Abstract
Purpose
To histologically examine early bone formation around transmucosal implants and to evaluate the influence of surface characteristics on early peri-implant bone healing using a miniature pig model. For this, commercially available dental implants with a rough zirconia (YTZP) surface were compared to surface-modified Ti control implants at 4 and 8 weeks after placement.
Methods
Immediately following the extraction of six mandibular premolars, 20 two-piece, tissue-level, screw-shaped YTZP implants (Patent™ Standard Zirconia Implant ø4.1 × 11 mm) with a modified rough blasted before sintering surface were inserted in four adult miniature pigs. In addition, four titanium (Ti) tissue-level implants (Straumann® Standard RN ø4.1 × 10 mm Roxolid®) with a moderate surface (SLActive®), one per animal, were placed as control implants. A histological analysis was performed on the hard tissues after 4 and 8 weeks of transmucosal healing.
Results
The results show a high rate of osseointegration of the test YTZP dental implants at 4 and 8 weeks following insertion. At 4 weeks, a bone-to-implant contact ratio (BIC) of 73.7% (SD ± 16.8) for the test implants (n = 10) and 58.5% for the first control implant was achieved. The second control implant had to be excluded from analysis. At 8 weeks, a BIC of 82.4% (SD ± 16.9) for the test implants (n = 9) and 93.6% (SD ± 9.1) (n = 2) for the control implant was achieved. No statistical difference was observed comparing 4 and 8 weeks YTZP data (p = 0.126).
Conclusions
The results indicate a predictable osseointegration of immediate zirconia implants with a modified YTZP implant surface and a high degree of BIC present at 4 weeks following insertion. After 8 weeks of healing both the zirconia implants and the Ti implants show a BIC indicating full osseointegration. Further studies involving a larger sample size with more time points are needed to confirm these results.
Similar content being viewed by others
Background
The long-term clinical success of dental implants, in terms of function and esthetics, relies on sustained tissue integration. Osseointegration, defined as direct bone apposition to the implant surface, can occur with implants made of various materials [1, 2, 3, 4, 5, 6, 7, 8, 9, 10]. A wide range of materials have been used for dental implants, with commercially pure (cp) titanium (Ti) as the most common. Surface modification of Ti implants by hydroxyapatite coating, sandblasting, and/or acid etching is usually performed to increase bone apposition [11]. Although cp Ti exhibits high biocompatibility and favorable mechanical properties, a disadvantage is the grayish color, which can lead to undesirable esthetics when mucosal tissue retracts and the Ti surface becomes exposed. In addition, the potential accumulation of Ti particles in local lymph nodes is a limitation [12, 13, 14, 15].
Initially, various ceramics were used as implant material; however, their use is currently insignificant [4, 16, 17, 18]. Yttria-stabilized zirconia (YTZP), a ceramic material with wide application and accepted long-term results in the field of orthopedic medical implants, has been introduced as a new dental implant material [19, 20, 21, 22, 23]. Its successful application was demonstrated in several preclinical and clinical studies as evidenced by excellent tissue integration and positive clinical outcomes [19, 20, 21, 22, 23, 24, 25, 26, 27].
Moreover, the inflammatory response induced by ceramic particles is considerably lower compared with that induced by Ti particles, clearly indicating the biocompatibility of such ceramics [28, 29].
However, there are few studies that have explored the healing mechanisms around zirconia implants, also referred to ZrO2 implants. Some studies indicate that smooth ZrO2 surfaces result in comparatively long healing periods, although these only included single time points without controls [19, 20, 23, 24].
The goal of this study was to qualitatively and quantitatively examine early bone apposition and the bone healing mechanisms of commercially available rough zirconia dental implants at 4 and 8 weeks after insertion and to compare the tissue reaction to surface-modified Ti control implants. The null hypothesis assumed was that the rough zirconia implant surfaces tested would perform similarly to well-documented reference titanium surfaces, with similar bone-to-implant contact (BIC) values as the primary outcome parameter and similar new bone formation as the secondary outcome parameter.
Methods
Study implants
Twenty commercially available two-piece screw-shaped tissue-level YTZP implants with a modified, roughened surface were tested (Patent™ Standard Zirconia Implant, 2-piece, REF 2S4111-2p ø4.1 × 11 mm EP 5.2, LOT 40290920c01; Zircon Medical Management AG) (Fig. 1a–d). The implants are produced from yttria-stabilized ZrO2 in a patented manufacturing process. The blasted before sintering (BBS) surface of the intraosseous portion of the YTZP implant has a surface roughness (Ra) of 5.7 µm. The transmucosal portion has a machined surface with a surface roughness of 1.25 µm. Straumann Ti implants [Straumann® Standard RN ø4.1 mm Roxolid® (85% Ti, 15% ZrO2) SLActive® 10 mm, REF 033.532 s, LOT EPW62, Straumann Group AG] with an intraosseous SLActive® surface roughness of Ra = 2.2 µm and a transmucosal portion with machined surface served as control. Surface roughness data are given by the respective manufacturer.
Specipig® animal model
The study was performed at Specipig Barcelona, a certified and authorized breeding, supplier and animal experimentation center. Ethical approval for this study was provided by the Direcció General del Medi Natural i Biodiversitat, Servei de Biodiversitat i Protecció dels Animals (C/Dr. Roux, 80, 08017 Barcelona, document IMP-115).
Four miniature pigs (species Sus scrofa domesticus and Specipig® miniature breed) were used for the study. The pigs were male, older than 20 months, and weighed more than 20 kg. They were identified by their ear tags. Antibiotics were administered on the day of surgery and continued for 1 week. Two pigs were randomly assigned to the 4-week, and the remaining to the 8-week healing group following surgery. The pigs were provided soft food for the first 4 weeks after surgery, after that, they were provided regular pellet-based food. Healing and well-being were monitored regularly.
Surgical procedure
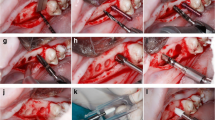
Under full sedation and local anesthesia, a flap was elevated in the mandibular premolar region to provide good visibility and access to the sites. All three premolars on each side were extracted without trauma (Fig. 2a). Because all removed teeth were twin-rooted, any remaining root fragments were carefully removed, and the extraction sockets were systematically curetted and rinsed using sterile saline solution in order to clean all sites properly. For each tooth position, implant site preparation was conducted in one of the sockets according to the manufacturer’s instructions, resulting in three osteotomies on each side of the mandible. Immediately after extraction, five test implants and one Ti control implant were randomly assigned and placed in each animal with an average insertion torque of 36 ± 9.8 Ncm. Neither surgical bone leveling nor socket grafting or membrane was applied before flap closure. Flaps were carefully adapted and closed with resorbable sutures (Vicryl 5-0 Ethicon, Johnson & Johnson). All test and control implants were subjected to transmucosal healing (Fig. 2b).
Scanning electron microscopic (SEM) evaluation of the YTZP implant surface
Three test YTPZ implants were removed from the original package and mounted on alumina stub holders. The implants were sputter-coated with a 6-nm-thick platinum layer, and examined by scanning electron microscopy (Zeiss Supra 40VP; Zeiss, Oberkochen, Germany).
Termination and preparation of ground sections for light microscopy
After 4 and 8 weeks of transmucosal healing, two animals at a time were killed and block sections were harvested.
The blocks were fixed by immersing in 10% neutral-buffered formalin and prepared for non-demineralized ground sections according to the technique of Donath and Breuner [30]. The specimens were washed with 0.01 M PBS buffer (Sigma-Aldrich) and dehydrated for approximately 4 days at each step in an ascending series of ethanol–pure water, with absolute ethanol (Sigma-Aldrich) for the final step.
The specimens were infiltrated with a graded series of ethanol and Technovit® 7200 VLC (Kulzer, Wehrheim, Germany) embedding resin for at least 12 days at standard temperature with constant shaking. The specimens were then placed into three consecutive containers of 100% Technovit® 7200 VLC for 24 h. Following dehydration and infiltration, the specimens were placed into embedding molds filled with fresh Technovit® 7200 VLC and polymerized with 450-nm light for 10 h, while cooling with running tap water to avoid temperatures exceeding 40 °C.
Polymerized blocks were sliced in the bucco-lingual direction using an Exakt cutting unit (Exakt, Norderstedt, Germany). The slices were reduced by microgrinding and polishing with an Exakt grinding unit to an even thickness of 80–120 µm. A final polish was applied with 0.1-μm diamond polishing paste. The sections were stained with Sanderson’s RBS (Dorn & Hart, Villa Park, US) and counterstained with acid fuchsin. The sections were cover-slipped for analysis using a Leica M205A stereo light microscope and a Leica DM6B light microscope.
Data analysis
The total percentage of bone-to-implant contact (BIC) as a primary outcome measurement was evaluated using ImageAccess software (Imagic, Glattbrugg, Switzerland). Both the lingual and buccal BIC per implant was measured beginning at the point of first BIC to the last point of BIC. The mean percentage of BIC and the standard deviation (SD) was calculated for test and control implants after 4 and 8 weeks.
Statistical analysis was performed using two-tailed t-tests for the comparison of the BIC values of the YTZP test implants 4 weeks versus 8 weeks following insertion. Crestal bone resorption was measured from the implant shoulder to the most coronal BIC and evaluated using a two-tailed t-test.
Results
The healing was uneventful, and no implants were lost during the healing phase. At 4 weeks, 10 implants from the test group and two from the control group were available. One control group implant was accidentally placed in the root remnant of a tooth and caused severe inflammatory reactions and bone resorption. This implant was excluded from further histologic evaluation.
After 8 weeks, nine implants from the test group and two implants from the control group were available for analysis. The histology of one test implant could not be completed because of a failure during the preparation of the section. Figure 3 presents selective light microscopic micrographs of representative specimens after 4 and 8 weeks of transmucosal healing. A high rate of osseointegration, already 4 weeks after implantation, was observed for both types of implants. At 8 weeks, all implants were completely osseointegrated.
The peri-implant alveolar crest was generally characterized by crestal bone resorption. For both implant types, crestal remodeling at the buccal aspect produced dehiscence defects, whereas gap-type bone defects occurred at lingual sites. Crestal bone resorption was measured from the implant shoulder to the most coronal BIC: after 4 weeks a mean bone loss of 5.1 mm (SD ± 0.26) at the lingual sides, and 6.3 mm (SD ± 0.62) at the buccal sides was observed; after 8 weeks a significant higher bone loss of 6.1 mm (SD ± 0.7) was found at the lingual sides (p = 0.037), and 6.7 mm (SD ± 0.45) at the buccal sides.
Histology and histomorphometry of the peri-implant bone
After 4 weeks of healing, the test and control implants exhibited direct osseous integration and presented a mean BIC of 73.7% (SD ± 16.8) (n = 10) for the test implants and 58.5% for the control implant. Distance osteogenesis from local bone towards the implant surface was observed. Moreover, contact osteogenesis starting from contact points between the local bone with the implant was observed directly on and along the surface. Both types of new bone formation were present in the test and control implants (Fig. 4a and c). Ongoing bone formation, indicated by the presence of a not yet mineralized, collagenous osteoid lined by numerous osteoblasts, was evident (Fig. 4b and d).
LM micrographs demonstrating the status of osseointegration after 4 weeks with YTZP implants (a, b) and Ti-SLA implants (c, d). Note the presence of distance osteogenesis (DO), outgoing from local bone, and contact osteogenesis (CO), outgoing from contact points between local bone with the implant. Also note the presence of an osteoid (O) lined with osteoblasts (OB), indicating ongoing bone formation (b, d). Scale bars: (a and c) 100 µm; (b and d) 300 µm
Test and control implants were completely osseointegrated after 8 weeks of healing, confirming the null hypothesis. As primary outcome measurement, mean BIC values of 82.4% (SD ± 16.9) for the nine test implants and 93.6% (SD ± 9.1) for the two control implants were observed. No statistical difference was observed comparing 4 and 8 weeks YTZP data (p = 0.126). The spaces between the osteotomy walls and the implant surface were filled with newly formed bone and small bone marrow chambers (Figs. 5a–d, 6a, b).
LM micrographs revealing the buccal peri-implant mucosa after 8 weeks. a Note the intimately adherent junctional epithelium (JE) and its apical end (aJE). b Higher magnification of a. Note the keratinized oral epithelium (OE) and the non-keratinized junctional epithelium (JE). c Note the collagenous fibers (black arrows) in the peri-implant connective tissue running parallel to the implant surface. Scale bars: 300 µm
Discussion
In this study, all evaluated test and control implants were clinically and histologically osseointegrated into our miniature pig model. A well-established method to evaluate the interface and the status between the bone and implant is the measurement of the BIC area by histomorphometric analysis. In the present study, BIC was measured at 4 and 8 weeks. At 4 weeks, the mean BIC for all YTZP test implants (73.7%) was higher compared to the Ti control implant (58.5%). At 8 weeks, the mean BIC ratio was comparable for both materials and in accordance with the criteria defined by Albrektsson et al. [31], indicating successful osseointegration. Other studies comparing zirconia implants to titanium controls confirm our findings: Gahlert et al. [32] report a BIC of 54.6% (ZrO2) and 44.1% (Ti), and Linares et al. [33] of 85.4% (ZrO2) and 84.3% (Ti), respectively, at 8 weeks.
Several studies describe a stronger bone response for zirconia implants with rough surfaces compared with Zirconia implants with machined surfaces [32, 34, 35]. A study conducted in mini pigs compared machined and sand-blasted zirconia implants and concludes that surface characteristics strongly influence bone integration [34]. In particular, ZrO2 implants were biomechanically and histomorphometrically compared with sand-blasted, large grit, acid-etched (SLA) Ti implants. The results indicated higher bone stability values for the SLA Ti implants, followed by the rough zirconia and the machined zirconia surface, suggesting that rough zirconia implants achieve higher anchorage in bone compared with machined zirconia implants.
In a mini pig study conducted by Gahlert et al. [32], the authors compared the bone tissue response of surface-modified Zirconia and Ti implants. For the test group, cylindrical low-pressure injection-molded zirconia implants were created, including an acid-etched surface. SLA Ti implants of identical shape served as controls. A histomorphometric analysis of bone density and BIC ratio revealed no significant differences between the two implant types. The interface between newly formed bone and both the zirconia and Ti surfaces revealed a thin layer of bone following the contour of the threads, thus indicating osteoconductive bone formation.
Another study with mini pigs compared one-piece yttria-stabilized ZrO2 implants with a sand-blasted surface (Ra = 1.0 µm) to SLA Ti implants (Ra = 2.75 µm) [35]. The ZrO2 implants were subjected to alternating submerged and non-submerged healing, whereas the Ti implants were all submerged. After a healing period of 4 weeks, both types of submerged implants achieved a BIC ratio of 53%. For the non-submerged implants, some epithelial downgrowth and crestal bone resorption was reported, resulting in a BIC of 48%. The upper-third of the implants exhibited bone formation by contact osteogenesis for both submerged and non-submerged implants. Apically to this zone, distant osteogenesis was observed in all groups.
Cionca and coworkers suggested that the surface on the intraosseous portion of a ZrO2 dental implant should be as rough as possible [36]. However, because of the tough material properties of zirconia and depending on the manufacturing process, there is significant variation in the surface roughness between the different manufacturers. The zirconia implant used in the present study, had an especially rough surface, which was approximately five times rougher than other documented ZrO2 implants [25, 37]. This could be one explanation for the markedly higher BIC value after 4 weeks of healing compared to other studies in the mini-pig mandible using smoother ZrO2 implants [34, 35].
Another experimental study conducted in mini pigs [38] compared ZrO2 implants with a modified ablative surface and acid-etched Ti implants following 1, 4, and 12 weeks of healing using scanning electron microscopy. At 1 week, a marked attachment of bone was detected, which was further increased to intimate bone contact after 4 weeks. At 12 weeks, osseointegration was complete. It is unclear whether the tissue observed after 1 week was indeed newly formed bone or the result of the implant insertion procedure. It is known, that during implant placement a rough implant surface acts like micro-grained sandpaper. It scratches along the walls of the cortical and trabecular bone and grinds bone at the interface resulting in several micron-thick smear layers consisting of bone debris and blood, covering part of the implant surface immediately following installation [39, 40, 41]. The presence of bone debris is of crucial importance to speed up the initial bone formation as described by Bosshardt et al. [41]. The bone debris guides new bone formation by distance osteogenesis to the implant surface.
In this study, bone resorption following extraction and implant installation was more pronounced on the buccal as compared to lingual sides. This is well in line with earlier findings reported by Botticelli and coworkers [42].
The results of the present study have to be interpreted cautiously given the small number of test and control implants. In general, a robust statistical analysis of the results could not be performed because of the small sample size. Unfortunately, one control implant was placed in the remnants of a tooth root, which created inflammation and bone resorption and had to be excluded from analysis, limiting the interpretability of the study results. BIC evaluation and interpretability could have been further influenced by the fact, that implant installation causes bone debris and a bone smear layer at the bone–implant interface, as discussed before. The rougher test implant surface (Ra = 5.7 µm) may have generated more osteogenic bone debris and a smear layer compared with the control implants (Ra = 1.25 µm) and increased the BIC at 4 weeks over proportional. On the other hand, a rougher implant surface may also be a source of enhanced bacterial adhesion and must be taken into account for clinical application [43, 44]. Therefore, larger studies are needed to confirm the present results.
Conclusion
The results indicate rapid and predictable osseointegration of immediately placed YTZP implants with a rough BBS surface. A high BIC value after 4 weeks of healing was maintained after 8 weeks. The results of this histologic study demonstrate a higher mean BIC on the tested rough zirconia implants compared with previous studies evaluating surface-modified ZrO2 implants in similar animal models. The evaluated BBS ZrO2 surface may be classified as highly osteoconductive.
Availability of data and materials
All data and materials are available through Peter Schupbach.
Abbreviations
- BBS:
-
Blasted before sintering
- BIC:
-
Bone-to-implant contact
- CO:
-
Contact osteogenesis
- DO:
-
Distance osteogenesis
- JE:
-
Junctional epithelium
- O:
-
Osteoid
- OB:
-
Osteoblast
- SLA:
-
Sand-blasted, large grit, acid-etched
- Ti:
-
Titanium
- Zirconia:
-
Zirconium dioxide ZrO2
- ZrO2 :
-
Zirconium dioxide
- YTZP:
-
Yttria-stabilized zirconia
References
Markle DH, Grenoble DE, Melrose RJ. Histologic evaluation of vitreous carbon endosteal implants in dogs. Biomater Med Devices Artif Organs. 1975;3(1):97–114.
Young FA, Spector M, Kresch CH. Porous titanium endosseous dental implants in Rhesus monkeys: microradiography and histological evaluation. J Biomed Mater Res. 1979;13(6):843–56.
Klawitter JJ, Weinstein AM, Cooke FW, Peterson LJ, Pennel BM, McKinney RV Jr. An evaluation of porous alumina ceramic dental implants. J Dent Res. 1977;56(7):768–76.
Pedersen KN. Tissue reaction to submerged ceramic tooth root implants. An experimental study in monkeys. Acta Odontol Scand. 1979;37(6):347–52.
Peterson LJ, Pennel BM, McKinney RV Jr, Klawitter JJ, Weinstein AM. Clinical, radiographical, and histological evaluation of porous rooted polymethylmethacrylate dental implants. J Dent Res. 1979;58(1):489–96.
De Lange GL, De Putter C, De Groot K, Burger EH. A clinical, radiographic, and histological evaluation of permucosal dental implants of dense hydroxylapatite in dogs. J Dent Res. 1989;68(3):509–18.
Gross HN, Holmes RE. Surgical retrieval and histologic evaluation of an endosteal implant: a case report with clinical, radiographic and microscopic observations. J Oral Implantol. 1989;15(2):104–13.
Steflik DE, McKinney RV Jr, Koth DL. Ultrastructural comparisons of ceramic and titanium dental implants in vivo: a scanning electron microscopic study. J Biomed Mater Res. 1989;23(8):895–909.
Berglundh T, Abrahamsson I, Lang NP, Lindhe J. De novo alveolar bone formation adjacent to endosseous implants. Clin Oral Implants Res. 2003;14(3):251–62.
Abrahamsson I, Berglundh T, Linder E, Lang NP, Lindhe J. Early bone formation adjacent to rough and turned endosseous implant surfaces. An experimental study in the dog. Clin Oral Implants Res. 2004;15(4):381–92.
Lazzara RJ, Testori T, Trisi P, Porter SS, Weinstein RL. A human histologic analysis of osseotite and machined surfaces using implants with 2 opposing surfaces. Int J Periodontics Restorative Dent. 1999;19(2):117–29.
Weingart D, Steinemann S, Schilli W, et al. Titanium deposition in regional lymph nodes after insertion of titanium screw implants in maxillofacial region. Int J Oral Maxillofac Surg. 1994;23(6 Pt 2):450–2.
Frisken KW, Dandie GW, Lugowski S, Jordan G. A study of titanium release into body organs following the insertion of single threaded screw implants into the mandibles of sheep. Aust Dent J. 2002;47(3):214–7.
Kim KT, Eo MY, Nguyen TTH, Kim SM. General review of titanium toxicity. Int J Implant Dent. 2019;5(1):10. https://doi.org/10.1186/s40729-019-0162-x.
Müller-Heupt LK, Schiegnitz E, Kaya S, Jacobi-Gresser E, Kämmerer PW, Al-Nawas B. Diagnostic tests for titanium hypersensitivity in implant dentistry: a systematic review of the literature. Int J Implant Dent. 2022;8(1):29.
McKinney RV Jr, Koth DL, Steflik DE. The single-crystal sapphire endosseous dental implant. I. Material characteristics and placement techniques. J Oral Implantol. 1982;10(3):487–503.
McKinney RV Jr, Koth DL, Steflik DE. The single crystal sapphire endosseous dental implant. II. Two-year results of clinical animal trials. J Oral Implantol. 1983;10(4):619–38.
Koth DL, McKinney RV Jr, Steflik DE. The single-crystal sapphire endosseous dental implant. III. Preliminary human clinical trials. J Oral Implantol. 1983;11(1):10–24.
Akagawa Y, Ichikawa Y, Nikai H, Tsuru H. Interface histology of unloaded and early loaded partially stabilized zirconia endosseous implant in initial bone healing. J Prosthet Dent. 1993;69(6):599–604.
Scarano A, Di Carlo F, Quaranta M, Piatelli A. Bone response to zirconia ceramic implants: an experimental study in rabbits. J Oral Implantol. 2003;29(1):8–12.
Schliephake H, Hefti T, Schlottig F, Gédet P, Staedt H. Mechanical anchorage and peri-implant bone formation of surface-modified zirconia in minipigs. J Clin Periodontol. 2010;37(9):818–28.
Kohal RJ, Klaus G. A zirconia implant-crown system: a case report. Int J Periodontics Restorative Dent. 2004;24(2):147–53.
Kohal RJ, Weng D, Bachle M, Strub JR. Loaded custom-made zirconia and titanium implants show similar osseointegration: an animal experiment. J Periodontol. 2004;75(9):1262–8.
Sennerby L, Dasmah A, Larsson B, Iverhed M. Bone tissue responses to surface-modified zirconia implants: a histomorphometric and removal torque study in the rabbit. Clin Implant Dent Relat Res. 2005;7(s1):s13–20.
Oliva J, Oliva X, Oliva JD. One-year follow-up of first consecutive 100 Zirconia dental implants in humans: a comparison of 2 different rough surfaces. Int J Oral Maxillofac Implants. 2007;22(3):430–5.
Mostafa D, Aboushelib M. Bioactive-hybrid-zirconia implant surface for enhancing osseointegration: an in vivo study. Int J Implant Dent. 2018;4(1):20. https://doi.org/10.1186/s40729-018-0129-3.
Vilor-Fernández M, García-De-La-Fuente AM, Marichalar-Mendia X, Estefanía-Fresco R, Aguirre-Zorzano LA. Single tooth restoration in the maxillary esthetic zone using a one-piece ceramic implant with 1 year of follow-up: case series [published correction appears in Int J Implant Dent. 2021 Nov 24;7(1):114]. Int J Implant Dent. 2021;7(1):26. https://doi.org/10.1186/s40729-021-00308-z.
Ichikawa Y, Akagawa Y, Nikai H, Tsuru H. Tissue compatibility and stability of a new zirconia ceramic in vivo. J Prosthet Dent. 1992;68(2):322–6.
Warashina H, Sakano S, Kitamura S, et al. Biological reaction to alumina, zirconia, titanium and polyethylene particles implanted onto murine calvaria. Biomaterials. 2003;24:3655–61.
Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J Oral Pathol. 1982;11(4):318–26.
Albrektsson T. On long-term maintenance of the osseointegrated response. Aust Prosthodont J. 1993;7:15–24.
Gahlert M, Röhling S, Wieland M, et al. Osseointegration of zirconia and titanium dental implants: a histological and histomorphometric study in the maxilla of pigs. Clin Oral Implants Res. 2009;20(11):1247–53.
Liñares A, Grize L, Muñoz F, et al. Histological assessment of hard and soft tissues surrounding a novel ceramic implant: a pilot study in the minipig. J Clin Periodontol. 2016;43(6):538–46. https://doi.org/10.1111/jcpe.12543.
Gahlert M, Gudehus Z, Eichhorn S. Biomechanical and histomorphometric comparison between zirconia implants with varying surface textures and a titanium implant in the maxilla of miniature pigs. Clin Oral Implants Res. 2007;8(5):662–8.
Stadlinger B, Hennig M, Eckelt U, et al. Comparison of zirconia and titanium implants after a short healing period. A pilot study in minipigs. Int J Oral Maxillofac Surg. 2010;39(6):585–92.
Cionca N, Hashim D, Mombelli A. Zirconia dental implants: where are we now, and where are we heading? Periodontol. 2017;73(1):241–58.
Roehling S, Schlegel KA, Woelfler H, Gahlert M. Zirconia compared to titanium dental implants in preclinical studies—a systematic review and meta-analysis. Clin Oral Implants Res. 2019;30(5):365–95.
Depprich R, Zipprich H, Ommerborn M, et al. Osseointegration of zirconia implants: an SEM observation of the bone-implant interface. Head Face Med. 2008;4:25.
Dhore CR, Snel SJ, Jacques SV, Naert IE, Walboomers XF, Jansen JA. In vitro osteogenic potential of bone debris resulting from placement of titanium screw-type implants. Clin Oral Implants Res. 2008;19(6):606–11.
Tabassum A, Walboomers F, Wolke JG, Meijer GJ, Jansen JA. Influence of the surgical technique and surface roughness on the primary stability of an implant in artificial bone with a density equivalent to the maxillary bone: a laboratory study. Clin Implant Dent Relat Res. 2011;13(4):269–78.
Bosshardt DD, Salvi GE, Huynh-Ba G, Ivanovski S, Donos N, Lang NP. The role of bone debris in early healing adjacent to hydrophilic and hydrophobic implant surfaces in man. Clin Oral Implants Res. 2011;22(4):357–64.
Botticelli D, Berglundh T, Lindhe J. Hard-tissue alterations following immediate implant placement in extraction sites. J Clin Periodontol. 2004;31(10):820–8.
Kligman S, Ren Z, Chung CH, Perillo MA, Chang YC, Koo H, Zheng Z, Li C. The impact of dental implant surface modifications on osseointegration and biofilm formation. J Clin Med. 2021;10(8):1641.
Roehling S, Astasov-Frauenhoffer M, Hauser-Gerspach I, Braissant O, Woelfler H, Waltimo T, Kniha H, Gahlert M. In vitro biofilm formation on titanium and zirconia implant surfaces. J Periodontol. 2017;88:298–307.
Acknowledgements
Editing, proofreading and submission support was provided by medtextpert, Switzerland.
Funding
This study was supported by Zircon Medical AG, Switzerland.
Author information
Authors and Affiliations
Contributions
RG: clinic and preparation of a draft manuscript. PS: histology, preparation of data and a draft manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval for this study was provided by the Direcció General del Medi Natural i Biodiversitat, Servei de Biodiversitat i Protecció dels Animals (C/Dr. Roux, 80, 08017 Barcelona, document IMP-115).
Consent for publication
Not applicable.
Competing interests
Peter Schupbach received financial support for the histologic evaluation from Zircon Medical AG. The study support had no influence on the study design, sample collection, analysis, and interpretation of data, nor in the writing of the report and the decision to submit this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Glauser, R., Schupbach, P. Early bone formation around immediately placed two-piece tissue-level zirconia implants with a modified surface: an experimental study in the miniature pig mandible. Int J Implant Dent 8, 37 (2022). https://doi.org/10.1186/s40729-022-00437-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40729-022-00437-z