Abstract
Background
We examined whether the pharmacodynamic drug–drug interaction between esaxerenone and trimethoprim enhances the hyperkalemic effect.
Methods
A retrospective observational study was conducted to identify patients >18 years undertaking esaxerenone alone or esaxerenone plus trimethoprim at Mie University Hospital from May 2019 to December 2022. We performed propensity score-matching (1:1) to compare between-group differences in the maximum change in serum potassium levels (ΔK) using the Mann–Whitney U test. For esaxerenone plus trimethoprim, Spearman's correlation coefficients were used to examine correlations between ΔK and variables, including changes in blood urea nitrogen (ΔBUN), serum creatinine levels (ΔCr), and weekly trimethoprim cumulative dose.
Results
Out of propensity score-matched groups (n=8 each), serum potassium levels significantly increased after administration of esaxerenone alone (4.4 [4.2 to 4.7] meq/L to 5.2 [4.7 to 5.4] meq/L, p=0.008) and esaxerenone plus trimethoprim (4.2 [4.0 to 5.1] meq/L to 5.4 [4.7 to 5.5] meq/L, p=0.023). ΔK did not significantly differ between the groups (esaxerenone alone; 0.6 [0.3 to 0.9] meq/L vs. esaxerenone plus trimethoprim; 1.0 [0.4 to 1.3] meq/L, p=0.342). ΔK positively correlated with ΔBUN (r=0.988, p<0.001) or ΔCr (r=0.800, p=0.017). There was a trend of correlation of ΔK with a weekly cumulative trimethoprim dose (r=0.607, p=0.110).
Conclusions
The hyperkalemic effect of the drug–drug interaction between esaxerenone and trimethoprim is not notable and related to renal function and trimethoprim dosage.
Similar content being viewed by others
Background
Esaxerenone (a mineralocorticoid receptor antagonist) blocks aldosterone binding to mineralocorticoid receptors in the distal convoluted tubule and collecting duct, thereby inhibiting sodium reabsorption and potassium secretion [1]. Randomized controlled trials have shown that esaxerenone lowers blood pressure and urinary protein level in patients with essential hypertension, type 2 diabetes, and microalbuminuria [2]. However, hyperkalemia occurs during esaxerenone treatment in approximately up to 10% of patients [2, 3], causing lethal arrhythmias and cardiac arrest.
Aldosterone-activated epithelial sodium channels are key regulators of potassium excretion [4]. Since animal studies demonstrated that trimethoprim inhibits epithelial sodium channels in a concentration-dependent manner [5], trimethoprim can amplify the hyperkalemic effect of esaxerenone in humans. Previous studies have suggested a higher risk of sudden death and hyperkalemia with the co-administration of spironolactone (a traditional mineralocorticoid receptor antagonist) and trimethoprim [6, 7]. In particular, esaxerenone has a high inhibitory capacity for mineralocorticoid receptors and a long half-life among mineralocorticoid receptor antagonists (3.7 nM and 18.6 h, respectively) [8]. We have identified that the hyperkalemic effect is obvious when esaxerenone and clarithromycin are co-administered, but there was no increase in serum potassium level in patients with eplerenone and clarithromycin, owing to the inhibition of cytochrome P450 3A4 metabolism for mineralocorticoid receptor antagonist by clarithromycin [9]. Therefore, we hypothesized that the hyperkalemic effect would be greater in patients who received esaxerenone and trimethoprim concomitantly than in those who received esaxerenone alone.
The objective of this study was to evaluate hyperkalemia due to the pharmacodynamic interaction between esaxerenone and trimethoprim.
Methods
Study design
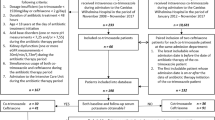
This single-center retrospective observational study enrolled all patients (>18 years) who received esaxerenone alone or esaxerenone plus trimethoprim at Mie University Hospital from May 2019 to December 2022. Exclusion criteria included non-measurement of serum potassium levels or serum potassium levels exceeding 5.5 meq/L at baseline, according to the Common Terminology Criteria for Adverse Events version 5.0. The Institutional Review Board of Mie University Hospital approved the study protocol in accordance with the Declaration of Helsinki (H2023-075).
Data collection
Data collection through electronic medical chart review included demographics, co-existing diseases, clinical laboratory data, test drugs (esaxerenone and trimethoprim), and concomitant medications known to alter serum potassium levels (angiotensin receptor neprilysin inhibitor, renin-angiotensin system inhibitors, loop diuretics, thiazide diuretics, and sodium-glucose co-transporter 2 inhibitors). The follow-up period was till the point of treatment termination with esaxerenone alone and esaxerenone plus trimethoprim, or December 2022. The blood samples were collected in the morning. The estimated glomerular filtration rate (eGFR) was calculated as follows [10]:
Outcomes
The primary outcome was the maximum change in serum potassium levels (ΔK, meq/L) from baseline to the maximum value at post-dose as calculated by the following equation:
When pseudo-hyperkalemia (e.g., blood collection technique or hemolysis) was present, we alternatively used remeasured serum potassium levels for the analysis. Likewise, the secondary outcome comprised changes in serum sodium levels (ΔNa, meq/L), blood urea nitrogen (ΔBUN, mg/dL), serum creatinine levels (ΔCr, mg/dL), and eGFR (ΔeGFR, mL/min/1.73m2) at the point of maximum potassium levels since trimethoprim potentially causes hyponatremia and pseudo-elevation of serum creatinine [11, 12].
Statistical analysis
Statistical analysis was conducted using JMP® Pro 16.2.0 (SAS Institute Inc., Cary, NC, USA). Statistical significance was a two-tailed p-value <0.05. Data were presented as median [interquartile range (IQR)] for continuous data or as numbers (%) for categorical data. Continuous data were compared using the Mann–Whitney U test (unpaired data) or Wilcoxon signed-rank test (paired data). The heterogeneity of categorical data was evaluated using the chi-square test. Spearman's correlation coefficients were used to analyze the correlations between continuous variables.
A 1:1 propensity score-matched pair was generated to adjust for potential confounders based on the propensity score as a probability of receiving esaxerenone plus trimethoprim in logistic regression analysis. The variables in the propensity score included sex, age, body mass index, serum potassium, serum sodium, serum chloride, albumin, blood urea nitrogen, eGFR, esaxerenone dosing schedule, angiotensin receptor neprilysin inhibitor or renin-angiotensin system inhibitors, loop and thiazide diuretics, and sodium-glucose co-transporter 2 inhibitors.
We compared the outcomes of within-group differences in each group and between-group differences between esaxerenone alone and esaxerenone plus trimethoprim. A sub-analysis was conducted to examine the correlations between ΔK and variables such as secondary outcomes and weekly cumulative trimethoprim dose in the esaxerenone plus trimethoprim group. Since the kidney plays an essential role in eliminating trimethoprim [13], the weekly cumulative trimethoprim dose normalized by eGFR was also used for sub-analysis.
Results
Clinical characteristics
Of the 185 patients who received esaxerenone (esaxerenone alone, n=177; esaxerenone plus trimethoprim, n=8), 72 met the exclusion criteria. Ultimately, 113 patients were included in the analysis (esaxerenone alone, n=105; esaxerenone plus trimethoprim, n=8). Serum albumin, blood urea nitrogen, and esaxerenone dosing schedules differed between the groups significantly (Additional data 1). After propensity score-matching, the clinical characteristics were well-balanced (n=8 each; Table 1). The dosing schedule of esaxerenone comprised a low dose (0.625 mg×1, 1.25 mg×1, and 2.5 mg ×1) in both groups. In the esaxerenone plus trimethoprim group, all patients prophylactically received cotrimoxazole containing 400 mg of sulfamethoxazole and 80 mg of trimethoprim in one tablet. The trimethoprim dosing schedule was daily (one tablet ×2 every day; n=1) and non-daily (half tablet ×1 twice a week; n=1 and one tablet ×1 thrice a week; n=6). The median follow-up duration was 186 [IQR: 47 to 292] days for esaxerenone alone and 95 [23 to 222] days for esaxerenone plus trimethoprim (p=0.25).
Outcome
Table 2 summarizes the study outcomes. Serum potassium levels significantly increased after esaxerenone alone from 4.4 [4.2 to 4.7] meq/L to 5.2 [4.7 to 5.4] meq/L (p=0.008) and esaxerenone plus trimethoprim from 4.2 [4.0 to 5.1] meq/L to 5.4 [4.7 to 5.5] meq/L (p=0.023). No significant difference in ΔK was found between the esaxerenone alone and esaxerenone plus trimethoprim (esaxerenone alone: 0.6 [0.3 to 0.9] meq/L vs. esaxerenone plus trimethoprim: 1.0 [0.4 to 1.3] meq/L, p=0.342).
Similarly, there were no significant difference in ΔNa (esaxerenone alone: -1 [-2 to 1] meq/L vs. esaxerenone plus trimethoprim: -1 [-3 to 1] meq/L, p=0.749), ΔBUN (esaxerenone alone: 5.7 [-0.7 to 7.0] mg/dL vs. esaxerenone plus trimethoprim: 4.1 [-0.5 to 11.0] mg/dL, p=0.674), ΔCr (esaxerenone alone: 0.23 [0.02 to 0.45] mg/dL vs. esaxerenone plus trimethoprim: 0.20 [-0.03 to 0.43] mg/dL, p=0.752), and ΔeGFR (esaxerenone alone: -3.4 [-8.3 to -0.9] mL/min/1.73m2 vs. esaxerenone plus trimethoprim: -9.8 [-14.7 to 1.4] mL/min/1.73m2, p=0.401). The serum sodium levels were comparable after esaxerenone alone from 140 [139 to 143] meq/L to 141 [139 to 142] meq/L (p=0.375) and esaxerenone plus trimethoprim from 141 [139 to 143] meq/L to 141 [138 to 143] meq/L (p=0.266). Blood urea nitrogen did not differ after esaxerenone alone from 25.1 [21.0 to 30.5] mg/dL to 30.5 [23.0 to 33.9] mg/dL (p=0.055) and esaxerenone plus trimethoprim from 21.2 [17.4 to 26.6] mg/dL to 28.5 [19.0 to 34.3] mg/dL (p=0.109). Serum creatinine levels significantly increased after esaxerenone alone from 1.29 [1.00 to 1.82] mg/dL to 1.65 [1.03 to 2.17] mg/dL (p=0.039), although there was no significant increase after esaxerenone plus trimethoprim from 1.00 [0.74 to 1.31] mg/dL to 1.31 [0.76 to 1.60] mg/dL (p=0.086). A significant decrease in eGFR was also observed in esaxerenone alone from 38.9 [27.8 to 45.4] mL/min/1.73m2 to 31.2 [23.4 to 41.0] mL/min/1.73m2 (p=0.039), whereas an insignificant decrease was detected after esaxerenone plus trimethoprim from 50.9 [38.8 to 63.3] mL/min/1.73m2 to 40.1 [30.0 to 60.9] mL/min/1.73m2 (p=0.055).
We confirmed significant correlations of ΔK with ΔBUN (Fig. 1B, r =0.988, p<0.001) and ΔCr (Fig. 1C, r=0.800, p=0.017). Additionally, ΔK tended to correlate with the weekly cumulative trimethoprim dose (Fig. 1E, r=0.607, p =0.110) and the weekly cumulative trimethoprim dose normalized by eGFR (Fig. 1F, r=0.603, p =0.114).
Factors influencing maximum serum potassium level changes in patients receiving esaxerenone and trimethoprim
Abbreviations: K, potassium; Na, sodium; BUN, blood urea nitrogen; Cr, creatinine; eGFR, estimated glomerular filtration rate; TMP, trimethoprim. The y- and x-axes represent maximum changes in serum potassium levels (ΔK) and changes in Na, BUN, Cr, and eGFR at the timing of maximum serum potassium level, and TMP dosing (weekly cumulative TMP and weekly cumulative TMP/eGFR), respectively. \(\triangle K(meq/L)=K\;at\;maximum\;value-K\;at\;baseline\) The maximum value was at the observation of the maximum potassium value. \(\text{eGFR }\left(\text{mL}/\text{min}/{1.73\text{m}}^{2}\right)=194\times {\text{age}}^{-0.287}\times {\text{serum creatinine}}^{-1.094}\times 0.739 \left(\text{if female}\right)\) [10]. A ΔNa, B ΔBUN, C ΔCr, D ΔeGFR, E Weekly cumulative dose of TMP, F Weekly cumulative dose of TMP/eGFR
Discussion
The hyperkalemic effects of esaxerenone alone and esaxerenone plus trimethoprim were comparable in a small size of the study. Moreover, ΔK significantly correlated with ΔBUN and ΔCr. In addition, the trimethoprim dose may have enhanced the hyperkalemic effect after treatment with esaxerenone and trimethoprim. These insights will help to continue the treatment of esaxerenone and avoid the risk of critical hyperkalemia due to the pharmacodynamic interaction between esaxerenone and trimethoprim.
A randomized controlled trial reported that the median increase in serum potassium levels was 0.2 meq/L [2]. In contrast, the hyperkalemic effect was notable in patients taking low-dose esaxerenone with and without trimethoprim (Table 2). We postulated that older age and reduced eGFR exacerbated the hyperkalemic effect. Compared with other landmark trials, the patients in our study were older (≥70 years vs. <70 years) [2]. Advancing age is a risk factor for hyperkalemia associated with spironolactone, a mineralocorticoid receptor antagonist [14]. Similarly, our study patients had lower eGFR (40 to 50 mL/min/1.73m2 vs. approximately 70 mL/min/1.73m2) [2]. In particular, a clinical trial set the exclusion criteria for eGFR <60 mL/min/1.73m2 [2]. Since the urinary excretion of potassium is determined by kidney function [15], clinicians should evaluate renal function following esaxerenone treatment.
Our finding contradicted the hypothesis. This reason is that the co-administration of trimethoprim at a prophylactic dose did not influence the hyperkalemic effects of esaxerenone. We speculated that the potential mechanism of drug–drug interaction involves aldosterone antagonism by esaxerenone [1] and epithelial sodium channel blockade by trimethoprim [5]. Some case reports have shown severe hyperkalemia in patients receiving trimethoprim under hypoaldosteronism [16, 17]. This phenomenon is similar to the effects of drug–drug interactions between esaxerenone and trimethoprim. In fact, esaxerenone at 1.25 to 5.0 mg ×1 secondary increased plasma renin activity and plasma aldosterone concentration in essential hypertension or primary aldosteronism [18, 19], which is reflective of hypoaldosteronism. Our study showed that the absolute ΔK was numerically larger in patients administered esaxerenone plus trimethoprim than in those administered esaxerenone alone, indicating that the pharmacodynamic interaction between esaxerenone and trimethoprim is clinically significant to a greater or lesser extent. From the viewpoint of potassium kinetics, total potassium has a positive correlation with body size that stores potassium in the body [20]. Indeed, we previously reported that a higher hyperkalemic risk of renin-angiotensin system inhibitors was observed in patients with reduced renal function in addition to low body size [21]. In this study, the group of esaxerenone and trimethoprim had -9.8 mL/min/1.73m2 of ΔeGFR from baseline (Table 2). The absolute ΔK might depend on the kidney function.
Trends toward reduced eGFR were observed in both the esaxerenone alone and esaxerenone plus trimethoprim groups. Trimethoprim inhibits creatinine secretion via organic cation transporter 2, which increases serum creatinine levels at an average of 0.05 to 0.2 mg/dL or 6 to 20% from baseline [22,23,24]. In the present study, ΔCr was within the reported range. The pseudo-elevation of serum creatinine by trimethoprim did not directly affect renal function [25,26,27]. The magnitude of ΔK was higher, especially when ΔCr exceeded an average elevation of serum creatinine level in pseudo-elevation (0.2 mg/dL) (Fig. 1C). Since the kidney handles the elimination of potassium and trimethoprim [13, 15], serum creatinine level is a valuable marker for estimating the magnitude of drug–drug interactions between esaxerenone and trimethoprim in case of ΔCr ≥0.2 mg/dL. Furthermore, ΔK significantly correlated with ΔBUN as well as ΔCr. Blood urea nitrogen homeostasis depends on diet (protein), catabolism, and glomerular filtration [28]. Unlike creatinine [29], urea reabsorption increases with decreasing urine flow rate mediated by vasopressin in the collecting duct [30]. Therefore, ΔBUN may indirectly reflect slow urine flow and low capacity for potassium excretion.
Sub-analysis revealed a mild but non-significant correlation between ΔK and weekly cumulative trimethoprim dose (Fig. 1E and F). Trimethoprim impairs potassium excretion in the urine by inhibiting epithelial sodium channels in a concentration-dependent manner [5]. Although a therapeutic dose of trimethoprim (>10 mg/kg/day) is likely to cause hyperkalemia powerfully [31], prophylactic doses (e.g., one tablet ×1) could also cause hyperkalemia [32, 33]. Almost all patients in the present analysis received non-daily administration of trimethoprim as prophylaxis. Recently, a low dose of cotrimoxazole at a daily dose of 100 mg sulfamethoxazole and 20 mg trimethoprim was found to be an acceptable option for Pneumocystis pneumonia prophylaxis after transplantation [34]. Therefore, it is required to confirm whether co-administration of a minimal dose of trimethoprim (i.e., 20 mg) is effective in avoiding the hyperkalemic effect by pharmacodynamic interaction between esaxerenone and trimethoprim.
This study has many inherent limitations when interpreting the findings. First, its retrospective observational nature leads to bias and confounding factors. Second, it was difficult to assess medication adherence using pill-count due to a retrospective nature although self-reported medication adherence was obtained from individual patients. Third, we could not analyze ΔK according to the esaxerenone dosing schedule as well as co-administration with sodium-glucose co-transporter 2 inhibitors, which is expected to reduce the risk of hyperkalemia through mineralocorticoid receptor antagonists [35]. Fourth, the dataset did not include important information such as diet composition, neurohormonal findings (e.g., renin and aldosterone), and urinary potassium. Finally, most of the patients had chronic kidney disease, which limits the generalizability of the findings.
Conclusions
The pilot data indicated that the hyperkalemic effect caused by the pharmacodynamic drug–drug interaction between esaxerenone and trimethoprim is weak although we cannot draw a clear conclusion because of a limited sample size. This hyperkalemic effect may increase in patients with reduced renal function or in those administered high doses of trimethoprim. Further study was required to validate the present findings using a large dataset.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author.
Abbreviations
- eGFR:
-
Estimated glomerular filtration rate
- ΔK:
-
Change in serum potassium levels
- ΔNa:
-
Changes in serum sodium levels
- ΔBUN:
-
Change in blood urea nitrogen
- ΔCr:
-
Change in serum creatinine levels
- ΔeGFR:
-
Change in estimated glomerular filtration rate
- IQR:
-
Interquartile range
References
Kolkhof P, Joseph A, Kintscher U. Nonsteroidal mineralocorticoid receptor antagonism for cardiovascular and renal disorders - New perspectives for combination therapy. Pharmacol Res. 2021;172:105859.
Ito S, Itoh H, Rakugi H, Okuda Y, Yoshimura M, Yamakawa S. Double-Blind Randomized Phase 3 Study Comparing Esaxerenone (CS-3150) and Eplerenone in Patients With Essential Hypertension (ESAX-HTN Study). Hypertension. 2020;75:51–8.
Rakugi H, Yamakawa S, Sugimoto K. Management of hyperkalemia during treatment with mineralocorticoid receptor blockers: findings from esaxerenone. Hypertens Res. 2021;44:371–85.
Meneton P, Loffing J, Warnock DG. Sodium and potassium handling by the aldosterone-sensitive distal nephron: the pivotal role of the distal and connecting tubule. Am J Physiol Renal Physiol. 2004;287:F593–601.
Velázquez H, Perazella MA, Wright FS, Ellison DH. Renal mechanism of trimethoprim-induced hyperkalemia. Ann Intern Med. 1993;119:296–301.
Antoniou T, Gomes T, Mamdani MM, Yao Z, Hellings C, Garg AX, Weir MA, Juurlink DN. Trimethoprim-sulfamethoxazole induced hyperkalaemia in elderly patients receiving spironolactone: nested case-control study. Bmj. 2011;343:d5228.
Antoniou T, Hollands S, Macdonald EM, Gomes T, Mamdani MM, Juurlink DN. Trimethoprim-sulfamethoxazole and risk of sudden death among patients taking spironolactone. Cmaj. 2015;187:E138–e143.
Tezuka Y, Ito S. The Time to Reconsider Mineralocorticoid Receptor Blocking Strategy: Arrival of Nonsteroidal Mineralocorticoid Receptor Blockers. Curr Hypertens Rep. 2022;24:215–24.
Hirai T, Ueda S, Ogura T, Katayama K, Dohi K, Hosohata K, Aoyama T, Matsumoto Y, Iwamoto T. Hyperkalemia by eplerenone or esaxerenone in the presence or absence of clarithromycin in hypertensive patients: a retrospective observational cohort study. J Hypertens. 2023;41:580–6.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
Tsapepas D, Chiles M, Babayev R, Rao MK, Jaitly M, Salerno D, Mohan S. Incidence of Hyponatremia with High-Dose Trimethoprim-Sulfamethoxazole Exposure. Am J Med. 2016;129:1322–8.
Chan WY, Clark AB, Wilson AM, Loke YK. The effect of co-trimoxazole on serum potassium concentration: safety evaluation of a randomized controlled trial. Br J Clin Pharmacol. 2017;83:1808–14.
Patel RB, Welling PG. Clinical pharmacokinetics of co-trimoxazole (trimethoprim-sulphamethoxazole). Clin Pharmacokinet. 1980;5:405–23.
Vardeny O, Claggett B, Vaduganathan M, Beldhuis I, Rouleau J, O’Meara E, Anand IS, Shah SJ, Sweitzer NK, Fang JC, Desai AS, Lewis EF, Pitt B, Pfeffer MA, Solomon SD. Influence of Age on Efficacy and Safety of Spironolactone in Heart Failure. JACC Heart Fail. 2019;7:1022–8.
Clase CM, Carrero JJ, Ellison DH, Grams ME, Hemmelgarn BR, Jardine MJ, Kovesdy CP, Kline GA, Lindner G, Obrador GT, Palmer BF, Cheung M, Wheeler DC, Winkelmayer WC, Pecoits-Filho R. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2020;97:42–61.
Montebello A, Gruppetta M. Cotrimoxazole-induced hyperkalaemia in a patient with known hypoaldosteronism. BMJ Case Rep. 2021;14:e239543.
Elisaf M, Terrovitou C, Tomos P, Siamopoulos KC. Severe hyperkalaemia after cotrimoxazole administration in a patient with hyporeninaemic hypoaldosteronism. Nephrol Dial Transplant. 1997;12:1254–5.
Uchida HA, Nakajima H, Hashimoto M, Nakamura A, Nunoue T, Murakami K, Hosoya T, Komoto K, Taguchi T, Akasaka T, Shiosakai K, Sugimoto K, Wada J. Efficacy and Safety of Esaxerenone in Hypertensive Patients with Diabetic Kidney Disease: A Multicenter, Open-Label. Prospective Study Adv Ther. 2022;39:5158–75.
Ishikawa T, Morimoto S, Ichihara A. Effects of mineralocorticoid receptor antagonists on sex hormones and body composition in patients with primary aldosteronism. Hypertens Res. 2022;45:496–506.
Boddy K, King PC, Hume R, Weyers E. The relation of total body potassium to height, weight, and age in normal adults. J Clin Pathol. 1972;25:512–7.
Hirai T, Yamaga R, Fujita A, Itoh T. Low body mass index is a risk factor for hyperkalaemia associated with angiotensin converting enzyme inhibitors and angiotensin II receptor blockers treatments. J Clin Pharm Ther. 2018;43:829–35.
Dijkmans BA, van Hooff JP, de Wolff FA, Mattie H. The effect of co-trimoxazole on serum creatinine. Br J Clin Pharmacol. 1981;12:701–3.
Roy MT, First MR, Myre SA, Cacini W. Effect of co-trimoxazole and sulfamethoxazole on serum creatinine in normal subjects. Ther Drug Monit. 1982;4:77–9.
Yamanaga S, Tanaka K, Kinoshita K, Kaba A, Fujii M, Ogata M, Hidaka Y, Kawabata C, Toyoda M, Uekihara S, Kashima M, Miyata A, Inadome A, Yokomizo H. Impact of Very Low-Dose Trimethoprim-Sulfamethoxazole on Serum Creatinine after Renal Transplantation: A Retrospective Study. Transplant Proc. 2020;52:1757–61.
Kastrup J, Petersen P, Bartram R, Hansen JM. The effect of trimethoprim on serum creatinine. Br J Urol. 1985;57:265–8.
Trollfors B, Wahl M, Alestig K. Co-trimoxazole, creatinine and renal function. J Infect. 1980;2:221–6.
Kainer G, Rosenberg AR. Effect of co-trimoxazole on the glomerular filtration rate of healthy adults. Chemotherapy. 1981;27:229–32.
Rafoth RJ, Onstad GR. Urea synthesis after oral protein ingestion in man. J Clin Invest. 1975;56:1170–4.
Dossetor JB, Creatininemia versus uremia. The relative significance of blood urea nitrogen and serum creatinine concentrations in azotemia. Ann Intern Med. 1966;65:1287-1299
Sands JM. Mammalian urea transporters. Annu Rev Physiol. 2003;65:543–66.
Caulder CR, Kocherla CS, Qureshi ZP, Magagnoli J, Bookstaver PB. Dose-Dependent Hyperkalemia Among Hospitalized, HIV-Infected Patients Receiving Sulfamethoxazole/Trimethoprim. Ann Pharmacother. 2020;54:852–7.
Prasad GVR, Beckley J, Mathur M, Gunasekaran M, Nash MM, Rapi L, Huang M, Zaltzman JS. Safety and efficacy of prophylaxis for Pneumocystis jirovecii pneumonia involving trimethoprim-sulfamethoxazole dose reduction in kidney transplantation. BMC Infect Dis. 2019;19:019–3944.
Al AdAwi RM, Albu-Mahmood Z, Abdelgelil M, Abdelaziz H, Stewart D, Awaisu A. Incidence of Co-Trimoxazole-Induced Hyperkalemia in a Tertiary Care Hospital. Risk Manag Healthc Policy. 2021;14:519–25.
Chen RY, Li DW, Wang JY, Zhuang SY, Wu HY, Wu JJ, Qu JW, Sun N, Zhong C, Zhu C, Zhang M, Yu YT, Yuan XD. Prophylactic effect of low-dose trimethoprim-sulfamethoxazole for Pneumocystis jirovecii pneumonia in adult recipients of kidney transplantation: a real-world data study. Int J Infect Dis. 2022;125:209–15.
Provenzano M, Puchades MJ, Garofalo C, Jongs N, D’Marco L, Andreucci M, De Nicola L, Gorriz JL, Heerspink HJL. Albuminuria-Lowering Effect of Dapagliflozin, Eplerenone, and Their Combination in Patients with Chronic Kidney Disease: A Randomized Crossover Clinical Trial. J Am Soc Nephrol. 2022;33:1569–80.
Acknowledgements
We would like to thank Editage for editing and reviewing the manuscript in the English language.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
TH and TI contributed to the study conception and design. Data collection was performed by TH and SU. Data analysis was performed by TH, TO, YK, and YS. The first draft of the manuscript was written by TH, TO, KK, KD, YK, YI, and TI and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We did not obtain consent to participate because of a retrospective observational study. The Institutional Review Board of Mie University Hospital approved the study protocol in accordance with the Declaration of Helsinki (H2023-075).
Consent for publication
We did not obtain consent to participate because of a retrospective observational study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hirai, T., Ueda, S., Ogura, T. et al. Hyperkalemic effect of drug–drug interaction between esaxerenone and trimethoprim in patients with hypertension: a pilot study. J Pharm Health Care Sci 10, 46 (2024). https://doi.org/10.1186/s40780-024-00366-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40780-024-00366-6