Abstract
Background
It is well known that molecular-targeted drugs, of which sorafenib (Nexavar®) is one, differ from previous anticancer drugs and cause various unusual adverse drug reactions. Treatment with sorafenib causes adverse drug reactions such as hand-foot skin reactions, hypertension, and diarrhea. Physicians spend a lot of time monitoring adverse drug reactions to sorafenib in outpatients. As such, at Okayama University Hospital, pharmacists and nurses have organized a medical supportive team to help physicians in this regard. However, the effectiveness of interventions for sorafenib-treated outpatients by this medical supportive team remains unclear. The purpose of this study was thus to clarify the effectiveness of interventions for sorafenib-treated outpatients by this medical supportive team.
Methods
We retrospectively studied 70 outpatients treated with sorafenib between May 2009 and December 2012 at Okayama University Hospital. These outpatients were classified into two groups, an intervention group (31 outpatients) and a non-intervention group (39 outpatients). We compared the duration of sorafenib treatment between these groups.
Results
The duration of treatment with sorafenib was significantly longer in the intervention group than in the non-intervention group. No outpatients in the intervention group discontinued sorafenib due to adverse drug reactions such as hand-foot skin reactions or diarrhea.
Conclusion
The duration of sorafenib treatment was significantly longer in the intervention group than in the non-intervention group. Our findings suggest that interventions by the medical supportive team consisting of health care professionals were effective in preventing the discontinuation of sorafenib.
Similar content being viewed by others
Background
Sorafenib (Nexavar®) is one of the oral molecular-targeted drugs used as systemic treatment in patients with unresectable hepatocellular carcinoma and advanced renal cell carcinoma [1-3]. It inhibits the activity of serine/threonine kinases of RAF, protein kinases of MEK and ERK, and blocks vascular endothelial growth factor receptors (VEGFR), platelet-derived growth factor receptors (PDGFR), the cytokine receptor c-KIT, and the receptor tyrosine kinase Flt-3 to inhibit tumor angiogenesis and tumor cell growth [4-6].
Molecular-targeted drugs were initially expected to cause less severe adverse drug reactions than previous anticancer drugs. However, they have been shown to cause various unusual adverse drug reactions. Sorafenib also causes many adverse drug reactions, such as hand-foot skin reactions, diarrhea, and hypertension [2,7,8]. Therefore, the dose of sorafenib has been decreased or sorafenib treatment has been discontinued [9,10]. In some hospitals, outpatients undergo interventions by a medical supportive team to prevent adverse drug reactions. We organized a medical supportive team consisting of pharmacists and nurse at Okayama University Hospital (Figure 1). Physicians do not have enough time in their limited consultations with outpatients to establish the adverse drug reactions of sorafenib. Pharmacists and nurse thus met with outpatients during the waiting time for blood sampling. We recorded suggestions on monitoring sheets regarding the problems encountered by patients, such as the grade of adverse drug reactions and afflictions. Furthermore, we also provided outpatients with some advice related to their problems, including adverse drug reactions or difficulties in daily life. If hand-foot skin reactions occurred, the team took pictures of the patient’s hand, foot, and skin, and posted them in an electronic medical record system. Thus, the medical supportive team supported physicians regarding the problems encountered by patients. Active participation of the medical supportive teams has been reported in the management of patients [11,12]. However, the effectiveness of interventions for patients by medical supportive teams has not been demonstrated. In our hospital, our medical supportive team initially intervented in cases with sorafenib treatment. In the present study, we examined whether a prolonged duration of sorafenib treatment in outpatients could be identified in association with interventions by the medical supportive team in our hospital.
Methods
Patients
We retrospectively screened outpatients with hepatocellular carcinoma and advanced renal cell carcinoma (70 patients) who had been treated with sorafenib between May 2009 and December 2012 at Okayama University Hospital. Outpatients were categorized according to age, gender, an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, previous therapy, metastatic site, and Child-Pugh classification for hepatocellular carcinoma patients. This study was approved by the Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences Ethics Committee (No. 650).
The roles of the medical supportive team for sorafenib treatment
Our team is composed of two pharmacists (one Japanese Society of Pharmaceutical Health Care and Sciences-certified oncology pharmacist and one board certified pharmacist in oncology pharmacy) and one nurse (certified nurse in cancer chemotherapy nursing). One pharmacist and the nurse always intervene per patient, and the frequency of intervention is once every month. We check the medical situation, dose, and adverse events before a doctor’s examination. The main work of our team is to consider the advisability of administrating particular anti-cancer agents and other prescription drugs based on patient consultation, and to make recommendations to the physicians in charge.
Outcome evaluation
We categorized outpatients into two groups based on whether or not they had been undergone on intervention by the medical supportive team in terms of the duration of sorafenib treatment. The intervention by the medical supportive team was decided according to the physician’s requirements. Patients were excluded from this study based on the guidelines for the appropriate use of sorafenib as follws: those with progressive disease, grade 2 hand-foot skin reactions more than 4 times, or grade 3 hand-foot skin reactions more than 3 times. When other non-hematotoxicities occurred, the sorafenib treatment had to be interrupted for more than 30 days. In addition, sorafenib treatment was also discontinued when the primary care physician considered continuation of the treatment to be unnecessary or a reduction in the sorafenib dose by 2 levels or more to be necessary. Adverse drug reactions were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 4.0.
Statistical analysis
Categorical variables were compared by Fisher’s exact test, and the means of the two groups were analyzed by independent t-test. The duration of treatment was analyzed using Kaplan-Meier survival curves with log-rank survival comparisons. Significance was set at P <0.05. Statistical analysis was performed with the Statistical Package for the Social Sciences software, version 21 (IBM SPSS, Inc. Chicago, IL, USA).
Results
Patient characteristics
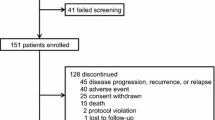
In this study, 72 outpatients were screened. Two patients were excluded from the analysis; one had not undergone an intervention by the medical supportive team and the other was only administered sorafenib for one day because of adverse drug reactions. Therefore, 70 outpatients were classified into two groups: an intervention group and a non-intervention group, according to interventions by the medical supportive team. No significant differences were observed in the baseline characteristics between the two groups, except for diagnosis (Table 1).
Duration of the sorafenib treatment
The duration of the sorafenib treatment was significantly longer in the intervention group than in the non-intervention group (P = 0.008) (Figure 2). In the non-intervention group, 7 patients stopped using sorafenib due to adverse drug reactions that were potentially tolerable. Table 2 shows the reasons for the discontinuation of sorafenib in the non-intervention group. The dosage of sorafenib was not decreased from 800 mg/day in three cases. However, sorafenib treatment was interrupted in 3 outpatients to maintain a dose of 800 mg. Furthermore, one outpatient refused to continue the sorafenib treatment due to anorexia. The dose of sorafenib was decreased to 600 mg/day in a patient with hypothyroidism; however, inn this case, sorafenib treatment was discontinued without endocrine therapy such as thyroid gland auxiliary therapy.
Activities of the medical supportive team for outpatients
Mean daily doses of sorafenib were lower in the intervention group (486.5 ± 198.6 mg (median, 480.8 mg)) than in the non-intervention group (581.8 ± 196.0 mg (median, 600 mg)) (P = 0.048). Furthermore, no significant differences were observed in the mean initial doses of sorafenib between the intervention group (709.7 ± 170.0 mg (median, 800 mg)) and the non-intervention group (702.6 ± 170.9 mg (median, 800 mg)). The medical supportive team spent an average of 37.1 ± 11.1 min per outpatient (median, 40 min), and the total number of interventions was 146. A total of 61 interventions in the intervention group concerned suggestions on medication to physicians and outpatients. Table 3 shows the medical supportive team’s suggestions to physicians and patients. The incidences of adverse drug reactions, apart from those of grades 3–4, such as hand-foot skin reaction, diarrhea, anorexia, hair loss, thrombocytopenia, hoarseness, increased serum amylase, fatigue, and stomatitis, were higher in the intervention group than in the non-intervention group (Table 4).
Discussion
In this study, the duration of treatment with sorafenib was significantly increased due to interventions by the medical supportive team for outpatients. The supportive team performed clinical management that included checking the adherence of patients to the use of moisturizing lotion for hand-foot skin reactions. If outpatients had not properly followed the instructions, we participated in pharmaceutical care including patient education for swabbing the ointment to avoid serious hand-foot skin reactions. Previous prospective or randomized trials had not determined the best management of hand-foot skin reactions caused by treatment with sorafenib. However, early use of a moisturizer was recommended to avoid hand-foot skin reaction symptoms [9,13,14]. Furthermore, the incidences of sorafenib-induced adverse drug reactions and hand-foot skin reactions were shown to be higher in Japanese patients than in European ones [10]. The medical supportive team performed clinical management that included four main activities; 1) checking adverse drug reactions before the physician’s consultation; 2) medication use evaluations based on laboratory data; 3) checking for adherence to sorafenib; and 4) providing drug information and lifestyle care points to patients in our hospital. It is hoped that the clinical activities of the medical supportive team will enhance the quality of sorafenib medication. In the present study, the discontinuation of sorafenib treatment by outpatients did not occur due to frequent communication with the medical supportive team. For this reason, the early stages of hand-foot skin reactions were detected by the medical supportive team.
On the other hand, information on the appropriate use of sorafenib suggests that treatment with it should be interrupted until recovery to Grades 0–1 when Grade 3 hand-foot skin reactions occur. Furthermore, if patients recover from this symptom, decreases of 400 mg in the dose (daily or alternate-day treatment) should be implemented when administration is resumed. The incidence of adverse drug reactions was higher in the intervention group than in the non-intervention group. These results together with the significantly low mean doses of sorafenib and higher incidence of adverse drug reactions in the intervention group suggest that the supportive team had a detailed interview with patients regarding adverse effects. Thus, interventions by the supportive team helped primary care physicians to reduce the sorafenib dose and appropriately prescribe supportive therapies. However, this study has the following limitations. Patients with advanced renal cell carcinoma were included in the non-intervention group. In patients with renal cell carcinoma, since molecular-targeted drugs other than sorafenib can be selected, there is a possibility that sorafenib treatment was readily discontinued. Some studies have suggested an association between prolongation of the sorafenib treatment period and clinical effects [15-17]. In addition, although there has been no evidence on the appropriate sorafenib dose in the Japanese, some studies have shown effects of sorafenib even at a small dose [18-20]. These findings suggest that measures against adverse effects taken by a supportive team prevent premature discontinuation of sorafenib treatment.
Conclusions
In conclusion, the medical supportive team-implemented management of pharmaceutical care contributed to continuation of the use of sorafenib in outpatients. Furthermore, the activities of the medical supportive team are important not only for outpatients treated with sorafenib, but also for those with other molecular-targeted drugs used in other hospitals.
Abbreviations
- ECOG PS:
-
Eastern cooperative oncology group performance status
- HCC:
-
Hepatocellular carcinoma
- RCC:
-
Renal cell carcinoma
- TACE:
-
Transarterial chemoembolization
- HAIC:
-
Hepatic arterial infusion chemotherapy
- RFA:
-
Radiofrequency ablation
- TAI:
-
Transcatheter arterial infusion
References
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM: Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007, 356:125–134.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J: Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008, 359:378–390.
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z: Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009, 10:25–34.
Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S: Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 2006, 5:835–844.
Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M: Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther 2008, 7:3129–3140.
Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA: BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004, 64:7099–7109.
Robert C, Soria JC, Spatz A, Le Cesne A, Malka D, Pautier P, Wechsler J, Lhomme C, Escudier B, Boige V, Armand JP, Le Chevalier T: Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol 2005, 6:491–500.
Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, Gore M, Desai AA, Patnaik A, Xiong HQ, Rowinsky E, Abbruzzese JL, Xia C, Simantov R, Schwartz B, O'Dwyer PJ: Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 2006, 24:2505–2512.
Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, Garbe C, Hauschild A, Puzanov I, Alexandrescu DT, Anderson RT, Wood L, Dutcher JP: Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist 2008, 13:1001–1011.
Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N, Kaneko S, Tsubouchi H, Suh DJ, Furuse J, Okusaka T, Tanaka K, Matsui O, Wada M, Yamaguchi I, Ohya T, Meinhardt G, Okita K: Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer 2011, 47:2117–2127.
Walko CM, Grande C: Management of common adverse events in patients treated with sorafenib: nurse and pharmacist perspective. Semin Oncol 2014, 41(Suppl 2):S17–S28.
Aimono Y, Nemoto M, Sato W, Saito Y, Aoyama Y, Joko F, Maruyama T, Kamoshida T: [Examination of the usefulness of the pharmacists' outpatient clinic for treatment with oral molecule-targeting drugs]. Gan to Kagaku Ryoho 2013, 40:901–905.
Degen A, Alter M, Schenck F, Satzger I, Volker B, Kapp A, Gutzmer R: The hand-foot-syndrome associated with medical tumor therapy - classification and management. J Dtsch Dermatol Ges 2010, 8:652–661.
Porta C, Paglino C, Imarisio I, Bonomi L: Uncovering Pandora's vase: the growing problem of new toxicities from novel anticancer agents. The case of sorafenib and sunitinib. Clin Exp Med 2007, 7:127–134.
Kawashima A, Takayama H, Arai Y, Tanigawa G, Nin M, Kajikawa J, Imazu T, Kinoshita T, Yasunaga Y, Inoue H, Nishimura K, Takada S, Nishimura K, Tsujimura A, Nonomura N: One-month relative dose intensity of not less than 50% predicts favourable progression-free survival in sorafenib therapy for advanced renal cell carcinoma in Japanese patients. Eur J Cancer 2011, 47:1521–1526.
Tanigawa G, Kawashima A, Yamaguchi S, Nishimura K, Miyoshi S, Kajikawa J, Meguro N, Yosioka T, Oka T, Hara T, Takayama H, Nonomura N: Clinical outcome and prognostic factors of sorafenib in Japanese patients with advanced renal cell carcinoma in general clinical practice. Jpn J Clin Oncol 2011, 41:1265–1270.
Okuwaki Y, Nakazawa T, Hidaka H, Shibuya A, Koizumi W: Late-onset benefit in progressive advanced hepatocellular carcinoma with continued sorafenib therapy: a case report. J Med Case Rep 2012, 6:38.
Nojiri S, Kusakabe A, Fujiwara K, Shinkai N, Matsuura K, Iio E, Miyaki T, Nomura T, Sobue S, Sano H, Hasegawa I, Ohno T, Takahashi Y, Orito E, Joh T: Clinical factors related to long-term administration of sorafenib in patients with hepatocellular carcinoma. Cancer Manag Res 2012, 4:423–429.
Wang SX, Byrnes A, Verma S, Pancoast JR, Rixe O: Complete remission of unresectable hepatocellular carcinoma treated with reduced dose of sorafenib: a case report. Target Oncol 2010, 5:59–63.
Ueda T, Imamura Y, Komaru A, Fukasawa S, Sazuka T, Suyama T, Naya Y, Nihei N, Ichikawa T, Maruoka M: Treatment outcomes of sorafenib for first line or cytokinerefractory advanced renal cell carcinoma in Japanese patients. Int J Urol 2010, 17:811–815.
Acknowledgements
We thank a native English speaker for checking the language and grammar used in this manuscript (Medical English Service, Kyoto, Japan).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MK and MA carried out the survey of electronic records, performed the statistical analysis, and drafted the manuscript. YK and TS conceived of the study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Makoto Kajizono and Megumu Aoyagi contributed equally to this work.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kajizono, M., Aoyagi, M., Kitamura, Y. et al. Effectiveness of medical supportive team for outpatients treated with sorafenib: a retrospective study. J Pharm Health Care Sci 1, 6 (2015). https://doi.org/10.1186/s40780-014-0005-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40780-014-0005-0