembryonic antigen (2.2 ng/mL) and CA19-9 (25.5 U/mL) levels. CT/MRCP showed a 1.5-cm mass in the lower remnant common bile duct (Fig. 5A and B). We suspected IPNB recurrence and planned surgical intervention. The patient underwent subtotal stomach-preserving pancreaticoduodenectomy. Intraoperative examination of the main pancreatic duct was negative for malignant cells. By gross appearance, the papillary tumor with rich mucus was apparent in the lower part of the common bile duct. Microscopically, the lesion resembled the lesion resected in the previous surgery. The tumor consisted of non-invasive and invasive portions, the former being similar to the primary lesion. Immunohistochemically, MUC1 and MUC2 were negative and MUC5AC and MUC6 were positive, indicating a gastric-type IPNB, as before. The latter part of the recurrent lesion showed conversion from the non-invasive to the invasive type, and was considered to have acquired MUC1-positive and MUC5AC-negative characteristics in the process (Fig. 6). Multiple metastases were detected in the posterior pancreatic lymph nodes. No nerve infiltration was observed, and the surgical margin in the permanent specimen was negative. A postoperative pancreatic fistula (International Study Group of Pancreatic Fistula grade B) was identified, but improved with antibiotic therapy. The patient was discharged at 28 days after surgery.
Contrast-enhanced computed tomography and magnetic resonance cholangiopancreatography findings for the recurrent lesion. A Contrast-enhanced computed tomography findings revealed a 1.5-cm mass in the lower remnant common bile duct at 16 months after the first surgery (arrow). B Magnetic resonance cholangiopancreatography findings identified the tumor as a defect in the lower remnant common bile duct (arrowhead)
Histopathological findings of the recurrent lesion. A Hematoxylin and eosin staining findings. On the left side of the image, the recurrent tumor shows papillary proliferation that resembles the finding shown in Fig. 5A. Mucus production was observed in the atypical epithelial cells, as with the primary lesion. On the right side of the image, the tumor is expanding beyond the mucosa. B The tumor with a papillary structure was almost MUC1-negative; however, the tumor that exhibited invasion was MUC1-positive. C, D The tumor was MUC2-negative and MUC5AC-positive
At approximately 31 months after the first operation, CT findings revealed an abdominal mass at the superior mesenteric plexus (Fig. 7), which was diagnosed as recurrent IPNB. The patient received recurrent chemotherapy with gemcitabine, cisplatin, and S-1. However, at 6 months after the initiation of the first chemotherapy, he developed hematological toxicity and, consequently, he was switched to gemcitabine monotherapy. He is still alive at 42 months after the first operation.
Discussion
We herein report the case of a patient with recurrent IPNB because of intraductal dissemination in the common bile duct at 12 months after curative resection. In this case, the primary and recurrent lesions were MUC1-/MUC2-negative and MUC5AC-/MUC6-positive, indicating gastric-type lesions. The standard treatment for IPNB is surgery, and previous studies have reported that a good prognosis can be obtained with R0 resection [7, 8]. However, the risk of recurrence is not low even after R0 resection [8, 9]. According to a report by Uemura et al. [7], 12 of 69 patients (17.4%) who underwent R0 resection relapsed. Regarding the mechanism of recurrence, IPNB has been reported to exhibit multicentric development [10]. In fact, metachronous IPMN recurrence in the pancreatic duct is known, and Izawa et al. reported multicentric IPMN recurrence with a genetical approach [11]. However, no reports have described multicentric IPNB recurrence with a genetical approach and, therefore, the mechanism of IPNB recurrence remains unclear. Yokode et al. [12] reported that the IPNB recurrence site is downstream of the initial lesion in most cases and dissemination is the main mechanism of IPNB recurrence concerning the flow of bile. They also reported that 84% of the first lesions were found in the IHBD, whereas 80% of the recurrent lesions were found in the EHBD. We searched PubMed using the terms “IPNB”, “recurrence”, and “biliary papillomatosis” and collected reports on cases of recurrent IPNB. A similar search was also conducted using ICHUSHI (http://login.jamas.or.jp/), a tool for searching for the medical literature written in Japanese. Table 1 [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27] summarizes the cases of recurrent IPNB; including the present case, a total of 18 cases of recurrent IPNB were collected. In all cases, the initial treatment was surgery. Of the 12 patients for whom the resection margin in the initial surgery was noted in the report, 11 (91.7%) underwent R0 resection, but all 12 experienced IPNB recurrence. Of the 18 cases, 14 (77.8%) had a recurrence pattern from the upper site of the bile duct to the lower site. Of these, six cases mentioned the pathological type of the primary and recurrent lesions, all of which were of the same type.
In our case, the initial and recurrent lesions were diagnosed as IPNB, both of which were of the relatively rare gastric type [4]. The fact that these gastric-type lesions recurred at a downstream site suggested that the mechanism of IPNB recurrence may be intraductal dissemination. If multicentric development was the main mechanism of recurrence, the site of recurrence would be random. In addition, the fact that the pathology of the primary and recurrent lesions was the same in all cases, for which the information on pathological type was available, can be attributed to dissemination along the flow of bile. Of the 18 cases presented in Table 1, eight had presence or absence of mucin production for the initial and recurrent tumors, all of which had upper-to-lower site recurrence. Moreover, the presence or absence of mucin production was consistent between the initial and recurrent tumors. Among IPNBs, the mucin-producing type was reported to be 80% and 20% in IHBD and EHBD lesions, respectively [28]. In these cases, mucin production was observed in seven (87.5%) out of eight cases of recurrent tumors in the EHBD. If IPNB has multicentric recurrence, a high rate of mucin-producing type IPNB in the EHBD, as shown in Table 1, would contradict the low rate of mucin-producing type IPNB in the EHBD, as mentioned in previous works. This contradiction indicates that intraductal dissemination along the flow of bile is one of the main mechanisms of IPNB recurrence.
IPNB includes various benign and malignant lesions. The malignancy conversion rate of IPNB is 41–83% [29]. In our case, the primary lesion was non-invasive but the recurrent lesion had converted to the invasive type. In nine of the 18 cases, in which invasive type (or not) was mentioned, three cases (33.3%) experienced conversion from non-invasive type to invasive type in primary recurrence. All of the conversion cases recurred after the second surgery. Of the six non-conversion cases, only one case (16.7%) recurred. It is possible that conversion from the non-invasive to invasive type is related to the high rate of the second IPNB recurrence. In fact, the invasive type was reported to have inferior recurrence free survival compared to the non-invasive type [28]. Thus, even if R0 resection is performed on a lesion without invasion findings, a recurrent lesion in the lower bile duct could be detected as an invasive tumor, leading to recurrence after the second surgery.
As aforementioned, the main treatment for IPNB is surgery, but postoperative follow-up examination is also important. R0 resection for IPNB has a lower recurrence rate than R1 resection [9], and a better prognosis [4, 6, 30]. However, there is a good chance of recurrence even if R0 resection is performed. Considering the high frequency and oncological conversion of recurrence in IPNB, regular follow-up examination is essential for the early detection of IPNB recurrence.
Conclusion
Based on our review of previous reports on IPNB recurrence, intraductal dissemination is considered one of the mechanisms underlying recurrence after multicentric development. Considering the high frequency and oncological conversion of recurrence in IPNB, regular follow-up examination is essential for better prognosis in patients with recurrent IPNB. Further studies to track and evaluate recurrent IPNB cases are warranted to comprehensively understand the oncology of IPNB.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- IPNB:
-
Intraductal papillary neoplasm of the bile duct
- CT:
-
Computed tomography
- LHD:
-
Left hepatic duct
- PHD:
-
Posterior segmental branch of the hepatic duct
- MRCP:
-
Magnetic resonance cholangiopancreatography
References
F Harada R Matsuyama R Mori T Kumamoto D Morioka M Taguri 2019 Outcomes of surgery for 2010 WHO classification-based intraductal papillary neoplasm of the bile duct: case-control study of a single Japanese institution’s experience with special attention to mucin expression patterns Eur J Surg Oncol 45 5 761 768
GW Jang S Hwang YJ Lee KH Kim KM Park CS Ahn 2012 Clinicopathological features of the intraductal papillary neoplasms of the intrahepatic bile duct Korean J Hepatob Pancreat Surg 16 4 138 141
Y Zen T Fujii K Itatsu K Nakamura H Minato S Kasashima 2006 Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas Hepatology 44 5 1333 1343
XS Wan YY Xu JY Qian XB Yang AQ Wang L He 2013 Intraductal papillary neoplasm of the bile duct World J Gastroenterol 19 46 8595 8604
SS Lee MH Kim SK Lee SJ Jang MH Song KP Kim 2004 Clinicopathologic review of 58 patients with biliary papillomatosis Cancer 100 4 783 793
FG Rocha H Lee N Katabi RP DeMatteo Y Fong MI D’Angelica 2012 Intraductal papillary neoplasm of the bile duct: a biliary equivalent to intraductal papillary mucinous neoplasm of the pancreas? Hepatology 56 4 1352 1360
S Uemura R Higuchi T Yazawa W Izumo Y Matsunaga M Shiihara 2021 Prognostic factors for surgically resected intraductal papillary neoplasm of the bile duct: a retrospective cohort study Ann Surg Oncol 28 2 826 834
J Shi X Wan Y Xie J Lin J Long W Xu 2020 CK20 and lymph node involvement predict adverse outcome of malignant intraductal papillary neoplasm of the bile duct Histol Histopathol 35 5 449 456
WJ Kim S Hwang YJ Lee KH Kim KM Park CS Ahn 2016 Clinicopathological features and long-term outcomes of intraductal papillary neoplasms of the intrahepatic bile duct J Gastrointest Surg 20 7 1368 1375
M Narita B Endo Y Mizumoto R Matsusue H Hata T Yamaguchi 2015 Multicentric recurrence of intraductal papillary neoplasms of bile duct in the remnant intrahepatic bile duct after curative resection Int J Surg Case Rep 12 123 127
T Izawa T Obara S Tanno Y Mizukami N Yanagawa Y Kohgo 2001 Clonality and field cancerization in intraductal papillary-mucinous tumors of the pancreas Cancer 92 7 1807 1817
M Yokode Y Yamashita Y Zen 2017 Biliary intraductal papillary neoplasm with metachronous multiple tumors - true multicentric tumors or intrabiliary dissemination: a case report and review of the literature Mol Clin Oncol 6 3 315 320
H Hirano M Nakamura T Yoshikawa T Araida T Azuma T Ohta 1996 A recent case of mucin-producing distal bile duct carcinoma recurred 3 years after resection of mucin-producing intrahepatic bile duct carcinoma Tan Sui 17 497 502 (in Japanese)
T Fujita T Ajiki H Sawa Y Mita H Hori Y Fujino 2005 A case of mucin-producing intrahepatic cholangiocarcinoma recurred 24 years after resection J Jpn Biliary Assoc 19 4 500 504
Y Nakanishi S Kondo S Hirano Y Ambo E Tanaka T Morikawa 2006 Recurrence of mucosal carcinoma of the bile duct, with superficial flat spread, 12 years after operation J Hepatobil Pancreat Surg 13 4 355 358
M Fujioka T Mitsui T Terada A Takehara A Uno M Kawaguchi 2007 A case of biliary papillomatosis with asynchronous recurrence J Biliary Tract Pancreas 28 231 236 (in Japanese)
LP Bechmann P Hilgard A Frilling B Schumacher HA Baba G Gerken 2008 Successful photodynamic therapy for biliary papillomatosis: a case report World J Gastroenterol 14 26 4234 4237
H Kurahara H Shinchi Y Mataki S Maeda S Natsugoe S Takao 2009 A long-term survival case of mucin-producing bile duct carcinoma treated with repetitive surgical procedure Jpn J Gastroenterol Surg 42 5 510 515 (in Japanese)
S Fukuda K Koide S Mukai K Oishi S Fujisaki M Arita 2010 A case of biliary papillomatosis with asynchronous recurrence after curative operation Jpn J Gastroenterol Surg 43 8 815 821 (in Japanese)
E Ito J Watanabe M Hatano F Kushihata Y Takada 2013 A case of intraductal papillary neoplasm of bile duct, which recurred 4 years after primary curative resection J Jpn Surg Assoc 74 791 796 (in Japanese)
H Sato Y Sato K Harada M Sasaki K Hirano Y Nakanuma 2013 Metachronous intracystic and intraductal papillary neoplasms of the biliary tree World J Gastroenterol 19 36 6125 6126
R Miyata J Kawamukai K Sakurai Y Terakawa D Matsui T Watanabe 2014 A case of intraductal papillary neoplasm that recurred twice J Biliary Tract Pancreas 35 11 1319 1326 (in Japanese)
K Ohgi T Sugiura H Kanemoto Y Okamura T Ito T Kuribara 2015 A case of intraductal papillary neoplasm of the bile duct recurred at the remnant lower bile duct after curative liver resection J Jpn Biliary Assoc 29 2 271 278 (in Japanese)
Y Kageyama R Yamaguchi S Watanabe K Aizu F Sato A Arimoto 2018 Intraductal papillary neoplasm of the bile duct with rapidly progressive multicentric recurrence: a case report Int J Surg Case Rep 51 102 106
E Marín-Serrano CA Barbado 2019 Intraductal papillary neoplasm of the bile duct: a recurring disease Rev Esp Enferm Dig 111 11 890 891
Patel A, Sonnenday CJ, Schulman AR. Recurrent extrahepatic cholangiocarcinoma after bile duct resection for intraductal papillary mucinous neoplasm of the bile duct [Video]. VideoGIE. 2019 Aug 22. GIE;4(11):519-21.
Y Satoh O Kainuma T Maruyama H Tanaka T Natsume A Miyazaki 2020 Intraductal papillary neoplasm of the bile duct asynchronously recurred at the remnant bile duct 3 years after operation for hilar cholangiocarcinoma Jpn J Gastroenterol Surg 53 4 344 351
T Matsumoto K Kubota H Hachiya Y Sakuraoka T Shiraki T Shimizu 2019 Impact of tumor location on postoperative outcome of intraductal papillary neoplasm of the bile duct World J Surg 43 5 1313 1322
P Apostolopoulos KA Ekmektzoglou K Paraskeva K Dimopoulos K Paparaskeva G Alexandrakis 2018 Intraductal papillary neoplasm of the bile duct: case report and review of the literature Acta Gastroenterol Belg 81 1 97 99
V Luvira A Pugkhem V Bhudhisawasdi C Pairojkul E Sathitkarnmanee V Luvira 2017 Long-term outcome of surgical resection for intraductal papillary neoplasm of the bile duct J Gastroenterol Hepatol 32 2 527 533
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
YN and TT conceived and designed this case report. The remaining authors (MN, RM, YO, TI, DT, KH, YK, KM, and TN) contributed to data collection, analysis, and interpretation. YN wrote the manuscript draft. TT, MN, and RM performed critical revision of the manuscript. TN provided the final approval of the version to be published. TT, MN, and RM took overall responsibility and guaranteed the scientific integrity of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics committee approval was not applicable as the information was analyzed in a retrospective manner and had no effect on treatment. Informed consent to participate was obtained from the patient.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nakayama, Y., Tomino, T., Ninomiya, M. et al. Recurrent intraductal papillary neoplasm of the bile duct due to intraductal dissemination: a case report and literature review. surg case rep 7, 238 (2021). https://doi.org/10.1186/s40792-021-01318-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-021-01318-0