Abstract
Background
Worldwide, many guidelines recommend the use of expressed breast milk (EBM) and maternal expression of breast milk for the prevention and treatment of neonatal hypoglycemia. However, the impact of both practices on neonatal hypoglycemia is unclear. This study aims to determine the effectiveness of EBM and maternal expression of breast milk in preventing and treating neonatal hypoglycemia.
Methods
We registered our review in PROSPERO (CRD42022328072). We systematically reviewed five databases and four clinical trial registries to identify randomized controlled trials (RCT), non-randomized studies of intervention (NRSI), and cohort studies that compared infants who received EBM to infants who did not, and similar study designs that compared infants whose mothers expressed breast milk to infants whose mothers did not. Two independent reviewers carried out screening, data extraction, and quality assessment. The quality of included RCT, NRSI, and cohort studies were respectively assessed with the Cochrane Risk of Bias 2, Risk Of Bias In Non-randomised Studies—of Interventions, and the Newcastle–Ottawa Scale tools. Results from studies on EBM were synthesized separately from those on maternal expression of breast milk. Meta-analysis was undertaken using Revman 5.4. and fixed-effect models.
Results
None of the ten included studies was specifically designed to determine the effect of EBM or maternal expression of breast milk on neonatal hypoglycemia. The effect of EBM on neonatal hypoglycemia was not estimable. There was no difference in the risk of hypoglycaemia among neonates whose mothers expressed breast milk compared to those whose mothers did not [RR (95%CI); one RCT: 0.92 (0.77, 1.10), high-certainty evidence; one cohort: 1.10 (0.74, 1.39), poor quality study].
Conclusions
There is insufficient evidence to determine the effectiveness of EBM for preventing or treating neonatal hypoglycemia. Limited data suggests maternal breast milk expression may not alter the risk of neonatal hypoglycemia. High-quality randomized controlled trials are needed to determine the effectiveness of EBM and maternal expression of breast milk for the prevention and treatment of neonatal hypoglycemia.
Similar content being viewed by others
Background
Neonatal hypoglycemia is the most common metabolic disorder among newborn infants, affecting 5% to 15% of these infants [1] and approximately one in two of all at-risk infants [2]. The risk of neonatal hypoglycemia is highest in the first few hours after birth. This is because clamping of the umbilical cord at birth leads to termination of transplacental glucose transfer coupled with continued endogenous production of insulin by the infant [3, 4]. The risk of hypoglycemia is increased in states associated with reduced glycogen stores, increased glucose utilization, and hyperinsulinemia [3, 5]. In these states, compensatory mechanisms like the production of counterregulatory hormones (cortisol and glucagon), which trigger gluconeogenesis and glycogenolysis thus resulting in endogenous glucose production, are often delayed [3]. Risk factors associated with such states include being small or large for gestational age, an infant of a diabetic mother, preterm, asphyxiated, and hypothermic [3, 5]. Hence, a prompt exogenous supply of glucose may be important in preventing neonatal hypoglycemia, especially in at-risk infants. Despite being the commonest metabolic disorder in newborn infants, there is no consensus on the threshold that define neonatal hypoglycaemia [3, 6]. However, its most widely accepted definition is blood glucose concentration less than 47 mg/dL (2.6 mmol/L), with variations even among pediatric professional organizations [6].
Hypoglycemia in the newborn, especially when severe, recurrent, or not promptly detected and treated, is associated with far-reaching poor perinatal and long-term neurodevelopmental outcomes [7, 8]. These include neonatal seizures, apnea, death, developmental delays, seizure disorder, visual-motor impairment, and executive dysfunction [5, 7,8,9]. Prevention and management options for neonatal hypoglycemia include breastfeeding [10], oral glucose gel [11], intravenous dextrose [4], medications such as hydrocortisone and glucagon [4], and feeding with formula milk or expressed breast milk (EBM) [10].
Both feeding EBM (mother’s or donor’s) to infants and the expression of breast milk by mothers to prevent or treat hypoglycemia are incorporated into many neonatal management guidelines worldwide [5, 10, 12,13,14]. While EBM provides ready non-formula feeds for the infant, the expression of breast milk, in addition, may be associated with improved lactogenesis [15]. Thus, these two interventions, although closely related, may potentially have independent effects on neonatal hypoglycemia. Both practices are recommended, increasingly encouraged, and practised for at-risk and hypoglycemic infants [10, 12,13,14, 16], yet their effectiveness in preventing and treating neonatal hypoglycemia is uncertain. Hence, this systematic review aims to review the evidence on the effectiveness of feeding EBM and maternal expression of breast milk for preventing and managing neonatal hypoglycemia.
Methods
We registered our study protocol in the International prospective register of systematic reviews (PROSPERO)-CRD42022328072 [17]. In addition to investigating the effectiveness of EBM for the prevention and treatment of neonatal hypoglycemia, our registered protocol was revised (expanded) to determine the effectiveness of maternal expression of breastmilk for the prevention and treatment of neonatal hypoglycemia as this is also a commonly recommended practice in neonatal care [12, 14]. Hence, our protocol was expanded to include relevant review questions, participants, interventions, and comparators [17]. Our review is reported following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines [18].
Eligibility criteria
We included studies that compared infants (≤ 28 days old) who received EBM (mother’s or donor’s) to infants who received no intervention or other interventions (breastfeeding, formula milk, dextrose gel, intravenous dextrose, placebo, or a combination of these) as well as studies that compared infants of mothers who expressed breast milk with infants whose mothers did not express breast milk. Randomized controlled trials (RCTs), quasi-RCTs, non-randomized studies of intervention (NRSI), cluster randomized trials, cohort and case–control studies, and abstracts (if they provided enough information) were included. There were no language or geographic restrictions. Study protocols and those without comparison groups were excluded.
Search strategy
We searched OVID MEDLINE, Embase (OVID), CINAHL Plus, Cochrane Library, and Scopus from inception to 19th May 2022, and trial registration repositories, Current Controlled Trials, Clinical Trials, Australian and New Zealand Clinical Trials Registry, and the World Health Organization International Clinical Trials Registry Portal (Additional file 1). In addition, we searched references of previous relevant reviews for additional studies for relevant articles. Results from the literature search were imported into Covidence software [19], where studies were screened. Two authors (OIO and JH/LL) independently reviewed all studies for eligibility. Any discrepancies were resolved after discussion or involving a third author (JH/LL).
Study selection
We included all RCTs, NRSI, and cohort studies that compared infants given EBM to those given no or other interventions and studies that compared infants whose mothers expressed breast milk with those whose mothers did not express breast milk. We did not identify any relevant case–control study.
The primary outcome was neonatal hypoglycemia (study-defined, i.e., as defined by study authors) after the intervention. Secondary outcomes were neonatal hypoglycemia (any blood glucose concentration ≤ 2.6 mmol/L), receipt of treatment for hypoglycemia (study-defined), number of episodes of hypoglycemia (study-defined), severity of hypoglycemia (lowest recorded blood glucose concentration or study-defined), separation from the mother for any treatment before discharge home (infant nursed in an environment not in the same room as the mother, e.g., for neonatal intensive care unit (NICU) admission or special care baby unit (SCBU) admission), separation from the mother for treatment of hypoglycemia before discharge home (infant nursed in an environment not in the same room as the mother, e.g., NICU admission or SCBU for treatment of hypoglycemia), injury attributable to hypoglycemia on brain imaging (study defined), duration of initial hospital stay, breast milk feeding exclusively (infant only receives breast milk without any other drink or food) from birth to discharge, breast milk feeding exclusively after discharge, breastfeeding (any) after discharge, exclusive breast milk feeding (infant only receives breast milk without any other drink or food) at six months after birth, cost of intervention (as measured by study), cost of neonatal care (as measured by the study).
Data extraction, synthesis, and analysis
Two authors (OIO and LL) independently extracted data using pre-designed data extraction forms. Data extracted include study design, location, year of publication, population, intervention used, control exposure, and whether the study was primarily designed to prevent or treat hypoglycemia. The risk of bias for outcomes was independently assessed by two authors (OIO and LL) using the Cochrane Risk of Bias -2 tool [20] for RCTs, Risk Of Bias In Non-randomized Studies of Interventions tool (ROBINS-I) [21] for NRSI, and the Newcastle–Ottawa Scale [22] for cohort studies. For RCTs and NRSI, the risk of bias was assessed for each outcome, while for cohort studies, the risk of bias was assessed for each study. For RCTs, study outcomes were assessed as having low, some concerns or high risk of bias [20], while for NRSI, they were assessed as having low, moderate, serious, or critical risk of bias [21]. Cohort studies were assessed as being of good or poor quality [22]. Discrepancies were resolved with discussion. We planned to assess publication bias by visual inspection of a funnel plot, plotting the study effect size against the sample size, but this was not possible because of few relevant studies.
We calculated the risk ratio (RR) with 95% confidence intervals (CIs) for dichotomous outcomes and the mean difference (MD) with 95% CIs were calculated for continuous outcomes. A p-value of < 0.05 denoted statistical significance. The median (first and third quartiles) were converted to mean (SD) for studies that report median [23]. The mean (SD) for studies with two or more EBM groups (e.g. raw vs. pasteurized) were merged to create a single group as recommended by the Cochrane Collaboration [24]. For studies that presented results using graphs, WebPlotDigitizer was used to extract numbers from the graph [25].
Meta-analysis is a valid, accurate and precise method for synthesizing estimates reported by at least two studies [26, 27]. Hence, for outcomes reported by a minimum of two studies, meta-analysis was undertaken using Revman 5.4 [28] and fixed-effect models. I2 and χ2 were calculated for each analysis and describe the percentage of variability in effect estimates due to heterogeneity. If we observed substantial heterogeneity (I2 > 50% and P < 0.10 in the χ2 test), we planned to explore possible causes in a sensitivity analysis.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) [29] was used to assess the certainty of evidence for RCTs reporting any of the following outcomes: neonatal hypoglycemia (study-defined), receipt of treatment for hypoglycemia (study‐defined, any treatment ‐ oral dextrose gel, intravenous dextrose, or other drug therapy) during the initial hospital stay, separation from the mother for any treatment before discharge home, separation from the mother for treatment of hypoglycemia before discharge home, breast milk feeding exclusively from birth to discharge, exclusive breast milk feeding at six months. Results from studies on EBM were synthesized and reported separately from those on expression of breast milk.
Results
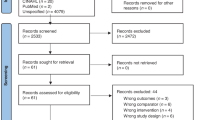
We identified 6 912 studies, of which six were additional papers identified through a review of references (Fig. 1). After removing duplicates, 3 761 studies were screened. After title and abstract screening, 3 663 studies were excluded. One study could not be retrieved despite contacting the authors. Of the remaining 97 studies for which we conducted full text review, we included 10 studies, two of which were included in the meta-analysis and the remaining eight were included in the qualitative analysis. None of the studies specifically investigated the use of EBM or breast milk expression for preventing and treating neonatal hypoglycemia. Of the three ongoing studies [30,31,32], one [30] is investigating the effectiveness of human donor milk for the treatment of neonatal hypoglycemia among breastfed infants.
Study characteristics
The included studies were five RCTs [16, 33,34,35,36], two NRSIs [37, 38], and three cohort studies [39,40,41] (Table 1). Five of the studies were about the effects of EBM and five about maternal expression of breast milk. The majority (90%) were conducted in high-income countries, while one (10%) was conducted in a low-middle income country (India). The publications spanned more than six decades (1958 to 2022), with sample sizes ranging from 20 to 656 infants.
The number of infants included in this review was 2 224. The five studies on EBM included 512 infants, of whom 281 received EBM (mother’s [211], donor [29], mother’s or donor [31], or unspecified [10]) and 231 received other interventions (formula [109], or a combination of breastfeeding, no milk and formula [122]). The five studies on breast milk expression included 1 712 infants, 744 mothers of whom expressed breast milk, and 968 did not. All mothers who expressed breast milk did so antenatally. Three studies included mothers with pre-gestational or gestational diabetes [16, 40, 41], one involved low-risk nulliparous individuals [36] and one involved mothers in an obstetric ward [38].
Risk of bias
The risk of bias by outcomes reported by RCTs and NRSI for studies on expressed breast milk and maternal expression of breast milk varied widely from low to high risk of bias (Table 2).
Similarly, the only cohort study that reported on EBM and a relevant outcome was of good quality, while the two cohort studies that reported on maternal expression of breast milk and relevant outcomes were of poor quality (Table 3).
Outcomes for studies on expressed breast milk
Neonatal hypoglycemia
One RCT [35] reported no hypoglycemic episodes in infants who were and were not given EBM, although authors did not report any blood glucose concentrations nor explain how hypoglycemia was defined (20 infants, RR – not estimable, very low certainty evidence, high risk of bias) (Fig. 2).
Duration of initial hospital stay
Three studies [33, 34, 37] compared the duration of hospital admission among infants given EBM and infants given other interventions. One RCT [33] reported no difference in the duration of initial hospital stay of infants who were fed EBM compared to infants who had other interventions (53 infants, MD [95% CI]: -9.33 [-32.07, 13.40] days, p = 0.42, some concerns about risk of bias) (Fig. 2). Similarly, one NRSI [37] reported the duration of hospital stay among infants fed expressed mother’s milk was not different from infants fed infant formula (143 infants, MD [95% CI]: -2.00 [-12.39, 8.39] days, p = 0.71, low risk of bias). One RCT [34] reported that among infants who developed an infection, the duration of initial hospital stay was shorter among infants given breast milk compared to infants given formula, but no supporting data or statistical measures were reported (62 infants, MD not estimable, high risk of bias).
Other outcomes
None of the other pre-specified outcomes were reported. However, one cohort study [39] reported that the change in blood glucose concentration was not different in infants fed EBM compared to infants who had other interventions (227 infants, MD [95% CI]: -1.4 [-3.7, 0.9]mg/dL, p = 0.25, good quality study). In addition, one RCT [35] reported that fasting blood glucose concentrations were lower at 24 h but higher at one to four weeks (measured weekly) after birth in infants fed EBM compared to infants fed formula (20 infants, high risk of bias, MD [95% CI] -0.52 [-0.77, -0.27] mmol/L, p < 0.0001 at 24 h; 1.02 [0.72, 1.32] mmol/L, p < 0.00001 at one week; 0.73 [0.49, 0.97] mmol/L, p < 0.00001 at two weeks; 1.14 [0.88, 1.40] mmol/L, p < 0.00001 at three weeks; 0.63 [0.36, 0.90] mmol/L, p < 0.00001 at four weeks).
Outcomes for studies on the expression of breast milk
Neonatal hypoglycemia
Two studies (one RCT [16] and one cohort [40]) reported that the risk of hypoglycemia was not different in infants whose mothers expressed breast milk compared to infants whose mothers did not (RCT- 630 infants, RR [95% CI]: 0.92 [0.77, 1.10], p = 0.38, high certainty evidence, low risk of bias; cohort—303 infants, RR [95% CI]: 1.01 [0.74, 1.39], p = 0.93, poor quality study) (Fig. 3).
Separation from mother for any treatment
Two RCTs [16, 36] reported that the risk of separation from the mother for any treatment was not different between infants whose mothers expressed breast milk compared to infants whose mothers did not (2 studies, 668 infants, RR [95% CI]: 1.08 [0.75, 1.54], p = 0.69, I2 = 0; P = 0.53, low certainty evidence, 1 RCT at low risk of bias, the other at high risk of bias). In contrast, one cohort study [41] reported that infants whose mothers expressed breast milk had a higher risk of being separated from their mother (SCBU admission) compared to infants whose mothers did not express breast milk (81 infants, RR [95% CI]: 2.75 [1.05, 7.23], p = 0.04, poor quality study) (Fig. 3).
Separation from mother for the treatment of hypoglycemia
One RCT [16] reported the risk of separation from the mother for the treatment of hypoglycemia was similar among infants whose mothers did compared to infants whose mothers did not express breast milk antenatally (89 infants, RR [95% CI]: 1.16 [0.69, 1.95], p = 0.57, low certainty evidence, low risk of bias) (Fig. 3).
Duration of initial hospital stay
One RCT [16] reported no difference in the duration of initial hospital stay among infants whose mothers expressed breast milk antenatally compared to infants whose mothers did not (632 infants, MD [95% CI]: -1.20 [-9.88, 7.48] days, p = 0.79, low risk of bias) (Fig. 3).
Breastfeeding outcomes
Two studies (one RCT [16] and one NRSI [38]) reported that infants of mothers who expressed breast milk compared to infants whose mothers who did not were not more likely to be exclusively breast fed at discharge [38] or until seven days if still an in-patient [16] (RCT—632 infants, RR [95% CI]: 1.15 [0.99, 1.33], p = 0.07), some concerns about risk of bias; NRSI—656 infants, RR [95% CI]: 1.01 [0.97, 1.05], p = 0.63, serious risk of bias) (Fig. 4). In contrast, a cohort study [40] reported that infants whose mothers expressed breast milk compared to infants whose mothers did not were more likely to be exclusively breastfed until discharge (313 infants, RR [95% CI]: 1.50 [1.29, 1.74], p < 0.00001, poor quality study).
Two RCTs [16, 36] reported no significant difference in exclusive breast milk feeding rates at three to four months among infants whose mothers expressed breast milk antenatally compared to infants whose mothers did not (604 infants, RR [95% CI]: 1.09 [0.95, 1.25], p = 0.20, I2 = 0%; P = 0.87, some concerns about risk of bias with both studies) (Fig. 4).
Two RCTs [16, 36] reported that the rates of any breastfeeding three to four months after birth were similar in infants whose mothers expressed breast milk antenatally compared to infants whose mothers did not (604 infants, RR [95% CI]: 1.01 [0.94, 1.08], p = 0.30, I2 = 7%; P = 0.84, some concerns about risk of bias with both studies) (Fig. 4).
Other outcomes
None of our other pre-specified outcomes were reported by any of the five studies on breast milk expression.
The certainty of each GRADE outcome was assessed as very low, low, or high [29] (Table 4).
Discussion
Our study has systematically reviewed the evidence for the effectiveness of giving EBM to infants and the mother’s expression of breast milk for prevention and treatment of neonatal hypoglycemia and other outcomes, including the duration of initial hospital stay, separation from the mother for any treatment or the treatment of hypoglycemia, and breastfeeding.
Despite the widespread practice and recommendations of feeding EBM to infants [5, 10, 12,13,14] and encouraging mothers to express breast milk [12, 14, 16] to prevent and treat neonatal hypoglycemia, we found no published study specifically designed to assess the effectiveness of these practices. However, a parallel group RCT is underway to determine the effectiveness of donor human milk supplementation in treating hypoglycemia in breastfed infants [30].
Breast milk, in addition to having adequate nutrients for optimal growth and development in the first six months of life, also has anti-infective, immunomodulatory, and anti-inflammatory benefits [42, 44], which are associated with improved short- and long-term health outcomes [43]. However, there have been conflicting reports on whether it increases blood glucose concentrations. Rees et al. [44] reported that among breastfed infants, there was a significant increase in blood glucose concentrations of 9.6 mg/dL when fed donor human milk (DHM) and 7.8 mg/dL when fed formula. In contrast, Harris et al. [39] reported a significant increase in blood glucose concentration following formula feeds but no change in the blood glucose concentration of hypoglycemic infants fed mother’s EBM in the first 48 h after birth. This could be because of the different sources of breast milk. There have been concerns about the adequacy of volume and hence available calories of mother’s milk in the first few days after birth, since lactation is often not well established in this period [45]. For example, Harris et al. [39] reported that the median breast milk volume (0.5 mL/kg) available to feed infants was substantially smaller than the median volume of formula (4.5 mL/kg) given to the infants. Since the greatest risk of neonatal hypoglycemia is in the first few days after birth, when maternal lactation may not be well established, to determine the effectiveness of EBM in preventing and treating neonatal hypoglycemia, future studies should consider the use of donor human milk as a supplement to mother’s milk, if required.
Our finding that breast milk expression had no significant effect on neonatal hypoglycemia may be surprising because the expression of breast milk provides milk feeds for the infant and also potentially improves the initiation and establishment of lactogenesis [46]. The authors of the RCT [16] that reported this outcome, however, noted that while the volume of milk expressed by mothers ranged from zero to 905mls, the number of expressing episodes ranged from one to 59 times. The wide variation in these variables may be responsible for the reported lack of benefit on neonatal hypoglycemia. Further studies are needed to determine the optimal frequency of expression and breast milk volume required to potentially prevent and treat neonatal hypoglycemia.
While the RCTs [16, 36] included in our review showed with low certainty that maternal expression of breast milk was not significantly associated with the separation of the infant from the mother for any treatment or for the treatment of hypoglycemia, the included cohort study [41] reported that infants whose mothers expressed breast milk had more than a two-and-a-half times higher risk of being separated from their mothers. The authors of this cohort study attributed this to the lower gestational age at birth in the group of infants whose mothers expressed breast milk. While it has been hypothesized that the expression of breast milk causes the release of oxytocin, which may lead to preterm birth, several other studies have not shown a significant reduction in the gestational age at birth of infants whose mothers expressed breast milk antenatally compared to infants who did not [16, 36, 40].
The NRSI [38] at high risk of bias showed a benefit of antenatal expression of breast milk on exclusive breastfeeding at discharge, and the RCT by Forster et al. [16] reported that antenatal expression of breast milk is effective in achieving exclusive breast milk feeding in the first 24 h after birth. However, other included studies (RCTs) showed neither benefit nor harm of antenatal breast milk expression on exclusive breastfeeding until discharge, or three-to-four months, or any breastfeeding at three-to-four months, suggesting that any possible short-term benefits of antenatal expression of breast milk on exclusive breastfeeding do not persist after the first few days.
Our systematic review and meta-analysis has some strengths. To the best of our knowledge, this is the first systematic review to determine the effectiveness of EBM and breast milk expression in preventing and treating neonatal hypoglycemia, although these are widely recommended and practised. As mechanisms that produce the desired outcomes may differ for these two interventions which are often linked, it is essential that studies on EBM and its expression are considered separately, as these are both recommended in many neonatal hypoglycemia management guidelines. Similarly, we have identified important knowledge and logistic gaps to be considered in future studies that may be designed to determine the effectiveness of EBM and the expression of breast milk in preventing and treating neonatal hypoglycemia.
Our study also has limitations. Although some studies with variable risk of bias in our review reported the prevalence of hypoglycemia, none were specifically designed to determine the effectiveness of the interventions for preventing and treating neonatal hypoglycemia. This underscores the need for more focused, high-quality studies. Similarly, all studies we reviewed on breastfeeding outcomes either had some concerns or were at high risk of bias for these outcomes, and our findings on breastfeeding outcomes need to be interpreted with this in mind. Thirdly, many outcomes of interest (number and severity of hypoglycemic episodes, injury attributable to hypoglycemia on neuroimaging, cost of intervention, and cost of neonatal care) were not reported in the included studies. Hence, we could not synthesize any evidence on these outcomes.
Conclusions
Given the few studies with variable risk of bias, we found insufficient evidence for the effectiveness of EBM for the prevention and treatment of neonatal hypoglycemia. There is high certainty evidence that breast milk expression may not alter the risk of neonatal hypoglycemia, and low certainty evidence of no benefit nor harm for the separation of the infant from the mother for any treatment or the treatment of hypoglycemia. Further high-quality RCTs are needed that are specifically designed to determine the effectiveness of EBM and breast milk expression in preventing and treating neonatal hypoglycemia and report on other important outcomes, including number and severity of hypoglycemic episodes, injury attributable to hypoglycemia on neuroimaging, cost of intervention, and cost of neonatal care.
Availability of data and materials
Data access requests are to be submitted to the Data Access Committee via researchhub@auckland.ac.nz. Data will be shared with researchers with a sound proposal on reasonable request.
Abbreviations
- EBM:
-
Expressed breast milk
- RCT:
-
Randomised controlled trial
- NRSI:
-
Non-randomised study of intervention
- PROSPERO:
-
International prospective register of systematic reviews
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- ROBINS-I:
-
Risk Of Bias In Non-randomized Studies of Interventions tool
- NICU:
-
Neonatal Intensive Care Unit
- SCBU:
-
Special care baby unit
- I2 :
-
Inconsistency Index
- GRADE:
-
Grading of Recommendations Assessment, Development and Evaluation
- DHM:
-
Donor human milk
References
Hay WW, Raju TN, Higgins RD, Kalhan SC, Devaskar SU. Knowledge gaps and research needs for understanding and treating neonatal hypoglycemia: Workshop report from Eunice Kennedy Shriver National Institute of Child Health and Human Development. J Pediatr. 2009;155(5):612–7.
Harris DL, Weston PJ, Harding JE. Incidence of neonatal hypoglycemia in babies identified as at risk. J Pediatr. 2012;161(5):787–91.
Edwards T, Harding JE. Clinical aspects of neonatal hypoglycemia: a mini review. Front Pediatr. 2021;8:562251. https://doi.org/10.3389/fped.2020.562251.
Sweet CB, Grayson S, Polak M. Management strategies for neonatal hypoglycemia. J Pediatr Pharmacol Ther. 2013;18(3):199–208.
Starship clinical guidelines. Hypoglycaemia in the neonate. 2019. Cited 2022 Sep 1. Available from: https://starship.org.nz/guidelines/hypoglycaemia-in-the-neonate/.
Shah R, Harding J, Brown J, Mckinlay C. Neonatal glycaemia and neurodevelopmental outcomes: A systematic review and meta-analysis. Neonatology. 2019;115(2):116–26.
Kamana KC, Sumisti S, Hua Z. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. 2015;9(66):14–20.
McKinlay CJD, Alsweiler JM, Anstice NS, Burakevych N, Chakraborty A, Chase JG, et al. Association of neonatal glycemia with neurodevelopmental outcomes at 4.5 years. JAMA Pediatr. 2017;171(10):972–83.
Kapoor D, Sidharth, Sharma S, Patra B, Mukherjee SB, Pemde HK. Electroclinical spectrum of childhood epilepsy secondary to neonatal hypoglycemic brain injury in a low resource setting: a 10-year experience. Seizure. 2020;79:90–4.
British Association of Perinatal Medicine. Identification and management of neonatal hypoglycaemia in the full term infant. 2017. Cited 2022 Aug 29; Available from: https://hubble-live-assets.s3.amazonaws.com/bapm/attachment/file/53/Identification_and_Management_of_Neonatal_Hypoglycaemia_in_the__full_term_infant_-_A_Framework_for_Practice_revised_Oct_2017.pdf.
Edwards T, Liu G, Hegarty JE, Crowther CA, Alsweiler J, Harding JE. Oral dextrose gel to prevent hypoglycaemia in at-risk neonates. Cochrane Database Syst Rev. 2021;: CD012152. https://doi.org/10.1002/14651858.CD012152.pub3.
Wight N, Marinelli KA. ABM Clinical Protocol #1: Guidelines for Blood Glucose Monitoring and Treatment of Hypoglycemia in Term and Late-Preterm Neonates. Breastfeed Med. 2014;9(4):173–9.
Hutt Valley Maternity Centre. Neonatal hypoglycaemia: prevention and management. 2021. Available from: http://www.huttmaternity.org.nz/health-professionals/policies-guidelines/neonatal-hypoglycaemia-guideline-2021.pdf.
Safer Care Victoria. Hypoglycaemia in neonates clinical guidelines. 2022. Cited 2022 Sep 1. Available from: https://www.safercare.vic.gov.au/clinical-guidance/neonatal/hypoglycaemia-in-neonates.
Fok D, Aris IM, Ho J, Chan YH, Rauff M, Lui JKC, et al. Early initiation and regular breast milk expression reduces risk of lactogenesis ii delay in at-risk singaporean mothers in a randomised trial. Singapore Med J. 2019;60(2):80–8.
Forster DA, Moorhead AM, Jacobs SE, Davis PG, Walker SP, McEgan KM, et al. Advising women with diabetes in pregnancy to express breastmilk in late pregnancy (Diabetes and Antenatal Milk Expressing [DAME]): a multicentre, unblinded, randomised controlled trial. Lancet. 2017;389(10085):2204–13.
Harding JE, Martis R, Oladimeji OI, Luling L. Expressed breast milk for the prevention and treatment of neonatal hypoglycaemia – a systematic review and meta-analysis. PROSPERO. 2022 [Cited 2022 Sep 1]. Available from: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=328072.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12.
Covidence. Covidence systematic review software [Computer Software]. Veritas health innovation. 2017. [Cited 2022 Aug 8]. Available from: www.covidence.org.
Higgins JPT, Sterne JAC, Savović J, Page MJ, Hróbjartsson A, Boutron I, Reeves B, Eldridge S. A revised tool for assessing risk of bias in randomized trials In: Chandler J, McKenzie J, Boutron I WV (editors). Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). Cochrane Methods. Cochrane Database of Systematic Reviews. 2019. Cited 2022 Sep 2. Available from: https://www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. 2016;355: i4919. https://doi.org/10.1136/bmj.i4919.
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2021. Cited 2022 Mar 24. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14(135):https://doi.org/10.1186/1471-2288-14-135.
Higgins JPT GS (editors). How to include multiple groups from one study. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. 2011. Cited 2022 Sep 15. Available from: https://handbook-5-1.cochrane.org/chapter_16/16_5_4_how_to_include_multiple_groups_from_one_study.htm.
Rohatgi A. WebPlotDigitizer - Extract data from plots, images, and maps. 2022 Cited 2022 Sep 21. Available from: https://automeris.io/WebPlotDigitizer/.
Valentine JC, Pigott TD, Rothstein HR. How many studies do you need? A primer on statistical power for meta-analysis. J Educ Behav Stat. 2010;35(2):215–47.
Ahn E, Kang H. Introduction to systematic review and meta-analysis. Korean J Anesthesiol. 2018;71(2):103–12.
RevMan Web 2016. Review manager Web (RevMan Web). The Cochrane Collaboration; 2016. Available from: http://community.cochrane.org/tools/review-production-tools/revman-5.
GRADE working group. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Schünemann H, Brozek J, Guyatt G OA, editor. 2013 Cited 2022 Mar 24. Available from: guidelinedevelopment.org/handbook.
Thoene M. Effectiveness of Donor Human Milk Supplementation for the Treatment of Hypoglycemia in the Breastfed Infant. ClinicalTrials.gov. 2019. Cited 2022 Sep 12. Available from: https://clinicaltrials.gov/ct2/show/NCT04030312.
Colaizy T, Walsh M, Shankaran S, Laptook AR, Cotten M, Carlton D, et al. Donor Milk vs. Formula in Extremely Low Birth Weight (ELBW) Infants. ClinicalTrials.gov. 2021. Cited 2022 Sep 12. Available from: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01535608/full.
Prollius A. The Effect of Prenatal Hand Expression on the Rate of Exclusive Breastfeeding to Two Months. ClinicalTrials.gov. 2021. Cited 2022 Sep 12. Available from: https://clinicaltrials.gov/ct2/show/NCT05066438?term=antenatal+breast+milk+expression&draw=2&rank=5.
Cristofalo EA, Schanler RJ, Blanco CL, Sullivan S, Trawoeger R, Kiechl-Kohlendorfer U, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. 2013;163(6):1592–5.
Narayanan I, Prakash K, Gujral VV. The value of human milk in the prevention of infection in the high-risk low-birth-weight infant. J Pediatr. 1981;99(3):496–8.
Schultz K, Soltesz G, Mestyan J. The metabolic consequences of human milk and formula feeding in premature Infants. Acta Pædiatrica. 1980;69(5):647–52.
Demirci JR, Glasser M, Himes KP, Sereika SM. Structured antenatal milk expression education for nulliparous pregnant people: results of a pilot, randomized controlled trial in the United States. Int Breastfeed J. 2022;17(1):50. https://doi.org/10.1186/s13006-022-00491-8.
Cossey V, Vanhole C, Verhaegen J, Schuermans A. Intestinal colonization patterns of staphylococci in preterm infants in relation to type of enteral feeding and bacteremia. Breastfeed Med. 2014;9(2):79–85.
Ingelman-Sundberg A. The value of antenatal massage of nipples and expression of colostrum. Int J Obstet Gynaecol. 1958;65(3):448–9.
Harris DL, Gamble GD, Weston PJ, Harding JE. What happens to blood glucose concentrations after oral treatment for neonatal hypoglycemia? J Pediatr. 2017;190:136–41.
Casey JRR, Banks J, Braniff K, Buettner P, Heal C. The effects of expressing antenatal colostrum in women with diabetes in pregnancy: a retrospective cohort study. Aust N Z J Obstet Gynaecol. 2019;59(6):811–8.
Soltani H, Scott AMS. Antenatal breast expression in women with diabetes: Outcomes from a retrospective cohort study. Int Breastfeed J. 2012;7:18. https://doi.org/10.1186/1746-4358-7-18.
Quitadamo PA, Comegna L, Cristalli P. Anti-Infective, Anti-Inflammatory, and Immunomodulatory Properties of Breast Milk Factors for the Protection of Infants in the Pandemic From COVID-19. Front Public Heal. 2021;8(8):589736. https://doi.org/10.3389/fpubh.2020.589736.
Kramer MS. “Breast is best”: the evidence. Early Hum Dev. 2010;86(11):729–32.
Rees DJ, Carr NR, Ponnapakkam AP. Effect of donor breastmilk vs formula supplementation on blood glucose levels in neonates at risk for hypoglycemia. Pediatrics. 2021;147:330–2.
Harding JE, Harris DL, Hegarty JE, Alsweiler JM, McKinlay CJ. An emerging evidence base for the management of neonatal hypoglycaemia. Early Hum Dev. 2017;104:51–6.
Uikey PA, Agrawal P, Khandale S. Antenatal breast milk expression at term increases postnatal lactational performance. Int J Reprod Contraception Obstet Gynecol. 2017;6:2438.
Acknowledgements
Alissa Hackett, University of Auckland’s Research Services Advisor who helped refine our search strategy.
Funding
This work was funded in part by grants from the New Zealand Ministry of Business and Employment and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health (NIH; grant R01HD091075). Some authors receive funding for other reasons: OIO – University of Auckland Senior Health Research Scholarship, JH and CC– funded partly by Health Research Council of New Zealand (19/690), and LL – Aotearoa Foundation (9909494). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the NIH.
Author information
Authors and Affiliations
Contributions
Conceptualization and initial design of study—all authors; search strategy and screening of studies – OIO, JH and LL; Data extraction and risk of bias assessment – OIO and LL; interpretation and review of data—OIO, JH and LL; initial draft—OIO; critical revision and approval of the final copy of the article – all authors; accountability for all aspects of review – all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Search strategy for databases and Clinical Trial Registries. Data contains the search strategy (key words and MESH terms) used in this systematic review.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Oladimeji, O.I., Harding, J.E., Crowther, C.A. et al. Expressed breast milk and maternal expression of breast milk for the prevention and treatment of neonatal hypoglycemia: a systematic review and meta-analysis. matern health, neonatol and perinatol 9, 12 (2023). https://doi.org/10.1186/s40748-023-00166-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40748-023-00166-0