Abstract
Background
Tracheoesophageal fistula (TEF) is a rare but life-threatening complication after esophagectomy. It has a high mortality rate and often leads to severe aspiration pneumonia. Various types of surgical repair procedures have been reported, but the optimal management of TEF is challenging and controversial. Treatment should be individualized to each patient.
Case presentation
A 66-year-old female underwent transthoracic esophagectomy with gastric tube reconstruction and an intrathoracic anastomosis for esophageal cancer. Three years later, she had hematemesis and was diagnosed with a gastro-aortic fistula due to a gastric ulcer. She underwent endovascular aortic repair urgently at another hospital. Two days later, she underwent total resection of the gastric tube, during which time an injury to the trachea occurred; it was repaired by patching the stump of the esophagus to the injury site. Two months later, descending aortic replacement was performed due to infection of the stent graft. Six months after the first operation, a TEF developed. The patient was referred to our hospital for further treatment. The fistula was ligated and divided via a cervical approach, and a pectoralis major muscle flap was used to cover the defect. Esophageal reconstruction with the pedunculated jejunum was performed via a subcutaneous route. The postoperative course was uneventful. The patient was discharged after 6 months of physical and dysphagia rehabilitation.
Conclusion
A TEF located near the cervicothoracic border was successfully treated with a pectoralis major muscle flap through a cervical approach. Total resection of a gastric conduit in the posterior mediastinum carries a risk of tracheobronchial injury; however, if such an injury occurs, surgeons should be able to repair the injury using a suitable flap depending on the injury site.
Similar content being viewed by others
Background
Tracheoesophageal fistula (TEF) is a rare but life-threatening complication after esophagectomy. It has a high mortality rate and often leads to severe aspiration pneumonia [1, 2]. Various types of surgical repair procedures have been reported, but the optimal management of TEF is challenging and controversial. Treatment should be individualized to each patient.
Herein, we report a patient with a TEF after total resection of a gastric conduit for gastro-aortic fistula due to a gastric ulcer, successfully repaired with a pectoralis major muscle flap through a cervical approach.
Case presentation
A 66-year-old woman with esophageal cancer underwent transthoracic esophagectomy with three-field lymph node dissection via a muscle-sparing thoracotomy as previously reported [3], with gastric conduit reconstruction and an intrathoracic anastomosis. A high-dose proton pump inhibitor (PPI) was administered postoperatively due to the patient’s history of gastric ulcers; she discontinued the medication of her own volition. Three years after surgery, she was admitted to a local hospital for mediastinitis due to a perforated gastric ulcer in the conduit (Fig. 1a). She was treated with antibiotics and fasting. Seventeen days later, she had hematemesis and was diagnosed with a gastro-aortic fistula due to a gastric ulcer (Fig. 1b, c). She was transferred to a nearby university hospital with shock status and underwent endovascular aortic repair urgently, using two GORE® TAG® devices (W.L. Gore & Associates, Flagstaff, AZ). Two days later, total resection of the gastric tube was performed via a right posterolateral thoracotomy. There was an abscess cavity between the gastric tube and the descending aorta which consisted of necrotic tissue and old blood. A 2-cm wall defect was found on the right wall of the middle of gastric tube. The clot-filled gastric tube was resected. The perforation of the descending aorta was left. During this operation, a tracheal injury occurred while the remnant esophagus was being separated from the trachea; this injury was subsequently repaired by patching the stump of the esophagus to the injury site. Tracheostomy and a feeding jejunostomy were also performed. The operative time was 8 h and 40 min, and the blood loss was 2380 ml. The remnant esophagus had been decompressed by nasal tube since the total resection of gastric conduit till the following reconstructive surgery. Two months later, a descending aortic replacement was performed due to infection of the stent graft. Stent graft was removed, and the descending aorta between the fifth vertebra and the eleventh vertebra level was replaced with rifampicin-soaked 24-mm J graft (JUNKEN MEDICAL, Tokyo, Japan). Infected vascular intima of the aorta around the gastro-aortic fistula was resected. The operative time was 8 h and 10 min. Purulent matter was found around the stent graft, and Candida albicans was recognized by the bacterial culture of the pus. Postoperative severe pneumonia due to methicillin-resistant Staphylococcus aureus (MRSA) occurred after aortic replacement and required artificial respirator. Postoperative pneumonia was gradually improved by antibiotics. Three months later, she was transferred to previous local hospital. When a TEF developed 6 months after the first operation, in spite of decompression of the remnant esophagus by nasal tube, she still had required respirator due to prolonged postoperative pneumonia. Her poor general condition could not allow reconstructive surgery. She could withdraw from respirator 5 months after the aortic replacement (7 months after the first operation). Though she suffered from repeated bouts of aspiration pneumonia, she could walk after rehabilitation for 5 months (1 year after the first operation), and transferred to our hospital for reconstructive surgery. This patient’s time course is summarized in Fig. 2.
a Gastrointestinal endoscopy showed an ulcerated lesion on the right wall of middle of gastric tube. b Horizontal and c sagittal enhanced computed tomography image showed an irregular ulceration on the anterior wall of the descending aorta, no extravasation, and absence of the descending aortic wall and gastric wall, suggesting sealed rupture of the descending aorta (yellow arrow)
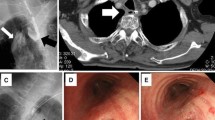
Bronchoscopy showed a fistula on the membranous portion of the trachea (Fig. 3a). Gastrointestinal endoscopy revealed a fistula in the stump of the esophagus (Fig. 3b). Computed tomography showed the TEF to be on the cervicothoracic border (Fig. 3c). The fistula was formed between the trachea and the esophageal stump on the cervicothoracic border (Fig. 3d). The cervical esophagus was accessed through a cervical approach, and the fistula was ligated and divided (Fig. 4a, b). A pectoralis major muscle flap was used to cover the defect and lay under the trachea (Fig. 4c, e). Esophageal reconstruction using the pedunculated jejunum with a microvascular anastomosis was performed via a subcutaneous route (Fig. 4d, e). The total operative time was 8 h and 54 min. Intraoperative blood loss was 453 ml. The postoperative course was uneventful. She was transferred to the previous hospital on postoperative day 14 and was discharged after 6 months of physical and dysphagia rehabilitation.
a Bronchoscopy showed the fistula on the membranous portion of trachea; a bubble arose from the fistula (white arrow). b Gastrointestinal endoscopy revealed a pinhole in the stump of the esophagus and the staple line (white arrow). c Sagittal computed tomography image showing the tracheoesophageal fistula on the cervicothoracic border (white arrow). d Schema of the tracheoesophageal fistula
a Cervical incision line. b The cervical esophagus was accessed through a cervical approach, and the fistula was ligated (white arrow). c A pectoralis major muscle flap was fixed to the fistula site, underneath the trachea. Pectoralis major muscle flap under the trachea (white arrow). d Esophageal reconstruction using the pedunculated jejunum with a microvascular anastomosis was performed via a subcutaneous route. e A schema of the operation
Discussion
In patients with TEF after esophagectomy, the latissimus dorsi muscle is the easiest muscle from which to fashion a flap for coverage, but it is often not usable as it is transected in a posterolateral thoracotomy. Though various repair techniques have been reported, the choice of which muscle to use for the repair depends on the location of the fistula. A pedicled posterior pericardial flap or diaphragmatic flap is useful for a fistula located near the carina or near a peripheral bronchus [4, 5]. A pectoralis major muscle flap or sternocleidomastoid muscle flap is useful for a fistula near or in the neck [6, 7]. The intercostal muscle flap can usually reach the whole intrathoracic trachea and bronchus and is a highly versatile option [8].
In this patient, there were several complicating factors including the surgical approach to the TEF given dense adhesions with two prior thoracotomies, flap choice, and conduit choice for esophageal reconstruction. Fortunately, the TEF was located on the cervicothoracic border, and a cervical approach was therefore chosen. A pectoralis major muscle or sternocleidomastoid muscle (SCM) flap can be suitable for repairing this tracheoesophageal fistula. A pectoralis major muscle flap is larger and thicker, and its covering area is wider. A SCM flap can be created easier in the same operative field. The larger pectoralis major muscle flap is considered to be more suitable for this patient to fill the dead space behind the membranous portion of the trachea. There was a problem about blood supply of SCM in this case. The upper third of SCM is supplied by branches of occipital artery. The middle third of SCM is supplied by branches of superior thyroid artery. The lower third of SCM is supplied by branches of the suprascapular artery [9]. There are two types of SCM flap: the superiorly based flap which is the commonly used and supplied from occipital artery and superior thyroid artery and the inferiorly based flap which is suitable for the lesion on the lower neck or upper mediastinum [10]. Though the inferiorly based sternocleidomastoid muscle flap was required according to the fistula position, the necrosis of the flap might occur because of no blood supply from branches of superior thyroid artery and suprascapular artery by previous cervical lymph node dissection. Therefore, the pectoralis major muscle was chosen over the SCM. With respect to esophageal reconstruction, small intestine reconstruction using the pedunculated jejunum with a microvascular anastomosis was chosen over a colonic conduit as there were dense adhesions of the transverse colon to the upper abdominal organs due to a previous total resection of the former gastric conduit.
During gastric conduit resection, dense adhesions around the conduit, particularly around the esophagogastrostomy in the upper mediastinum [11], make dissection difficult and a tracheobronchial injury a possibility. In this case, a tracheal injury occurred while the remnant esophagus was being dissected from the trachea via a thoracotomy. The injury was repaired with the remnant esophagus patch and resulted in a delayed TEF. Because the patient had previously undergone a muscle-sparing thoracotomy for esophageal cancer, thus preserving the latissimus dorsi muscle, a posterolateral thoracotomy with a latissimus dorsi muscle flap or intercostal muscle flap would have been a good option for repair of the initial tracheobronchial injury. When the tracheal injury occurred, the surgeons should have created an intercostal muscle flap to repair the injury and then performed a cervical esophagostomy. A pectoralis major muscle flap or a sternocleidomastoid muscle flap could also have been an option as the injury was near the neck.
The primary cause of this gastro-aortic fistula was discontinuation of PPI medication of her own volition. The frequency of peptic ulcer in the reconstructed gastric tube was reported to be 6.6–19.4% [12,13,14,15]. The mortality of patients with perforation of gastric tube ulcer was 56.5% in the review of Japanese literature and 84.6% in the review of the English literature [16]. Once gastric tube ulcer develops to gastro-aortic fistula, it causes a fatal result. We recommend PPI medication for patients with gastric tube reconstruction after esophagectomy.
Conclusions
A TEF located near the cervicothoracic border was successfully treated with a pectoralis major muscle flap through a cervical approach. Because total resection of a gastric conduit in the posterior mediastinum carries a risk of tracheobronchial injury, thoracotomy with creation of an intercostal muscle flap should be performed in preparation for a tracheobronchial injury in such situations. If such an injury occurs, surgeons should be able to repair the injury using a suitable flap depending on the injury site.
Abbreviations
- PPI:
-
Proton pump inhibitor
- SCM:
-
Sternocleidomastoid muscle
- TEF:
-
Tracheoesophageal fistula
References
Lambertz R, Holscher AH, Bludau M, Leers JM, Gutschow C, Schroder W. Management of tracheo- or bronchoesophageal fistula after Ivor-Lewis esophagectomy. World J Surg. 2016;40(7):1680–7. PubMed PMID: 26913731.
Yasuda T, Sugimura K, Yamasaki M, Miyata H, Motoori M, Yano M, et al. Ten cases of gastro-tracheobronchial fistula: a serious complication after esophagectomy and reconstruction using posterior mediastinal gastric tube. Dis Esophagus. 2012;25(8):687–93. PubMed PMID: 22292530.
Miyata K, Fukaya M, Itatsu K, Abe T, Nagino M. Muscle sparing thoracotomy for esophageal cancer: a comparison with posterolateral thoracotomy. Surg Today. 2016;46(7):807–14. PubMed PMID: 26311005.
Yasuda T, Makino T, Shiraishi O, Sogabe S. Pedicled posterior pericardial repair of tracheoesophageal fistula after chemoradiotherapy for esophageal cancer. J Thorac Cardiovasc Surg. 2016;151(6):e95–7. PubMed PMID: 26948167.
Mineo TC, Ambrogi V. The diaphragmatic flap: a multiuse material in thoracic surgery. J Thorac Cardiovasc Surg. 1999;118(6):1084–9. PubMed PMID: 10595982.
Shichinohe T, Okushiba S, Morikawa T, Kitashiro S, Manase H, Kawarada Y, et al. Salvage of a massive esophago-tracheal fistula resulting from a stenting treatment. Dis Esophagus. 2006;19(4):299–304. PubMed PMID: 16866865.
Arimoto J, Hatada A, Kawago M, Nishimura O, Maebeya S, Okamura Y. Closure of esophagotracheal fistula after esophagectomy for esophageal cancer. Gen Thorac Cardiovasc Surg. 2015;63(11):636–9. PubMed PMID: 26189183.
Deshpande G, Samarasam I, Banerjee S, Gnanamuthu RB, Chandran S, Mathew G. Benign esophagorespiratory fistula: a case series and a novel technique of definitive management. Dis Esophagus. 2013;26(2):141–7. PubMed PMID: 22486830.
Kierner AC, Aigner M, Zelenka I, Riedl G, Burian M. The blood supply of the sternocleidomastoid muscle and its clinical implications. Arch Surg. 1999;134(2):144–7. PubMed PMID: 10025452.
Yugueros P, Woods JE. The sternocleidomastoid myocutaneous flap: a reappraisal. Br J Plast Surg. 1996;49(2):93–6. PubMed PMID: 8733347.
Akita H, Doki Y, Ishikawa O, Takachi K, Miyashiro I, Sasaki Y, et al. Total removal of the posterior mediastinal gastric conduit due to gastric cancer after esophagectomy. J Surg Oncol. 2004;85(4):204–8. PubMed PMID: 14991878.
Yang L. Surgical treatment for carcinoma of gastric stump—a report on 13 patients. Zhonghua Zhong Liu Za Zhi. 1992;14(2):123–6. PubMed PMID: 1618080.
Koide N, Hiraguri M, Nishio A, Hanazaki K, Adachi W, Shikama N, et al. Ulcer in the gastric tube for esophageal replacement: a comparison of 12 esophageal cancer patients with or without postoperative radiotherapy. J Gastroenterol Hepatol. 2001;16(2):137–41. PubMed PMID: 11207892.
Maier A, Tomaselli F, Sankin O, Anegg U, Fell B, Renner H, et al. Acid-related diseases following retrosternal stomach interposition. Hepato-Gastroenterology. 2001;48(39):899–902. PubMed PMID: 11462952.
Motoyama S, Saito R, Kitamura M, Suzuki H, Nakamura M, Okuyama M, et al. Prospective endoscopic follow-up results of reconstructed gastric tube. Hepato-Gastroenterology. 2003;50(51):666–9. PubMed PMID: 12828056.
Ubukata H, Nakachi T, Tabuchi T, Nagata H, Takemura A, Shimazaki J, et al. Gastric tube perforation after esophagectomy for esophageal cancer. Surg Today. 2011;41(5):612–9. PubMed PMID: 21533931.
Author information
Authors and Affiliations
Contributions
MF, YS, HF, KI, and YK performed the surgery. YS and HF took charge of postoperative care in our hospital. AH took charge of conservative therapy for mediastinitis due to a perforated gastric ulcer in the conduit and postoperative physical and dysphagia rehabilitation in the local hospital. YS prepared the manuscript. MF and MN assisted in drafting the manuscript and reviewed the article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was carried out in accordance with the principles of the Declaration of Helsinki.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sakatoku, Y., Fukaya, M., Fujieda, H. et al. Tracheoesophageal fistula after total resection of gastric conduit for gastro-aortic fistula due to gastric ulcer. surg case rep 3, 90 (2017). https://doi.org/10.1186/s40792-017-0371-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-017-0371-6