Abstract
Thin films reinforced with chitosan and cellulose nanocrystals (CNC) were produced using the casting process. In this study, the impact of plasticisers and sizing agents such as glycerol and polyvinyl alcohol (PVA) respectively on morphological, structural, thermal, and mechanical properties was investigated. The results showed the blends of CNC/PVA/glycerol gave better results when compared to films produced by blends of chitosan/PVA/glycerol films and chitosan/CNC/PVA/glycerol films. The UV spectroscopy showed 65% transmittance for chitosan/PVA/glycerol films, while the film of CNC/PVA/glycerol showed transmittance of 40%. The transmittance of chitosan/CNC/PVA/glycerol showed 75%. The films formed by the combination of CNC/PVA/glycerol showed better stress/strain properties than other films. The films of all combinations showed good thermal stability between the range of 350 and 450 °C. The morphological study using SEM revealed smooth texture for all the films. The study suggests that the films produced may be used for the food packaging applications due to its thermal stability and stress/strain properties.
Similar content being viewed by others
Introduction
About 140 million aeons back, initial monocotyledonous terrestrial plants gave way to seagrasses, a form of polyphyletic angiosperm plant (Pfeifer 2021). The fact that large seagrass beds serve as a habitat for micro invertebrates makes them one of the most crucial ecosystems on the world. Knowledge about the breakdown of polysaccharides in stringy seagrass can assist researchers to better comprehend the extended constancy of certain polysaccharide units. Seagrass cell walls primarily consist of cellulose, just like those of angiosperm land plants (Pfeifer and Classen 2020).
The Decapoda order includes Portunus segnis and Penaeus kerathurus, which are both members of the Portunidae and Penaeidae families. Blue crabs and shrimp are the only crustaceans with coloured shells covering their bodies. The western Atlantic Ocean and the southeast Indian coast are both home to the P. segnis species. It spreads swiftly and has recently been found in Japan, the North and East Mediterranean, and the eastern Atlantic. It is currently widely distributed in the Mediterranean, especially in Tunisian jackets, causing several issues with fishing. Due to their excellent organoleptic features, blue crabs are also harvested and commercialised for use as human food (Hamdi et al. 2017).
The most prevalent naturally occurring polymer on the globe is cellulose, and it will soon overtake all other chemical resources in importance (Schurz 1999). For the expanding need for naturally safe and biocompatible materials, it is seen as a virtually infinite source of raw materials. Nevertheless, the full capability of cellulose has not yet been realised due to the nonexistence of an ecologically approachable method and the small quantity of popular solvents that swiftly disperse cellulose (Qi et al. 2009). In modern years, cellulose nanocrystals (CNC) have become a resourceful nanomaterial that can serve as a strengthening agent. Cellulose, the most prevalent natural polymer, is acid hydrolysed to produce cellulose nanocrystals, in which the crystalline parts are retained, while the amorphous areas are detached. The scientific community is becoming more and more interested in these nanocrystals because of their excellent characteristics, including their nanometric size, vast surface area, great strength, non-toxicity, high crystallinity, and biodegradability (Costa et al. 2021; Mujtaba et al. 2017).
There are many uses for chitosan, including chelating heavy metals to prevent water pollution, membrane separation in biotechnology and medicine, and food packaging (Dong et al. 2004; Muzzarelli 1996). Various businesses, including those in the pharmaceutical, cosmetic, paper, and food sectors, use polyvinyl alcohol (PVA) either unaided or in conjunction with another polymers (Azuma et al. 1992). Due to their exceptional biocompatibility and useful physical properties, polyvinyl alcohol/chitosan blends have gained popularity as a recyclable polymer blend that can be used in eco-friendly materials like wrapping, membrane separation, dye adsorption, and biomedical materials for sustained release, improved comfort, reduced discomfort, and tissue engineering (Ali and Abderraouf 2017: Tripathi et al. 2010).
Several researchers have talked about using chitosan/PVA as a packaging material. The ability of PVA and the nanofillers made from chitosan to create films is what gives the finished films their antibacterial properties (Bano et al. 2014). The food products in the films are preserved, thanks to this function. Extraction of nanofillers, such as CNC, is economical, ecologically approachable, and has a high mechanical strength (Azizi et al. 2014a, b), and PVA nanoparticles have been utilised for drug delivery (Dey et al. 2021; Reddy et al. 2016). However, there has not been much emphasis on the usage of chitosan nanoparticles as nanofillers in food packaging in the past. There are several different substrates, including chitosan, nanoclays, polylactic acid (PLA), polyethylene terephthalate (PET), polyethyleneimine (PEI), and polyvinyl alcohol (PVA), which can be used to produce dense, ultrathin nanocellulose films (Ferrer et al. 2017; Vilarinho et al. 2018). However, due to its sluggish process and limited suitability for large samples, it is still a long way from being employed in industry (Anukiruthika et al. 2020). Biobased plastics are often created from a variety of raw materials sourced mostly from plants as well as microbial sources such polyhydroxyalkanoates (PHAs) and polylactic acid, which can be degraded by microorganisms (Yaradoddi et al. 2020).
Packaging for food and beverages has long been made of plastic. Due to their superior mechanical forte and gas barricade capabilities, plastics have proven to be perfect for use in food packing purposes. Alarms have been raised concerning the utilisation of plastic in food packaging, though plastics’ inability to biodegrade, which causes environmental contamination, is the initial cause for concern. Plastic pollution produced from petroleum and synthetic polymers is not instantly broken down by microbes. As a result, landfills may become overflowing with plastic waste, harming the environment (Nayigiziki 2016). Due to their detrimental effects on social health and the environs, scientists in food and packaging industries around the world have recently begun to examine many petroleum-based composite films. We need to lessen the detrimental effects of petroleum-based products if we want to decrease our reliance on fossil fuels. Combining a synthetical polymer with a biopolymer is one approach to overcoming the issues with composite films made of petroleum. Chitosan and polyvinyl alcohol (PVA), for instance, can be combined to create a biosynthetic polymer blend that has demonstrated promise in a few studies (El-Hefian et al. 2010; Nakano et al. 2007).
The polymers such as chitosan and CNC can be used to create certain value-added goods, including thin films for use in food packaging. The current study’s objective is to separate CNCs from waste seagrass materials and chitosan from Portunus pelagicus shells discovered on the Thondi shore. The study also aims to characterise these thin films utilising equipment such as Fourier transform infrared spectroscopy (FTIR), X-ray powder diffractometry (XRD), U-V spectroscopy, thermogravimetric analysis (TGA), universal testing machine (UTM), and scanning electron microscopy (SEM) and thereby to identify the films with better characteristic properties with respect to packaging materials.
Materials and methods
Sample collection
The chitosan samples were obtained from crabs which were collected from a crab processing facility in Thondi (9.74°N, 79.01°E). Crab shells were crushed and pulverised before being used to extract chitosan. Cellulose nanocrystals were recovered from deteriorating seagrass along Thondi’s shore. The build-up of rotting seagrass on the Thondi coast is typical, and these seagrass samples were collected, rinsed, and dried in the sun for cellulose nanocrystal processing.
Extraction of chitosan
The removal of chitin from carcases comprises several processes, including decolourisation, demineralisation, and deproteinisation. By refluxing the sample in 10 ml of NaClO at 100 °C for 10 min, the sample was decoloured. This process was replicated for maximum discolouration. By demineralizing the samples in 20 ml of 1-M hydrochloric acid for 15 min at 70 °C, the minerals that were present in the shells were taken out. By soaking the sample in 20 ml of 1 M (NaOH) for 20 min at 100 °C, the material was deproteinised. The sample was thoroughly rinsed with distilled water while waiting for the pH neutralisation and then dehydrated at room temperature to extract the chitin. The chitin was then intermingled in 15 ml of 45% NaOH for 1 day at 110 °C. The finished product was permeated and thoroughly rinsed in distilled water to bring the pH level back to normal (Kaya et al. 2015: Rasti et al. 2017. The extracted chitosan samples were characterised in the previous study (Varma and Vasudevan, 2020).
Extraction of cellulose nanocrystals
The cellulose from the dead seagrass samples is extracted using a modified version of the process given by Szymaska-Chargot et al. (Szymanska-Chargot et al. 2017). The samples of seagrass were first dried and pulverised. The sample was then boiled in water for 10 min to remove sugar, phenolic chemicals, and water-soluble polysaccharides. After that, 1-M hydrochloric acid is used to acid hydrolyse the residue. The sample (30 g) was subjected to 100 ml of 1-M hydrochloric acid at 85 °C for 30 min. The highest amount of pectic polysaccharides is removed by repeating this process. The following stage involves alkaline hydrolysis using 1-M sodium hydroxide. The sample is permitted to react with 1-M sodium hydroxide for 30 min at 85 °C. To guarantee that all the hemicellulose is removed, this technique is carried out three times. The cellulose extraction process is completed with the bleaching treatment, which entails exposing the sample to 1–2% sodium hypochlorite for an hour at 95 °C. By repeating the decolorising procedure twice, cellulose is produced. The cellulose is repeatedly rinsed under running water until the pH balances out.
Acid hydrolysis was used to extract cellulose nanocrystals, as propositioned by Sai and Mitra (2020), with minor modifications. The 5 g of cellulose that was collected was given the chance to react with 100 ml of water and 55 wt.% H2SO4 in a 100-ml volume. The samples are incubated at 45 °C for 30 min while being vigorously agitated. After 30 min, the process was ended by putting in 1 l of distilled water. The pH of the mixture was then brought to neutrality by unremitting centrifugation, and water dialysis after the precipitate was spun for 10 min at 10,000 rpm. The precipitate is then sonicated at 225 W for 10 min. For further research, the created suspension of cellulose nanocrystals is put away at 4 °C. The extracted cellulose nanocrystals were characterised in the previous study (Varma and Vasudevan, 2022).
Preparation of composite films with polymer blends
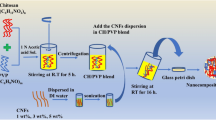
The composite films were made using chitosan, cellulose nanocrystals, polyvinyl alcohol, and glycerol utilising the casting technique (Annu and Ahmed 2021). The PVA solution was created by refluxing 5 mg of PVA in 90 ml of distilled water. The liquid was stirred and blended by continuous whirling at 45 °C for 30 min. Chitosan solution was created by blending 2 mg of chitosan with 2% acetic acid in 10 ml. These two mixtures of PVA solution and chitosan solution were stirred in a magnetic stirrer. The solution was stirred again with the addition of 2-ml glycerol. In a hot air oven set to 50 °C, the resultant solution was then cast onto Petri plates and dried for 4 h. This technique produced a film, which was carefully removed from the Petri plate.
The second film was created by combining cellulose nanocrystals, PVA, and glycerol. Like before, the PVA solution was created. The obtained PVA solution was combined with 2 mg of cellulose nanocrystals in glycerol at 50 °C for 30 min using a magnetic stirrer. The finished mixture was decanted onto a Petri plate and permitted to dry for 4 h at 50 °C in a hot air oven. The samples were removed from the Petri dish and saved for later examination.
The final film was created using chitosan, cellulose nanocrystals, PVA, and glycerol mixtures. The PVA and chitosan solutions were created using the procedure mentioned above. The PVA and chitosan solutions, 2-mg CNC, and 2-ml glycerol were all stirred at the same time. Once the mixture has been blended, the solution is cast onto a petri plate and retained in a hot air oven for 4 h at 50 °C. The film is peeled and stored until further characterisation.
Characterisation of polymer films
Fourier transform infrared spectroscopy (FTIR)
Using a Perkins-Elmer spectrometer, the biocomposite films were submitted to KBr-aided FTIR examination (Spectrum RX1, MA, USA). In the frequency range of 4000–400 cm−1, the FTIR was analysed at a resolution of 4 cm−1.
X-ray powder diffractometry (XRD)
X'Pert PRO PAN analytical was used to detect the crystalline quality of the biocomposite films (The Netherlands). Cu K = 1.5406 Å was used to run the instrument at 40 kV and 30 mA. The minimum step size (2θ) used during X-ray diffraction (XRD) measurement is 0.001°. The XRD scan covered an angle range of 0 to 80° (2θ), where the starting position of 2θ was 10.0231 and the end position was 80.9231.
UV–vis spectroscopy
The light barrier qualities of the three film samples were investigated using UV spectroscopy. Using a UV–visible spectrophotometer, the samples were exposed to wavelengths ranging from 100 to 1100 nm (Lambda 365 Perkin Elmer).
Thermogravimetric analysis (TGA)
The change in weight of the samples was measured using a Mettler Toledo TGA 2. Temperatures scale from 0 to 600 °C was used to evaluate thermal stability. The temperature was increased at a specific rate of 20°/min, and the gas used for the analysis was nitrogen because of its inert nature, and it allows to maintain a controlled atmosphere.
Universal testing machine (UTM)
The tensile strength of the film samples was measured with the universal testing machine (UTM) (Tinius Olsen, Poland). The film samples were cut such that the length of the films was 6 cm and the width was 3 cm, and then the samples were introduced to the UTM for the tensile strength analysis.
Scanning electron microscopy (SEM)
For examination, the samples were placed on carbon tape and spin coated with a gold coating. The TESCAN Oxford was used to create the SEM micrographs, and the SEM micrographs were taken at various magnifications.
Results
Fourier transform infrared spectroscopy (FTIR)
The bands of FTIR for the different polymer films were perceived in the scale of 4000 to 400 cm−1. The major peaks in the biocomposite films prepared by the chitosan/PVA/glycerol blends are found to be present at 3661 (OH stretching), 3642 (NH stretching), 1744 (C = O bond stretch), and at 889 cm−1 (C–H rocking). Similarly, in the case of CNC/PVA/glycerol blends, the peaks are seen at 2953 (C–H bond), 2151 (O–H groups), 1723 (C = O), 1116 (sulphate ester bond), and 858 cm–1 (C–C stretching). The films prepared with the blends of chitosan/CNC/PVA/glycerol combinations revealed major peaks at 1731 (C = O) and 1144 cm–1 (saccharide structure like cellulose) (Fig. 1).
X-ray powder diffractometry (XRD)
The spectra of XRD as shown by the figure reveals the peaks of 2θ at 19.0339° in the case of films generated with the blends of chitosan/PVA/glycerol. Similarly, in the case of film produced with the blends of CNC/PVA/glycerol, the peaks of 2θ were seen at 18.52°, and for the films prepared by the blends of chitosan/CNC/PVA/glycerol, the 2θ peaks were observed at 18.52° (Fig. 2).
UV–vis spectroscopy
The optical barricade characteristics of the film were estimated with the UV–vis spectroscopy, and the films produced by the blends of chitosan/PVA/glycerol showed the percentage of transmittance to be 65%, and the films produced by the mix of CNC/PVA/glycerol revealed the percentage of transmittance to be 40%, and the percentage of transmittance of the films produced with the blends of chitosan/CNC/PVA/glycerol was observed to be 75% (Fig. 3).
Thermogravimetric analysis (TGA)
The thermogravimetric analysis of the films made with the blends of chitosan/PVA/glycerol mixtures reveals that the initial weight loss of the films was witnessed between 50 to 170 °C. The next phase of weight loss was witnessed between 170 and 360 °C, and the total loss of mass of the film were observed at 500 °C. In the case of films generated by the mix of CNC/PVA/glycerol mixtures, the initial loss of mass was seen between 50 and 150 °C. The second stage of loss of mass in these films was observed between 150 and 300 °C, and the final loss of mass was observed at 500 °C. The films generated by the blends of chitosan/CNC/PVA/glycerol mixtures showed the initial mass loss at 40 to 120 °C. The second stage of loss of mass was seen between 120 and 250 °C, and the final mass loss was observed at 520 °C (Fig. 4).
Universal testing machine (UTM)
The ultimate tensile strength (UTS) of the films produced by the blends of chitosan/PVA/glycerol mixtures were calculated to be 1.51 MPa. The film showed the breaking distance at 206 mm with a total elongation percentage of 2060%. Similarly, the UTS of the film produced by the CNC/PVA/glycerol films was observed at 12.6 MPa, where the breaking distance was seen at 44.3 mm with an elongation percentage of 185%. The film generated with the blends of chitosan/CNC/PVA/glycerol showed the UTS to be 4.21 MPa, and the breaking distance was observed at 123 mm. Here, the elongation percentage of the film was observed at 490% (Fig. 5).
Scanning electron microscopy (SEM)
The scanning electron microscopy of all the films showed similar morphological characteristics. The SEM analysis reveals a smooth texture along with few solid substances which suggests an incomplete homogenisation of the various blends (Fig. 6).
Discussions
The CH–OH oxidation of the PVA to C = O groups during composting may be responsible for the peak perceived at 1744 cm−1 in the case of film created from a combination of PVA/chitosan/glycerol. This is only a little amount of evidence for the cause of some PVA degradation (Hernández Berrío et al. 2022: Jayasekara et al. 2003). The band found at the 850 cm−1 has been appointed as C–H rocking in PVA (Abdullah, et al. 2022). The stretching, wagging, bending, and rocking vibration of CH bonds from methylene or methyl groups at 2943 cm−1 is detected in CNC/PVA/glycerol film (Popescu 2017). The carbonyl groups left over after the creation of PVA by the hydrolysis of polyvinyl acetate or oxidation throughout its manufacture are what cause the peaks at 1720 cm−1 to occur (Gulati, et al. 2019). Peaks at 1124 cm−1 and 1160 cm−1 show that sulphate ester linkages, which are produced by the hydrolysis of sulphuric acid for CNC preparation, are present (Jayaramudu et al. 2018). Similarly, the C–C stretching vibration is responsible for the point seen at 842 cm−1 (Choo et al. 2016). The FTIR spectra of CNC/PVA/glycerol films show C–O stretching 1723 cm−1 (Jiao et al. 2021) and linear C–H stretching of PVA, and gelatin was identified at the 2933 cm–1 (Oyeoka, et al. 2021).
Three distinct peaks can be seen in the unadulterated PVA/CS film: the crystalline phase at 2θ = 11.3°, the amorphous condition with the major peak centering at 2θ = 19.4°, and the shoulder peak with a reduced concentration at 2θ = 22.8° (Azizi et al. 2014a, b). Tan et al. (2021) witnessed the 2θ value of 19.7° which are the characteristic peak of PVA. The three usual peaks for the PVA/CS = 50/50 film protected with 0.5 wt.% of CNCs were shown to be the crystalline phase at 2 θ = 11.3, the amorphous stage with the major halo of the distinctive peak centred at 2 θ = 19.5, and one more with a lower intensity at 2 θ = 23.0 (Bodin et al. 2007).
Such films can be an excellent UV barrier due to their low UV transmission level. Therefore, when used on food systems, the produced films may reduce UV light-induced lipid oxidation (Cazón et al. 2018). Chitosan, on the other hand, enhances the opacity of the films, while PVA increases their transparency. The produced films showed ideal transparency levels that were comparable to those of synthetic polymers (Han and Floros 1997).
The thermal stability of the films produced is found to have similarities when compared with the previous studies. The first stage of loss of mass in the study conducted by Kusmono and Lubis (2021) found the initial loss of mass at 50 to 100 °C, and this loss of mass was found to be caused by the dehydration of low-molecular-weight compounds. The study also showed the second stage of mass loss at 140 to 395 °C which was caused by the degradation of low volatile molecular compound. The films underwent an initial mass reduction phase due to temperature increase, observed between 50 and 170 °C, attributed to the removal of water molecules. Furthermore, the decrease in weight could be attributed to the removal of loosely bound water and evaporation of low-molecular-weight compounds in the films (Kusmono and Lubis 2021). The second phase occurred within the temperature range of 170 to 360 °C, where weight loss is likely connected to the decomposition of PVA, glycerol, CNC, and chitosan. The primary processes involved in CNC decomposition between 240 and 335 °C include the cleavage of cellulose glycosidic bonds, rearrangement, dehydration, and breakdown reactions involving C = O, water, and low-volatility molecular compounds (Kusmono and Lubis 2021). In the same research, the primary decomposition of chitosan occurred around 140–310 °C through the depolymerization of chitosan chains, including deacetylation and cleavage of glycosidic linkages through dehydration and delamination. The third phase occurred within the temperature range of 395–600 °C, associated with the oxidation and breakdown of char into lower molecular weight gas products.
According to Hernández et al. (Hernández Berrío et al. 2022) glycerol, the plasticiser that offers the maximum elongation is exactly proportional to concentration. Compared to PVA films, chitosan films were found to be the least stretchy. Blend films exhibited a significant plastic distortion that significantly diminished as the chitosan ratio rose (Bonilla et al. 2014). Cellulose nanocrystals enhanced the tensile strength and elongation at break of polyvinyl alcohol. This improvement was partly ascribed to CNCs’ high crystallinity, which increased the intrinsic chain stiffness and rigidity (Khoshkava and Kamal 2014). Additionally, the PVA matrix may be partially plasticized by CNCs’ hydrophilicity, increasing its ductility and elongation at break. Based on the results of the three mechanical testing, the CNC, chitosan nanoparticles (CNP), mix, and PVA films were optimised. The findings demonstrate that the tensile strength was considerably increased by the additional supplementation of nanofillers (Dey et al. 2021).
According to Ravindra et al. (2017), the PVA/chitosan/papaya latex film has a surface that is covered in numerous non-spherical granules of assorted sizes. These granules are not visible in the films that were prepared, and the SEM analysis revealed a surface that was a little rougher than the other blend films, indicating a more hydrophilic top surface. Nanocellulose from cotton and linen linter combined to polyvinyl alcohol films creates porous structures (Ibrahim et al. 2010). Choo et al. (2016) examined the surface of a PVA/chitosan film following synthesis using TEMPO-mediated oxidised cellulose nanofibre (TOCN) and discovered that the film had a flat surface and indicated uniform dispersion of CNC, PVA, and chitosan matrix.
Conclusions
Fourier transform infrared spectroscopy, XRD, TGA, UV–vis spectroscopy, UTM, and SEM were used to successfully characterise thin films made of chitosan, cellulose nanocrystals, polyvinyl alcohol, and glycerol. The current study found that the mechanical and thermal properties of the film formed by the combination of CNC/PVA/glycerol showed enhanced properties than that of the other films, from this current study, which was formed by combining chitosan/PVA/glycerol and chitosan/CNC/PVA/glycerol films. Chemical bonds were detected in the material by FTIR analysis, and the thin films generated were found to be crystalline by XRD examination. These films’ thermal stability was better according to the TGA, but their elongation percent was higher according to the UTM study. According to UV–vis spectroscopy, the CNC/PVA/glycerol mix film had the lowest transmittance competition percentage, indicating that it would be a good choice for packaging. The outcome of the SEM analysis showed films with smooth texture. When creating novel composites for potential advancements and applications, many elements, including as quality, utility, and cost, must be taken into consideration to fully reap the advantages of cellulose and its derivatives in the food wrapping industry. Additionally, more investigation is needed into its blending with different natural polymers and its uses as carriers.
Availability of data and materials
Not applicable.
References
Abdullah AM, Aziz SB, Brza MA, Saeed SR, Al-Asbahi BA, Sadiq NM, Ahmed AAA, Murad AR (2022) Glycerol as an efficient plasticizer to increase the DC conductivity and improve the ion transport parameters in biopolymer based electrolytes: XRD, FTIR and EIS studies. Arab J Chem 15:103791–103804. https://doi.org/10.1016/j.arabjc.2022.103791
Ali M, Abderraouf G (2017) Synthesis and characterization of the composite material PVA/chitosan/5% sorbitol with different ratio of chitosan. Int J Mech Mech Eng 17:15–28
Annu AA, Ahmed S (2021) Eco-friendly natural extract loaded antioxidative chitosan/polyvinyl alcohol based active films for food packaging. Heliyon 7:e06550–e06559. https://doi.org/10.1016/j.heliyon.2021.e06550
Anukiruthika T, Sethupathy P, Wilson A, Kashampur K, Moses JA, Anandharamakrishnan C (2020) Multilayer packaging: advances in preparation techniques and emerging food applications. Compr Rev Food Sci Food Saf 19:1156–1186. https://doi.org/10.1111/1541-4337.12556
Azizi S, Ahmad MB, Hussein MZ, Ibrahim NA, Namvar F (2014a) Preparation and properties of poly(vinyl alcohol)/chitosan blend bionanocomposites reinforced with cellulose nanocrystals/ZnO-Ag multifunctional nanosized filler. Int J Nanomedicine 9:1909–1917. https://doi.org/10.2147/IJN.S60274
Azizi S, Ahmad MB, Ibrahim NA, Hussein M, Namvar F (2014b) Cellulose nanocrystals/ZnO as a bifunctional reinforcing nanocomposite for poly(vinyl alcohol)/chitosan blend films: fabrication, characterization and properties. Int J Mol Sci 15:11040–11053. https://doi.org/10.3390/ijms150611040
Azuma Y, Yoshie N, Sakurai M, Inoue Y, Chûjô R (1992) Thermal behaviour and miscibility of poly(3-hydroxybutyrate)/poly(vinyl alcohol) blends. Polymer 33:4763–4767. https://doi.org/10.1016/0032-3861(92)90690-X
Bano I, Ghauri MA, Yasin T, Huang Q, Palaparthi ADS (2014) Characterization and potential applications of gamma irradiated chitosan and its blends with poly(vinyl alcohol). Int J Biol Macromol 65:81–88. https://doi.org/10.1016/j.ijbiomac.2014.01.015
Bodin A, Ahrenstedt L, Fink H, Brumer H, Risberg B, Gatenholm P (2007) Modification of nanocellulose with a xyloglucan-RGD conjugate enhances adhesion and proliferation of endothelial cells: implications for tissue engineering. Biomacromol 8:3697–3704. https://doi.org/10.1021/bm070343q
Bonilla J, Fortunati E, Atarés L, Chiralt A, Kenny JM (2014) Physical, structural and antimicrobial properties of poly vinyl alcohol-chitosan biodegradable films. Food Hydrocoll 35:463–470. https://doi.org/10.1016/j.foodhyd.2013.07.002
Cazón P, Vázquez M, Velazquez G (2018) Composite films of regenerate cellulose with chitosan and polyvinyl alcohol: evaluation of water adsorption, mechanical and optical properties. Int J Biol Macromol 117:235–246. https://doi.org/10.1016/j.ijbiomac.2018.05.148
Choo K, Ching YC, Chuah CH, Julai S, Liou NS (2016) Preparation and characterization of polyvinyl alcohol-chitosan composite films reinforced with cellulose nanofiber. Materials 9:644–658. https://doi.org/10.3390/ma9080644
Costa SM, Ferreira DP, Teixeira P, Ballesteros LF, Fangueiro TJA, R, (2021) Active natural-based films for food packaging applications: the combined effect of chitosan and nanocellulose. Int J Biol Macromol 177:241–251. https://doi.org/10.1016/j.ijbiomac.2021.02.105
Dey D, Dharini V, Selvam SP, Rotimi SE, Mahesh KM, Jayaramudu J, Nath GU (2021) Physical, antifungal, and biodegradable properties of cellulose nanocrystals and chitosan nanoparticles for food packaging application. Mater Tod Proceed 38:860–869. https://doi.org/10.1016/j.matpr.2020.04.885
Dong H, Cheng L, Tan J, Zheng K, Jiang Y (2004) Effects of chitosan coating on quality and shelf life of peeled litchi fruit. J Food Eng 64:355–358. https://doi.org/10.1016/j.jfoodeng.2003.11.003
El-Hefian EA, Nasef MM, Yahaya AH (2010) The preparation and characterization of chitosan / poly (vinyl alcohol) blended films. E-J Chem 7:1212–1219. https://doi.org/10.1155/2010/626235
Ferrer A, Pal L, Hubbe M (2017) Nanocellulose in packaging: advances in barrier layer technologies. Ind Crops Prod 95:574–582. https://doi.org/10.1016/j.indcrop.2016.11.012
Gulati K, Lal S, Diwan PK, Arora S (2019) Investigation of thermal, mechanical, morphological and optical properties of polyvinyl alcohol films reinforced with Buddha coconut (Sterculia alata) leaf fiber. Int J Appl Eng Res 14:170–179
Hamdi M, Hammami A, Hajji S, Jridi M, Nasri M, Nasri R (2017) Chitin extraction from blue crab (Portunus segnis) and shrimp (Penaeus kerathurus) shells using digestive alkaline proteases from P. segnis viscera. Int J Biol Macromol 101:455–463. https://doi.org/10.1016/j.ijbiomac.2017.02.103
Han JH, Floros JD (1997) Casting antimicrobial packaging films and measuring their physical properties and antimicrobial activity. J Plast Film Sheeting 13:287–298. https://doi.org/10.1177/875608799701300405
Hernández Berrío YDC, Realpe Jiménez Á, de Ávila MG (2022) Effect of glycerol, sunflower oil, and glucose on the physico-chemical and mechanical properties of chitosan/polyvinyl alcohol-based films. Polym Bull 79:6389–6407. https://doi.org/10.1007/s00289-021-03803-w
Ibrahim MM, El-Zawawy WK, Nassar MA (2010) Synthesis and characterization of polyvinyl alcohol/nanospherical cellulose particle films. Carbohydr Polym 79:694–699. https://doi.org/10.1016/j.carbpol.2009.09.030
Jayaramudu T, Ko HU, Kim HC, Kim J, Muthoka R, Kim J (2018) Electroactive hydrogels made with polyvinyl alcohol/cellulose nanocrystals. Materials 11:1615–1628. https://doi.org/10.3390/ma11091615
Jayasekara R, Harding I, Bowater I et al (2003) Biodegradation by composting of surface modified starch and PVA blended films. J Polym Environ 11:49–56. https://doi.org/10.1023/A:1024219821633
Jiao Y, Lu Y, Lu K, Yue Y, Xu X, Xiao H, Li J, Han J (2021) Highly stretchable and self-healing cellulose nanofiber-mediated conductive hydrogel towards strain sensing application. J Colloid Interface Sci 597:171–181. https://doi.org/10.1016/j.jcis.2021.04.001
Kaya M, Baran T, Karaarslan M (2015) A new method for fast chitin extraction from shells of crab, crayfish and shrimp. Nat Prod Res 29:1477–1480. https://doi.org/10.1080/14786419.2015.1026341
Khoshkava V, Kamal MR (2014) Effect of drying conditions on cellulose nanocrystal (CNC) agglomerate porosity and dispersibility in polymer nanocomposites. Powder Technol 261:288–298. https://doi.org/10.1016/j.powtec.2014.04.016
Kusmono WMW, Lubis FI (2021) Fabrication and characterization of chitosan/cellulose nanocrystal/glycerol bio-composite films. Polymers (basel) 13:1096–1109. https://doi.org/10.3390/polym13071096
Mujtaba M, Salaberria AM, Andres MA, Kaya M, Gunyakti A, Labidi J (2017) Utilization of flax (Linum usitatissimum) cellulose nanocrystals as reinforcing material for chitosan films. Int J Biol Macromol 104:944–952. https://doi.org/10.1016/j.ijbiomac.2017.06.127
Muzzarelli RAA (1996) Chitosan-based dietary. Carbohydr Polym 29:309–316. https://doi.org/10.1016/S0144-8617(96)00033-1
Nakano Y, Bin Y, Bando M, Nakashima T, Okuno T, Kurosu H, Matsuo M (2007) Structure and mechanical properties of chitosan/poly(vinyl alcohol) blend films. Macromol Symp 258:63–81. https://doi.org/10.1002/masy.200751208
Nayigiziki FX (2016) Physical characterization and antimicrobial properties of pva-cellulose nanofiber based films. Master of Science Dissertation, University of Missouri
Oyeoka HC, Ewulonu CM, Nwuzor IC, Obele CM, Nwabanne JT (2021) Packaging and degradability properties of polyvinyl alcohol/gelatin nanocomposite films filled water hyacinth cellulose nanocrystals. J Bioresour Bioprod 6:168–185. https://doi.org/10.1016/j.jobab.2021.02.009
Pfeifer L (2021) “Neptune balls” polysaccharides: disentangling the wiry seagrass detritus. Polymers (basel) 13:4285–4302. https://doi.org/10.3390/polym13244285
Pfeifer L, Classen B (2020) The cell wall of seagrasses: fascinating, peculiar and a blank canvas for future research. Front Plant Sci 11:1–13. https://doi.org/10.3389/fpls.2020.588754
Popescu MC (2017) Structure and sorption properties of CNC reinforced PVA films. Int J Biol Macromol 101:783–790. https://doi.org/10.1016/j.ijbiomac.2017.03.168
Qi H, Chang C, Zhang L (2009) Properties and applications of biodegradable transparent and photoluminescent cellulose films prepared via a green process. Green Chem 11:177–218. https://doi.org/10.1039/b814721c
Rasti H, Parivar K, Baharara J, Iranshahi M, Namvar F (2017) Chitin from the mollusc chiton: extraction, characterization and chitosan preparation. Iran J Pharm Res 16:366–379. https://doi.org/10.22037/ijpr.2017.1963
Ravindra C, Saraswati M, Bhagyavana M, Deepak K (2017) Miscibility and thermal study of PVA/chitosan/papaya latex blend films. Int Res J Eng Technol 4:2424–2432
Reddy AB, Manjula B, Jayaramudu T, Sadiku ER, Anand BP, Periyar SS (2016) 5-Fluorouracil loaded chitosan–PVA/Na+MMT nanocomposite films for drug release and antimicrobial activity. Nanomicro Lett 8:260–269. https://doi.org/10.1007/s40820-016-0086-4
Sai Prasanna N, Mitra J (2020) Isolation and characterization of cellulose nanocrystals from Cucumis sativus peels. Carbohydr Polym 247:1–37. https://doi.org/10.1016/j.carbpol.2020.116706
Schurz J (1999) “Trends in polymer science” a bright future for cellulose. Prog Polym Sci 24:481–483. https://doi.org/10.1016/S0079-6700(99)00011-8
Szymanska-Chargot M, Chylinska M, Gdula K, Koziol A, Zdunek A (2017) Isolation and characterization of cellulose from different fruit and vegetable pomaces. Polymers (basel) 9:495–511. https://doi.org/10.3390/polym9100495
Tan R, Li F, Zhang Y, Yuan Z, Feng X, Zhang W, Liang T, Cao J, De Hoop CF, Peng X (2021) High-performance biocomposite polyvinyl alcohol (PVA) films modified with cellulose nanocrystals (CNCs), tannic acid (TA), and chitosan (CS) for food packaging. J Nanomater 2021:1–9. https://doi.org/10.1155/2021/4821717
Tripathi S, Mehrotra GK, Dutta PK (2010) Preparation and physicochemical evaluation of chitosan/poly(vinyl alcohol)/pectin ternary film for food-packaging applications. Carbohydr Polym 79:711–716. https://doi.org/10.1016/j.carbpol.2009.09.029
Varma R, Vasudevan S (2020) Extraction, characterization, and antimicrobial activity of chitosan from horse mussel Modiolus modiolus. ACS Omega 5:20224–20230. https://doi.org/10.1021/acsomega.0c01903
Varma R, Vasudevan S (2022) Synthesis and characterization of cellulose and cellulose nanocrystals from dead seagrass-towards the wealth from waste concept. Celull Chem Technol 56:39–47. https://doi.org/10.35812/CelluloseChemTechnol.2022.56.03
Vilarinho F, Sanches Silva A, Vaz MF, Farinha JP (2018) Nanocellulose in green food packaging. Crit Rev Food Sci Nutr 58:1526–1537. https://doi.org/10.1080/10408398.2016.1270254
Yaradoddi JS, Banapurmath NR, Ganachari SV, Soudagar MEM, Mubarak NM, Hallad S, Hugar S, Fayaz H (2020) Biodegradable carboxymethyl cellulose based material for sustainable packaging application. Sci Rep 10:1–13. https://doi.org/10.1038/s41598-020-78912-z
Funding
The authors did not receive support from any organisation for the submitted work.
Author information
Authors and Affiliations
Contributions
RV, methodology, validation, formal analysis, investigation, and writing — original draft; SV — conceptualization, methodology, validation, investigation, and writing — review and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Varma, R., Vasudevan, S. Synthesis of composite films using polymer blends of chitosan and cellulose nanocrystals from marine origin. J Mater. Sci: Mater Eng. 19, 6 (2024). https://doi.org/10.1186/s40712-024-00145-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40712-024-00145-z