Abstract
Background
Rectal leiomyosarcoma (LMS) is an extremely rare disease. Previously, LMS was not properly distinguishable from gastrointestinal stromal tumor (GIST) until c-kit, a characteristic marker of GIST, was discovered in 1998. No standard therapeutic strategy for gastrointestinal LMS has been established except for surgical resection because of its rarity. Rectal LMS is often accompanied by symptoms, which can enable detection at a small size. However, when a large LMS is detected at the lower rectum, it is difficult to excise due to the narrow pelvic space.
Case presentation
We present the case of an 86-year-old man with a large LMS. The LMS was asymptomatic and incidentally found at the lower rectum when he visited another hospital for management of benign prostatic hypertrophy. An abdominoperineal resection of the rectum was performed with combined resection of both seminal vesicles and a part of the prostate because tumor invasion was suspected. We used the hybrid method of laparoscopic and transanal total mesorectal excision (TaTME) approaches to achieve negative surgical margins. Late-onset urethral injury occurred in the postoperative course, which was successfully treated with a urethral catheter. The patient was discharged and received no adjuvant therapy. Local recurrence did not occur, but multiple lung metastases were detected 4 months later and the patient died 12 months after the surgery.
Conclusions
This is the first report of the hybrid method of laparoscopic and TaTME approaches to remove a large LMS at the lower rectum.
Similar content being viewed by others
Background
Gastrointestinal leiomyosarcoma (LMS) is a rare entity that could not be accurately diagnosed until c-kit, a characteristic marker of gastrointestinal stromal tumor (GIST), was discovered in 1998 [1]. Rectal LMS is usually detected at a smaller size compared with colonic LMS because there are often symptoms in rectal LMS [2]. However, when a large tumor is found in the lower rectum, it is difficult to excise because of the narrow pelvic space. Transanal total mesorectal excision (TaTME) has been reported to be useful for removal of large pelvic tumors [3, 4]. Here, we present a case of large LMS in the lower rectum. The cavity of the lesser pelvis was almost completely occupied by the tumor. There has been no report about a large LMS in the lower rectum which was removed by the hybrid method of laparoscopic and TaTME approaches.
Case presentation
The patient was an 87-year-old man who had regularly visited another hospital for management of benign prostatic hyperplasia. His medical history included hypertension. In November 2013, a rectal mass was incidentally identified. Colonoscopy showed a large submucosal tumor at the lower rectum (Fig. 1). Pathological findings from biopsy specimen showed spindle cells arranged in irregular bands. Mitotic count was 3 per 10 high-power fields, and there was no tumor necrosis. Immunohistochemical staining for h-caldesmon, alfa-SMA, and desmin was positive, while staining for DOG-1, CD117 (c-kit), CD34, and S-100 was negative. Ki-67 index was 40%. The rectal mass was diagnosed as a rectal LMS. The tumor was very large and considered difficult to be removed by surgical intervention in that hospital. However, there were no other effective therapies except for surgical resection. In January 2014, he was therefore referred to our hospital for surgical treatment.
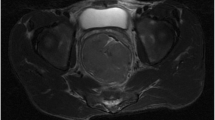
Digital rectal examination revealed a large mass at the anterior wall of the lower rectum, and the inferior edge of the tumor was located 2 cm from the anal verge. Laboratory findings were within normal ranges. Contrast-enhanced computed tomography (CT) showed that the size of the tumor was 7.5 cm in diameter and that there were no signs of distant metastasis (Fig. 2). Abdominal magnetic resonance imaging (MRI) showed that the tumor originated from the anterior wall of the lower rectum and that it was suspected to have invaded the left seminal vesicle and prostate (Fig. 3). The tumor was close to the urinary bladder, but a cystoscope showed no signs of tumor invasion. 18F-fluorodeoxyglucose positron-emission tomography showed no distant metastasis.
We made a diagnosis of a rectal LMS with suspected invasion of the left seminal vesicle and prostate. In March 2014, we performed an abdominoperineal resection of the rectum combined with both seminal vesicles and part of the prostate. We used the hybrid method of laparoscopic and TaTME approaches for better surgical view because it was apparently difficult to achieve negative surgical margins due to the large tumor located within a narrow pelvic space (Fig. 4). In this method, we used five trocars for laparoscopic surgery and a multiple access port for TaTME. A 12-mm trocar was inserted through an umbilical incision, and then the pneumoperitoneum was created. Another 12-mm trocar was placed in the right lower abdominal region, and three 5-mm trocars were inserted in the left lower abdominal and bilateral lateral regions. Pathological findings from the resected specimen showed that the tumor was a pleomorphic LMS and that tumor cells had invaded the left seminal vesicle and prostate with negative surgical margin. No lymph node metastasis was found. Immunohistochemical staining for calponin, alfa-SMA, and desmin was positive, while staining for DOG-1, c-kit, CD34, and S-100 was negative (Fig. 5). Tumor necrosis was found in the surgical specimen. The LMS was classified as grade 2 in Federation Nationale des Centres de Lutte le Cancer (FNCLCC) grading and stage IIb in TNM classification. In the postoperative course, the patient suffered from dysuria and needed intermittent self-catheterization. Late-onset urethral injury occurred 30 days after the operation, which was successfully treated with a urethral catheter. The patient was discharged 42 days after the operation. The patient received no adjuvant therapies such as chemotherapy and radiotherapy. Four months later, multiple lung metastases were detected on CT, although local recurrence was not found. The patient died 12 months after the operation.
Discussion
It had been difficult to distinguish gastrointestinal LMS from GIST until c-kit was discovered in 1998 [1]. That is, the previously diagnosed gastrointestinal LMS is considered to include what is currently termed GIST [5]. According to the recent literature, the incidence of LMS was reported to be 1/50 to 1/65 of the incidence of GIST [2, 5]. LMS originates from smooth muscle cells and can arise anywhere in human body, although the main locations of LMS are the retroperitoneum, lower extremities, and the uterus [6]. Therefore, gastrointestinal LMS is very rare and its characteristics are still unclear.
To our knowledge, there are only 10 cases, including our case, in the English-language literature since 1998 in which immunohistochemical findings have been reported [2, 5, 7] (Table 1). The patients were three men and seven women. Median age was 65 years, ranging from 24 to 88. Average tumor size was 3.8 cm. Polypectomy was performed in two cases, anterior resection in three cases, abdominoperineal resection in four cases, and the surgical method was not reported in one case. Common characteristics of three recurrent cases were non-polypoid appearances such as Borrmann type 2- and plaque-like lesions. Our case had the largest tumor size, and there has been no report in which a large LMS in the lower rectum was removed by the hybrid method of laparoscopic and TaTME approaches.
FNCLCC grading includes three factors; tumor differentiation, mitotic count, and tumor necrosis [8]. The Union for International Cancer Control/the American Joint Committee on Cancer staging system for soft tissue sarcoma assesses the following factors; tumor size, tumor depth, lymph node metastasis, distant metastasis, and histological grade [9]. However, the characteristics of gastrointestinal LMS have not been fully clarified because of its rarity [6]. Yamamoto et al. [5] reported that tumor-specific overall survival rate was 51.6% and that tumor size (≥5 cm) was significantly associated with a poor prognosis. They also suggested that tumor depth and necrotic area might be associated with patient survival. In our case, tumor size and gross appearance could be associated with a poor prognosis, while mitotic count was not. We surmised that patients with at least one risk factor might have a poor prognosis.
There has been no standard therapeutic strategy for gastrointestinal LMS. The effect of chemotherapy or radiotherapy is still unclear, whereas some antitumor drugs such as doxorubicin, ifosfamide, and eribulin are suggested to be effective for soft tissue sarcomas [10–12]. Surgical resection plays a critical role in the treatment for gastrointestinal LMS [6]. Despite the fact that lymph node metastasis is uncommon in soft tissue sarcoma, lymph node dissection seems to be necessary for gastrointestinal LMS because lymph node metastasis has been reported [5].
In our case, we performed abdominoperineal resection using the hybrid method of laparoscopic and TaTME approaches. It was considered difficult that the elderly patient underwent a total pelvic exenteration surgery and took care of a urostomy together with a colostomy. We assume that the TaTME approach is useful for the resection of a large pelvic tumor. Surgical margins were negative for tumor cells by this approach, but it was very close (<1 mm). There have been some reports on the effectiveness of radiotherapy against local recurrence [6, 13, 14], while radiotherapy was reported to be a risk factor for LMS [15]. Adjuvant radiotherapy or chemotherapy was not performed because there is no established adjuvant therapy for rectal LMS and the elderly patient did not hope to receive adjuvant therapies. However, local recurrence was not found irrespective of the short surgical margins. The late-onset urethral injury occurred after surgery. We conceived that the self-catheterization might make a negative impact on the occurrence. Four months after the surgery, multiple lung metastases were found. There have been some reports on the efficacy of chemotherapy in other LMS cases [6, 10, 12, 16, 17]. It also might prevent the recurrence of rectal LMS and contribute to prolong patient survival.
Conclusions
We reported a case of LMS in the lower rectum. It was successfully removed by the hybrid method of laparoscopic and TaTME approaches. This method can be useful to achieve negative surgical margins in the resection of large pelvic tumors.
Abbreviations
- CT:
-
Computed tomography
- FNCLCC:
-
Federation Nationale des Centres de Lutte le Cancer
- GIST:
-
Gastrointestinal stromal tumor
- LMS:
-
Leiomyosarcoma
- MRI:
-
Magnetic resonance imaging
- TaTME:
-
Transanal total mesorectal excision
References
Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–80.
Aggarwal G, Sharma S, Zheng M, Reid MD, Crosby JH, Chamberlain SM, et al. Primary leiomyosarcomas of the gastrointestinal tract in the post-gastrointestinal stromal tumor era. Ann Diagn Pathol. 2012;16(6):532–40.
Hasegawa S, Okada T, Hida K, Kawada K, Sakai Y. Transperineal minimally invasive approach for extralevator abdominoperineal excision. Surg Endosc. 2016;30:4620–1.
Wachter N, Worns MA, Dos Santos DP, Lang H, Huber T, Kneist W. Transanal minimally invasive surgery (TAMIS) approach for large juxta-anal gastrointestinal stromal tumour. J Minim Access Surg. 2016;12(3):289–91.
Yamamoto H, Handa M, Tobo T, Setsu N, Fujita K, Oshiro Y, et al. Clinicopathological features of primary leiomyosarcoma of the gastrointestinal tract following recognition of gastrointestinal stromal tumours. Histopathology. 2013;63(2):194–207.
Duffaud F, Ray-Coquard I, Salas S, Pautier P. Recent advances in understanding and managing leiomyosarcomas. F1000Prime Rep. 2015;7:55.
Miettinen M, Furlong M, Sarlomo-Rikala M, Burke A, Sobin LH, Lasota J. Gastrointestinal stromal tumors, intramural leiomyomas, and leiomyosarcomas in the rectum and anus: a clinicopathologic, immunohistochemical, and molecular genetic study of 144 cases. Am J Surg Pathol. 2001;25(9):1121–33.
Coindre JM, Trojani M, Contesso G, David M, Rouesse J, Bui NB, et al. Reproducibility of a histopathologic grading system for adult soft tissue sarcoma. Cancer. 1986;58(2):306–9.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4.
Pautier P, Floquet A, Chevreau C, Penel N, Guillemet C, Delcambre C, et al. Trabectedin in combination with doxorubicin for first-line treatment of advanced uterine or soft-tissue leiomyosarcoma (LMS-02): a non-randomised, multicentre, phase 2 trial. Lancet Oncol. 2015;16(4):457–64.
Judson I, Verweij J, Gelderblom H, Hartmann JT, Schoffski P, Blay JY, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol. 2014;15(4):415–23.
Schoffski P, Chawla S, Maki RG, Italiano A, Gelderblom H, Choy E, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet. 2016;387(10028):1629–37.
Tuan J, Vitolo V, Vischioni B, Iannalfi A, Fiore MR, Fossati P, et al. Radiation therapy for retroperitoneal sarcoma. Radiol Med. 2014;119(10):790–802.
Roeder F, Ulrich A, Habl G, Uhl M, Saleh-Ebrahimi L, Huber PE, et al. Clinical phase I/II trial to investigate preoperative dose-escalated intensity-modulated radiation therapy (IMRT) and intraoperative radiation therapy (IORT) in patients with retroperitoneal soft tissue sarcoma: interim analysis. BMC Cancer. 2014;14:617.
Horiguchi H, Takada K, Kamihara Y, Ibata S, Iyama S, Sato T, et al. Radiation-induced leiomyosarcoma of the prostate after brachytherapy for prostatic adenocarcinoma. Case Rep Oncol. 2014;7(2):565–70.
Sagara K, Takayoshi K, Kusumoto E, Uchino K, Matsumura T, Kusaba H, et al. Favorable control of rapidly progressive retroperitoneal pleomorphic leiomyosarcoma with multimodality therapy: a case report. BMC Res Notes. 2014;7:377.
Kasper B, Ouali M, van Glabbeke M, Blay JY, Bramwell VH, Woll PJ, et al. Prognostic factors in adolescents and young adults (AYA) with high risk soft tissue sarcoma (STS) treated by adjuvant chemotherapy: a study based on pooled European Organisation for Research and Treatment of Cancer (EORTC) clinical trials 62771 and 62931. Eur J Cancer. 2013;49(2):449–56.
Funding
No funding was received for this case report.
Authors’ contributions
KH performed the surgery, TS diagnosed pathology, NH drafted the manuscript, KK revised it critically, and YS approved the final manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hoshino, N., Hida, K., Kawada, K. et al. Transanal total mesorectal excision for a large leiomyosarcoma at the lower rectum: a case report and literature review. surg case rep 3, 13 (2017). https://doi.org/10.1186/s40792-017-0289-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-017-0289-z