Abstract
Calcium carbonate precipitation using a urease enzyme, referred to herein as Enzyme-Induced Carbonate Precipitation (EICP) is a technique for soil improvement. In this technique, a mixed solution composed of reagents and the urease enzyme, which produces calcite, is utilized as the grouting material. Recently, alternative materials to the urease enzyme have been examined to resolve the cost issue of using the urease enzyme. In this study, several tests were conducted to compare commercial urease and soybean-derived crude urease. A comparison of their hydrolysis rates was done through urease activity tests. The microscopic structures and mineralogy of the precipitated materials, produced during various loading periods, were investigated through SEM and XRD analyses. Moreover, the reinforcing effect of the grouting solutions on the treated soil specimens was evaluated by measuring the unconfined compressive strength (UCS) of the treated samples. Interestingly, the precipitated CaCO3 was vaterite and calcite when using soybean urease, while it was mostly calcite when using the urease enzyme. Higher UCS values were obtained with the soybean urease samples because the precipitated CaCO3 seemed to be concentrated at the inter-grain contacts. It was concluded that soybean powder shows great efficacy as a replacement for commercially produced enzyme urease in soil-improvement techniques mediated by carbonate precipitation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, enzyme-induced carbonate precipitation (EICP) has become an innovative and potential technique for soil improvement [1,2,3,4] comparing to microbially induced carbonate precipitation (MICP), which uses bacteria to induce carbonate precipitation [5,6,7]. As the practical application of MICP is still difficult, due to the bacteria cultivation issue and the limitation of the ground conditions [1, 8], the application of the carbonate precipitation method, utilizing purified urease enzyme induction (i.e., EICP) as a sandy soil-improvement technique, has been extensively studied by several researchers [1,2,3,4, 9,10,11,12,13,14,15]. EICP can significantly improve the strength of the soil [2, 14, 16, 17] and reduce the permeability and porosity of the soil [3, 16]. In this technique, a grouting solution is injected into a sand sample and CaCO3 compounds are formed. The formation of CaCO3 compounds in the soil may provide ties between the sand grains, limiting their movement, and thus, enhancing the soil strength. The deposited CaCO3 compounds fill the voids between the soil grains, helping to reduce the soil permeability and porosity [3, 7]. In the EICP technique, the grouting solution is composed of urease, a calcium source, and urea. The urease, which is the catalyst for the hydrolyzation of urea into ammonium and carbonate ions (Eq. 1), is usually derived from a purified commercial enzyme (i.e., from jack beans: 020-83242-Kishida Co., Ltd [3]. 9002-13-5 Sigma Chemical Co., Ltd. [9], U2125-Fisher Chemical [18]). The carbonate ions combine with the calcium ions, supplied in the form of a calcium salt solution (e.g., CaCl2), to produce calcium carbonate precipitation (Eq. 2). The mechanism of the improvement in soil strength using the carbonate precipitation method is shown in Fig. 1.
Since the EICP technique usually utilizes a purified urease enzyme, it can be costly. This is a major barrier to the method’s application in the field [19]. Just the commercial enzyme itself can constitute almost the total material cost [20]. Therefore, finding new inexpensive materials to replace the commercial enzyme is considered essential for developing this technique. This issue may be resolved by utilizing natural resources of urease [21]. Several researchers have investigated alternative natural materials to the commercial enzyme. Some potential plant seeds, such as those of jack beans, watermelon, legumes, and soybeans, have been examined as an alternative to EICP with commercial enzyme. Watermelon seeds were finely ground using a mortar and pestle, and the crushed seeds were stirred at 500 rpm with 10 mL of distilled water for 1 h. A crude extract of crushed watermelon was obtained after filtering and collecting the filtrate. Dilrukshi et. al [22] reported that the possibility of precipitation mechanisms from watermelon showed that the effect of urease activity on estimated UCS is different for concentrations of CaCl2-urea solution 0.3 M to 0.7 M. It is best to maintain low urease activity for low CaCl2-urea solution concentrations and high urease activity for solutions with high concentrations of CaCl2-urea, to maintain the rate of urea hydrolysis and finally maintain the rate of calcium carbonate precipitation. Otherwise, calcium carbonate started to precipitate before injecting the solution into the specimen if a low concentration of urea is immediately hydrolyzed by a high urease activity. Consequently, the voids between the sand particles are filled with pre-precipitated calcium carbonate (PPCC) rather than precipitated in the specimen, which may not be favorable to the forming of effective bonds between the sand particles. Lee and Kim [23] also evaluated EICP using yellow soybeans and compared its efficiency with MICP using bacteria. In order to prepare the urease solution for EICP in that study, various amounts of yellow soybean powder were mixed with 1 L of distilled water. The mixed solution was then centrifuged at a rate of 3,000 rpm for 20 min at 4 °C, and the crude extract of yellow soybean was collected. Compared with the untreated samples, the UCS increment ratios of the treated samples fell in the range of 1.58–2.72. It was concluded that yellow soybeans can be a urease source for EICP in terms of carbonate precipitation efficiency. However, there is a problem with the dehydration of the solution in the specimen pores, which may cause a delay in the reaction and bring about the need for longer loading times to obtain a satisfactory reinforcing effect [23]. Recently, Khodadadi et al. [24] introduced a crude and purified extract from four different plant sources (i.e., jack beans, jack bean meal, soybeans, and watermelon seeds) as sources for bio-cementation via EICP. Generally, a crude extract in that study was obtained via three stages of chemical extractions. In the first stage of enzyme extraction, 50 g of each species was soaked in 200 mL of an extraction solution overnight at pH 7.5 and 4 °C. In the second stage of enzyme extraction, referred to herein as the first fractionation step, acetone fractionation was used to precipitate (i.e., salt-out) urease from the crude extract. Then, in the third stage of enzyme extraction, referred to herein as the second fractionation step, the enzyme extract was further purified using acetone fractionation. The possibility of precipitation mechanisms from four different plant above showed that the level of enzyme purity can affect the efficacy of biocementation via EICP and it was confirmed that using crude extracts for EICP was considerably less expensive than using commercial enzyme products. Moreover, higher UCS results were obtained for the soil samples treated with jack bean crude extract than for those treated with the commercial enzyme products. The study implies that the organic impurities (such as proteins) in crude extracts may enhance the effectiveness of EICP for bio-cementation, although further investigation is required.
Soybean powder is a promising and inexpensive material with the potential to replace the commercially available urease enzyme in the EICP technique [25,26,27]. Since soybeans are included in the bean family, one of the urease-rich families [28], they may be a great replacement material for the purified commercial enzyme. The urease activity for soybean crude urease was estimated at 74.2 U/g (1 U activity corresponded to 1 µmol/L urea hydrolysed per minute), indicating that it is sufficient to be used for replacing the urease enzyme on the EICP technique [25, 26]. Moreover, the total protein in the soybean crude extract is 4863 mg which is much higher than that in other plant urease resources (i.e., watermelon) and close to that in jack beans, which is the most common commercial urease source [24]. The presence of protein can serve as a modifier of EICP solutions to enhance its strength and effectiveness [29].
Despite the great potential of soybean powder as a commercial urease enzyme replacement in the EICP technique, no comparative studies between commercial enzymes and soybean crude urease can be found in the literature. Soybean crude urease (SCU) is a crude enzyme extracted from soybean powder used as a catalyst. Moreover, studies on the use of SCU in the EICP technique are limited to several types of soil [23, 26, 27], and SCU has not been used thus far for sandy soil. Thus, the main objectives of this study are to investigate the efficacy of EICP using soybean powder (referred to as EICP-SCU in this study) for the improvement of sandy soil and to compare it with EICP using commercial enzymes (referred to as EICP-CU in this study) through several experiments. Another objective of this study is to investigate the reaction time when using soybean urease to achieve a satisfactory condition since there is an issue with the loading process. To examine the efficacy of EICP using soybean powder, the urease activity and amounts of calcium carbonate precipitation were measured and compared with EICP using purified urease through ammonia measurement tests and tube tests, respectively. In addition, mineralogical and microstructural analyses of the precipitated materials acquired from the tube tests were conducted through XRD and SEM, respectively. The reinforcing effect was also investigated by conducting unconfined compressive strength (UCS) tests. Finally, by combining the evaluations of the mineralogical and microstructural analyses and the reinforcing effect, the improvement mechanisms of the treated samples were thoroughly investigated.
Materials and method
Materials
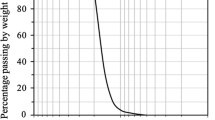
Urea, calcium chloride, soybean powder, and distilled water were used to produce the grouting solution. The urea and calcium chloride, with purification of more than 99%, were obtained from Kanto Chemicals Co. Inc., Japan. As an alternative material, soybean powder (Gasol Soybean Flour 200 gr) was obtained from PT. Gasol Organik, South Jakarta, Indonesia. It is mainly used to make porridge for baby foods. In addition, a purified commercial enzyme urease (16040-1210, Junsei Chemical, Tokyo, Japan), fabricated from jack bean seeds, was used for comparison purposes. Keisha number 4, considered silica sand, was obtained from Kitanihon Sangyo Co. Ltd, Japan, and used for the soil samples. The specific dry density, D50, uniformity coefficient (Cu), and coefficient of gradation (Cc) were 2.63 g cm−3, 0.755 mm, 1.3, and 0.96, respectively. The sand used in this study was categorized as poorly graded sand (SP) based on the Unified Soil Classification System [30]. Figure 2 shows the sand in the study and the grain size distribution of the sand used in this study.
Crude urease extraction
The extraction procedure for the soybean urease used in this study was based on the method introduced by Gao et al. [26]. The detailed procedure for extracting the soybean crude urease from the soybean powder is presented in Fig. 3. The soybean powder was stored in a refrigerator to maintain its activities. The storage of enzymes is an important issue, due to the urease activity of the soybean crude extract at 4 °C, a period of lag phase existed in the percolation phase of the EICP solution, which ensured the relatively uniform distribution and consequently the uniformity of the CaCO3 precipitation in the soil [10]. Therefore, storage of enzymes is required to inhibit the fast accumulation of CaCO3 precipitation in the surficial soil. The soybean crude urease was created by adding the soybean powder and distilled water to a conical flask at specific concentrations. The solution was mixed for six minutes with a magnetic stirrer to obtain a homogenous suspension. Next, the mixed suspension was centrifuged by a centrifuge apparatus at a rate of 3,000 rpm for 20 min at room temperature (20 ± 1 °C). Then, the clear supernatant containing the crude urease enzyme was collected and used as the EICP material in the following experiments.
Urease activity test
A comparison of the urease activity between soybean crude urease (SCU) and commercial urease enzyme (CU) was conducted. The urease activity test method was adopted from a method developed by Whiffin et. al with the ammonia direct measurement using the Nessler method [5]. In this method, the amount of ammonium present at the end of hydrolysis indicates that the reaction has reached completion. The tests were performed by mixing the urease solutions (e.g., soybean crude urease and purified commercial enzyme) and the testing solution containing urea. The concentrations of SCU were in the range of 10–50 g L−1, while those of the CU were in the range of 0.5–3 g L−1. Each testing sample was prepared by mixing 10 mL of the urease solution with 90 mL of the solution containing 150 mmol L−1 of the urea. After 5, 10, and 15 min of mixing, 1 mL from each sample was diluted 100-fold using DI water. Then, 1 mL of the diluted solution was used as a sample to determine the ammonia concentration. The Nessler method using Hanna HI97733 device was used to determine the ammonium concentration. In the solutions, the urea was hydrolyzed to ammonium and carbonate ions by the urease, which increased the ammonium ion. The enzyme activity (U) of the solution was calculated by dividing the slope of the initial linear part of the ammonium-time curve by urease concentration in the mixing solution (illustrated in Fig. 4).
Efficiency tests
Efficiency tests through tube tests were conducted to evaluate the efficiency of the soybean crude extract (SCU) and to find the appropriate concentration for an EICP material by evaluating the mass of the precipitated calcium carbonate. The test procedure was adapted from a method developed by Neupane et al. [2]. In this method, the amount of precipitated calcium carbonate directly was measured to find the appropriate concentration for an EICP material. Certain concentrations of the urease and reagent (Urea-CaCl2) solutions were prepared separately. 20 mL of the urease solution (both soybean crude urease and purified commercial enzyme) was mixed with 20 ml of the testing solution containing 1 M of urea and 1 M of calcium chloride. Then, the EICP solutions, each totalling 40 mL, were left for the designated number of days at room temperature (20 ± 1 °C). During the experiments, the tubes were stirred at 50 rpm by a rotating table. After each specified number of days, the evolution of the mass of the precipitated materials over time was measured. The EICP solutions were filtered using filter paper with a 20-µm pore size (Whatman No. 41) to collect the precipitated materials. The materials retained in the filter paper and the tubes were oven-dried at 80 °C for 24 h to calculate the total amount of precipitated materials by combining both masses. It should be noted that the precipitated materials of EICP-SCU contain not only calcium carbonate, but also a fraction of residual soybean powder particles. Although the SCU solution was processed through centrifugation, there were some undissolved powders in the solution [31, 32]. Lofianda et al. [32] evaluated the amount of actual calcite inside the precipitated materials using various concentrations of soybean crude urease. The ratio of calcite-soybean from the study was subtracted to the current study results to find the actual CaCO3 mass of the EICP-SCU. The precipitation ratio, namely, the ratio of the total evaluated mass from the test to the maximum theoretical mass of CaCO3, was also evaluated for a better analysis. Two sets of tube tests were performed to investigate the evolution of the calcium carbonate's mass influenced by the urease concentration and the loading time. Each set consist of three samples as repeatability. The detailed experimental conditions and procedures are presented in Table 1 and Fig. 5, respectively.
Soil-strength tests
To evaluate the strength effects of the EICP-SCU and EICP-CU on the treated soil samples, the EICP-treated samples were tested under the various experimental conditions listed in Table 2. The samples were prepared for the unconfined compressive strength (UCS) tests and calcite distribution analysis.
Sample preparation and UCS testing
The strength of the EICP-SCU was compared with that of the EICP-CU by conducting UCS tests. The experimental procedure developed by Putra et al. [11] was adopted in this study for the sample preparation. Figure 6 shows the UCS sample preparation. The PVC cylinders were 5 cm and 10 cm in diameter and height, respectively. Firstly, a 325 g of Keisha sand number 4 was poured into the PVC cylinders using a funnel to obtain a relative density of 50 ± 3%. The inner part of the PVC cylinder has been covered with a thin plastic plate to facilitate sample removal. Then, one pore volume of the EICP solution (approximately 75 mL), containing SCU or CU, urea, and the CaCl2 solution was introduced to the cylinders containing the sand samples by pouring the solution with a syringe tube. There was no addition of the EICP solution during the loading time (one cycle injection). After loading times of 7 and 14 days, the EICP-treated samples were removed from the PVC cylinders. Before the UCS tests were conducted, the surface of each EICP-treated sample was flattened with a spatula. Two tests were performed for each condition to check the reproducibility. After the UCS tests, the treated samples were rinsed with distilled water and oven-dried at 100 °C for 24 h. To obtain visual evidence of the calcium carbonate precipitation, SEM analysis was conducted on one small, treated sample.
Carbonate content measurement
The amount of precipitated calcium carbonate in each treated soil specimen was evaluated through acid digestion tests. For these tests, approximately 2 g of the treated sample was taken from three parts, namely, the top, middle, and bottom (Fig. 7) to represent a distribution of calcium carbonate in the treated sample soil. 100 mL of 1-M hydrochloric acid was mixed with 900 mL of distilled water and then added to each of the samples. The acid solution was poured onto the soil sample three times, in 20-mL increments, until no more of the calcium carbonate crystal inside the sample could be dissolved. Then, the samples were rinsed twice with distilled water before they were put into a 100 °C oven for 24 h. The weights of the samples before and after they reacted with the hydrochloric acid solution were compared to calculate the amount of precipitated calcium carbonate. The calcite distribution inside of the soil column sample was also evaluated using a larger PVC cylinder. The UCS PVC cylinder was 5 cm in diameter and 10 cm in height, whereas the cylinder prepared here was 10 cm in diameter and 20 cm in height (Table 2). The preparation procedure was the same as that given in the previous section with some adjustments made to the EICP solution and the amount of soil required. 2600 g of Keisha sand was poured into the cylinder to achieve a relative density of 50%, followed by the addition of 600 mL of the EICP solution. The samples were left for 7 days for both EICP-SCU and EICP-CU. After the loading time, the soil-treated samples were rinsed with 1 PV (600 mL) of distilled water and oven-dried at 100 °C for 24 h. The acid digestion tests were then immediately conducted.
Microscale identification analysis
X-ray powder diffraction (XRD) was used with Co-Ku radiation and scanning electron microscopy (SEM) to evaluate the mineral crystal phase and morphology of the samples, respectively. The samples were derived from tube tests after the mass had been evaluated, and UCS test samples, after the strength had been measured. The XRD used in this study was PANalytical X'Pert PRO MPD (Kaneko Techno Research Co., Japan) and the data were analyzed using the QualX program. QualX is a computer program that can identify crystal phases by powder diffraction data [33]. SEM was conducted on small amounts of the samples using a JSM-IT100 SEM/EDX apparatus (JEOL Ltd. Japan). The samples were sputter-coated with platinum (Pt) for 90 s using JEC-3000FC (JEOL Ltd. Japan) to prevent charging.
Results
Urease activity
The results of the urease activity for both the soybean crude urease (SCU) and purified commercial urease enzyme (CU) are depicted in Fig. 8. As is apparent in the figure, the urease activity increases with the increase in concentrations for both SCU and CU. The activities of SCU fell in the range of 1.75–6.90 U mL−1 by adding 10–50 g L−1 of soybean powder. On the other side, the urease activities of 0.5–3 g L−1 CU were calculated in the range of 1.63–7.95 U mL−1. Urease activity here (U mL−1) is defined as the 1 mL of enzyme concentration that catalyzes 1 µmol L−1 of urea per minute. The unit yield of SCU and CU, which is defined as the total urease activity divided by the initial mass of raw material used for extraction, was estimated at 2711 U g−1 and 132 U g−1, respectively. The urease activity of the CU was almost 20 times bigger than that of the SCU. However, using soybean crude urease at a high concentration (more than 20 g L−1) may be sufficient and high enough for replacing the commercial enzyme in the EICP technique. The efficacy of SCU then will be evaluated in the aqua phase experiment namely the tube test.
Efficiency test
The tube tests were conducted in two series and duplicate sets. (Due to the consistency in the results, triplicate sets were not considered essential). Tube test series 1 was conducted to find the appropriate concentration of SCU as an EICP material by comparing it to the EICP-CU. Then, after establishing the appropriate concentration of SCU, tube test series 2 was performed to evaluate the precipitation properties, including the precipitated mass, mineral crystal, and morphology influenced by the different loading times.
Evolution of precipitated mass with various concentrations
The optimum SCU used as EICP materials was evaluated based on the result of tube test series 1 (Table 1). Figure 9 shows the evolution of the precipitated mass influenced by several concentrations of both urease sources (SCU and CU) with three days of loading times. As shown in the figure, the precipitated mass and ratio are linearly related to the concentrations of the added SCU and CU. The higher concentration of urease added into EICP reagents resulted in more CaCO3 precipitated. However, the higher concentration of SCU doesn’t always indicate increasing the precipitated materials such as 50 g L−1 SCU case. These results are in line with the results of previous studies [27, 32]. The decrease in the 50 g L−1 SCU is because the high amount of undissolved powder in the solution may inhibit the precipitation reaction. The high soybean powder concentration resulted in a high amount of undissolved material, as a result, the precipitation reaction became less effective because the undissolved material can affect the calcite formation. The amount of precipitation of EICP-SCU with the urease concentration is 10 times by EICP-CU (10–50 g L−1), is more than 10%, and approaches the highest precipitation ratio of 62% at the concentration of 40 g L−1. On the other hand, the precipitation ratio generated by EICP-CU started from 16% at the CU concentration of 0.5 g L−1 and reached the maximum of 68% at the urease content of 5 g L−1. The precipitation mass of 20–40 g L−1 of SCU and 2–4 g L−1 CU tend to be similar. Furthermore, to achieve ease of application of the flowable solution into the soil, the SCU content of 20 g L−1 was selected and will be used for further testing by comparing it with a CU content of 2 g L−1.
Evolution of precipitated mass with time
A comparison between soybean crude urease (SCU) and commercial urease enzyme (CU) in the evolution of the precipitated mass and ratio for each loading time is depicted in Fig. 10. The results appear to be similar, but the characteristic trends between them are different. After 7 days of the loading time, the increase of precipitation material for the EICP-CU is greater than that for the EICP-SCU. These results imply that soybean crude urease requires a longer time to generate precipitated materials than commercial enzyme urease. This is because there is less activity for the SCU than that for the CU which might affect the precipitation rates. The CU has approximately 5.55 U mL−1 urease activity in the 2 g L−1 concentration compared to the 2.57 U mL−1 activity of 20 g L−1 SCU, resulting in faster urea hydrolyzation. Therefore, the discrepancy in the precipitation rates between them may be further investigated by simply adjusting the activity and concentration of soybean crude urease or commercial urease. It also concludes that using the high activity of the urease can enhance the calcite precipitation rate.
XRD analysis of precipitated materials
This study focused on the crystal phase of calcium carbonate that may form calcite, vaterite, and/or aragonite. The XRD analysis results of the precipitated materials from both the EICP-SCU and the EICP-CU are depicted in Fig. 11. The precipitated materials produced by various loading durations were analyzed. The peak intensities of calcite, which is the most stable polymorph of calcium carbonate, are clearly observed in both the EICP-SCU and the EICP-CU. In addition, the results confirmed from the EICP-SCU that vaterite, which is the least stable polymorph of the calcium carbonate, is precipitated. The XRD patterns of vaterite and aragonite exhibit significant resemblance and may exhibit partial overlapping. The presence of aragonite cannot be present in this study. The absence of aragonite can be attributed to the absence of magnesium sources in the reagent used for EICP. The formation of aragonite is known to be influenced by the presence of magnesium ions [11, 34]. Therefore, the distinct peaks obtained from the analyses are most likely to be vaterite. Moreover, it should be noted that the peak intensities of vaterite decrease with the increased loading time. In the aqueous solution, extending the duration of the reaction results in a decrease in the vaterite content [35, 36], which may indicate a phase change of the calcium carbonate from vaterite to calcite [37, 38]. This hypothesis should be verified through a visual analysis using SEM tests, which will be described in the next section. In addition, it should also be noted that no minerals, other than calcite and vaterite, were clearly observed from the XRD analysis.
SEM analysis of precipitated materials
An SEM analysis was conducted to visually observe the morphology of the precipitated materials, which supplementarily confirms the XRD analysis results, clearly showing the precipitation of calcite and vaterite. The SEM results of both the EICP-SCU and the EICP-CU are depicted in Fig. 12.
The SEM images of the EICP-SCU materials show that the precipitates in the tubes are mainly composed of spherical crystals (Fig. 8a, c, and e). These results coincide with those of previous works reporting spherical shapes in the precipitates when using plant-based crude extracts [24, 39]. However, agglomerated rhombohedral crystals are also seen, especially in the samples observed after 7, 14, and 21 days (Fig. 12e, g, and i, respectively). Calcite crystals may also have formed (indicated by red arrows) even in the samples observed after only 1 and 3 days, as explained by the results of the XRD tests, but the vaterite covered them and made them nearly impossible to see. On the other hand, the precipitates in the tubes containing the EICP-CU solution are mainly composed of agglomerated rhombohedral shapes (Fig. 12 b, d, f, h, and j). In brief, the predominant crystals observed in the tubes containing soybean crude urease are vaterite, while those in the tubes containing commercial urease are calcite. However, the vaterite crystals are gradually transformed to the more stable phase of CaCO3 (i.e., calcite) with an increase in the loading time. The presence of vaterite in the EICP-SCU materials may be due to the presence of certain content in the soybean crude urease. Khodadadi et al. [24] investigated the protein content in some plant-based urease sources and showed that the total protein in the soybean crude solution was higher than that in two other materials (i.e., jack bean meal and watermelon seeds). The presence of protein may cause the imperfection of calcite crystal morphology and prevent the formation of calcite crystals [40]. In addition, the formation of spherical vaterite can most likely be attributed to the supersaturation of the aqueous solution. In liquid–liquid systems, vaterite particles precipitate at room temperature under moderately supersaturated conditions [41,42,43]. A supersaturated solution is a solution that contains more than the maximum amount of solute that is capable of being dissolved at a given temperature. Since the SCU prescribed in this work is at a high concentration (20 g L−1) and the soybean powder was not completely dissolved in the solutions. The solubility of soybean powder is unclear and will be evaluated in a future study to check the hypothesis.
Analysis of strength effect
The applicability and efficacy of EICP-SCU solutions for soil improvement were investigated through soil strength tests. The soil specimens treated by both methods (EICP-SCU and EICP-CU) were prepared under two different experimental conditions to evaluate the sample strength and mineral distribution. The treated sample strength was analyzed through UCS tests, the mineral distribution in the sample was analyzed by measuring the CaCO3 content, and a visual investigation of the calcium carbonate precipitates within the soil was done by an SEM analysis.
Unconfined compressive strength
The relation between the UCS values for the EICP-SCU samples and the EICP-CU samples and the loading times are shown in Fig. 13a. In addition, the relation between the UCS values and the evaluated CaCO3 percentages obtained from the current study and, for comparison’s sake, other data available in the literature, are summarized in Fig. 13b. In order to facilitate the application of the flowable solution into the soil as discussed in “Evolution of precipitated mass with various concentrations” section, a SCU content of 20 g L−1 was selected and compared with a CU content of 2 g L−1 for the UCS test. The average UCS values of the soil samples treated by EICP-SCU were 191.2 kPa and 507.7 kPa for loading times of 7 and 14 days, respectively. In comparison, the average UCS values of the soil samples treated by the EICP-CU were about 30.1 kPa and 25.5 kPa for loading times of 7 and 14 days, respectively. The loading duration did not influence the UCS evolution of the EICP-CU samples, while it exerted a significant influence on that of the EICP-SCU samples. It is congruent with the study from [23] and that the sand treated by soybean urease had optimum UCS value at 14 days of loading time. Some other EICP-soybean studies also have reported that increasing the loading time brings a significant effect to the UCS value of treated sand [44] reported an increase in UCS value of 90% from 100 kPa in 3 days to 190 kPa in 7 days of loading time [32] also evaluated those 7–14 days of loading time can enhance the UCS value from 47 to 60 kPa. Increasing the loading duration may have caused the formation of more CaCO3 in the EICP-SCU samples and resulted in the higher UCS values of the 14-day samples. Overall, using soybean crude urease as the EICP material could enhance the strength of bare sand, which is consistent with the work by Lee and Kim [23] who have used yellow soybean powder for treating clayey sand.
The average UCS value of the soil samples treated by the EICP-SCU was more than five times that of the soil samples treated by the EICP-commercial enzyme. The soil samples treated by the EICP-commercial enzyme in this study are quite small than those in the previous study. However, it was reported that all the treated samples had almost similar calcium carbonates content. These results may support the argument that the use of crude extract urease provides more impurities due to the organic composition, such as protein in the EICP solution, and then influences the enhancement of the soil strength. It is congruent with the study from [24], the UCS values of the soil samples stabilized with jack been crude extract were reported to be 2–5 times higher than the UCS values of the soil samples stabilized with the more purified commercial urease (i.e., Sigma Aldrich urease). Figure 13b also indicates that, at a similar CaCO3 mass, the UCS values of the soybean-treated samples were greater than those in other studies done with a commercial enzyme urease [4, 14, 21]. Furthermore, this phenomenon should also be investigated through microstructural analysis.
The precipitated mineral distribution may also affect the strength of the treated sample. Therefore, in this section, the CaCO3 distribution in the treated sample will be analyzed. The vertical distribution of the CaCO3 content at three different locations (namely, bottom, middle, and top) is depicted in Fig. 14. As is shown in Fig. 14, the calcite distribution in the soil treated by the EICP-SCU was decreasing when it reached the top of the sample as was also occurring in the case of the EICP-CU sample. However, there is a difference in the middle and bottom parts of the treated sample. While the calcite content decreased in the middle of the sample treated by the EICP-SCU, contrastingly it increased in the sample treated by the EICP-CU. Moreover, in the bottom part of the treated sample, the CaCO3 of the EICP-SCU was concentrated and consisted of a greater amount compared to the EICP-CU sample. These results could not confirm which EICP solution would bring the better uniform distribution in the soil sample, which means the difference in strength between the EICP-SCU and the EICP-CU may not be affected by the calcite distribution. Reliance will be placed upon an SEM analysis to investigate the phenomenon, which will be described in the following section.
SEM analysis of treated samples
In this section, an SEM analysis was conducted to investigate the improvement mechanism of soil samples treated by the EICP solution. SEM images of the soil samples treated by both the EICP-SCU and the EICP-CU are depicted in Fig. 15. The analysis was focused on the appearance and size of the crystals of calcium carbonate precipitated on the soil particles. The SEM images of the soil samples treated with the EICP solution show precipitation similar to that of the SEM images of the precipitated materials generated from the tube tests (see Fig. 12). The SEM images of the soil samples treated by the EICP-SCU show the precipitation of spherical vaterite and rhombohedral calcite on the sand particle surfaces. On the other hand, the SEM images of the soil samples treated with the EICP-CU show the precipitation of rhombohedral calcite distributed over the sand particle surfaces. However, the appearance, size and shape of the precipitated calcium carbonate on the soil particles were slightly different from those of the tube test materials. Moreover, no agglomeration crystals were observed on the treated sand samples. This may have been caused by the fact that, when mineralization occurs on the surface of organic templates such as silica sand, the size and shape of the templates, even the chemical structure, and concentration of organic additives, control the form of the mineralized materials [45].
It was observed in the SEM images that, when the EICP-SCU solution was added to the soil samples, relatively large calcite crystals appeared that had formed mainly at the grain-to-grain contacts. Previous studies have also reported this phenomenon when adding organic materials, such as dry milk powder, to a solution [15]. Adding dry milk powder as a stabilizer to an EICP solution can increase the strength of the treated samples to that greater than samples treated without dry milk powder. The study has also reported that adding dry milk powder to an EICP solution can cause the formation of relatively large calcite and that the precipitated calcite will be concentrated at the inter-grain contacts.
Discussions
Further data are needed to clarify and explain the strength improvement phenomenon in the EICP-SCU samples that have superior strength to the EICP-CU samples. Figure 16 is provided to show the maximum magnification of SEM images for both EICP-SCU and EICP-CU samples for better analysis. In the figure, larger precipitated calcite crystals can clearly be seen for the EICP-SCU samples, collected in several group blocks, while this phenomenon is not found for the EICP-CU samples. The precipitated calcite crystals in the EICP-CU samples are likely to be distributed evenly on the sand particle surfaces and to be of the same crystal size. It is strongly believed that this is the main factor in the difference in strength between the EICP-SCU and the EICP-CU samples. The precipitated calcite crystals in the EICP-SCU samples also seem to have strong bonds between the soil particles, which is evidenced by the presence of fractures in the crystals. These fractures may illustrate connections to other parts of the attached calcite in the soil particles. It is also hypothesized that the difference in the higher concentration of protein content in the EICP-SCU solution is strongly assumed to be the main factor in the kinetic differences in the soil-treated samples.
Largely magnified images, focusing on the precipitated calcite that connects the soil grain particles in the soil treated by EICP-SCU, are depicted in Fig. 17. These images may provide a better understanding of the difference in strength between the EICP-SCU samples treated for 7 and 14 days. As is clearly shown in the figure, there is a notable difference in the size of the calcite in the connecting spaces. The calcite that formed over 14 days seems to be much larger and shows an increase in the covered connecting area in comparison to the calcite that formed over 7 days. Even though there is a less significant increment in the calcite content in the soil samples (see Fig. 13b), the difference in the geometry of the precipitates may be the main reason why the 14-day sample gained superior strength to the 7-day sample. The formed calcite may get larger and stronger as the loading time increases. The study was done by [46] also confirmed that increasing the experimental time enlarges the geometry size of connected precipitated calcite in porous media. It also verifies that the increments in the calcite content in the 14-day soil samples compared to the 7-day samples may not only be due to the addition of precipitated calcite but also due to the increase in crystal size.
The higher strength of the soil samples treated by EICP using plant-based crude extract (SCU) compared to the soil samples treated with commercially urease enzyme (CU) (shown in Fig. 13) indicates that there is something notable in the soybean crude extract that changed and modified the precipitation morphology, playing an important role in the soil strengthening and resulting in the soybean crude extract bringing more efficacy and effectivity. One of the possible reasons may be that the impurities and protein availability in the soybean crude extract allowed the EICP samples treated by the SCU to provide superior performance. However, the protein content was not measured in the current study, and therefore, future studies of protein content and impurities level are recommended. In addition, based on the previous result, utilizing soybean crude urease in the EICP technique has an issue in the reaction time. ECIP-soybean crude urease (EICP-SCU) required a longer time to achieve its satisfactory condition compared to the EICP-commercial enzyme (EICP-CU). Conversely, this issue may help the distribution of precipitated minerals, as its features of delay in the process of calcite formation. Therefore, EICP-SCU is expected to have a much well distribution of calcite than the EICP-CU. However, the distribution of calcium carbonate precipitation using soybean crude extract over large scale has not been done yet in this study. Therefore, further research to evaluate the distribution of precipitated materials within the large soil samples size is required.
Despite the superior performance of EICP-SCU, soybean crude extract is considered a highly inexpensive potential source for enzyme biocementation. As a comparison, 1 g of the soybean powder used in this study costs approximately $ 0.008, while the commercial urease enzyme (Junsei chemical urease) used in this study costs around $ 2.67. Both prices of the urease enzyme were purchased from the market in November 2021. There is such a big difference in cost between these materials. Even though the required amount of soybean crude urease is 10 times that of commercial urease enzyme, the cost is still considerably cheaper. The authors have estimated the approximate cost of EICP using both sources (SCU and CU) from the results of tube tests and referring to the costs estimated by [23]. It was estimated that EICP-SCU using 20 g of soybean powder costs approximately $3.27 at most ($ 0.18 for 20 g of soybean, $0.56 for using centrifuge device for 20 min, and $2.53 for labor) to precipitate 87.5 g of CaCO3 within 14 days in the 1 L of EICP solution. On the contrary, the EICP-CU required 2 g of enzyme urease which costs approximately $5.34 to produce the same amount of CaCO3 at the same condition. This shows the cost competitiveness of EICP when using the soybean crude extract.
Conclusions
The efficacy of soybean crude urease in carbonate precipitation as a soil improvement technique was evaluated. A series of laboratory experiments were conducted to examine the potential of using soybean powder as a new material. The urease tests showed that the activity of soybean crude urease linearly increases with the increased concentration of soybean powder. The results indicated that the urease activity of the soybean crude urease at a high concentration (more than 20 g/L) seems to be high enough for use in soil treatment. The tube tests confirmed the efficacy of using soybean urease that could produce calcium carbonate crystals. Interestingly, vaterite precipitation was observed when employing the soybean crude urease. However, prolonging the loading duration was seen to possibly result in a decrease in vaterite content and to bring about the formation of more stable calcite. The UCS test series showed that the strength of the EICP-SCU treated samples increased with an increase in the loading time. Moreover, the measured UCS values of the EICP-SCU samples were much higher than those of the EICP-CU samples. This should be attributed to the precipitation of CaCO3 concentrated at the grain-to-grain contacts in a larger size and collected in several blocks. All the test results lead to the conclusion that soybean powder is a material showing great efficacy as a replacement for commercially purified enzyme urease in the carbonate precipitation of soil-improvement techniques. Soybean powder is easy to obtain since it is commercially available everywhere. In addition, crude urease can be extracted simply by mixing soybean powder with water; and therefore, the processing fee is also inexpensive. It is concluded that soybean powder is a promising material for EICP in terms of its efficacy and cost-effectiveness.
Data availability statement
Not applicable.
References
Neupane D, Yasuhara H, Kinoshita N, Ando Y (2015) Distribution of mineralized carbonate and its quantification method in enzyme mediated calcite precipitation technique. Soils Found 55:447–457. https://doi.org/10.1016/j.sandf.2015.02.018
Neupane D, Yasuhara H, Kinoshita N, Unno T (2013) Applicability of enzymatic calcium carbonate precipitation as a soil-strengthening technique. J Geotech Geoenviron Eng 139:2201–2211. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000959
Yasuhara H, Neupane D, Hayashi K, Okamura M (2012) Experiments and predictions of physical properties of sand cemented by enzymatically-induced carbonate precipitation. Soils Found 52:539–549. https://doi.org/10.1016/j.sandf.2012.05.011
Oliveira PJV, Freitas LD, Carmona JPSF (2017) Effect of soil type on the enzymatic calcium carbonate precipitation process used for soil improvement. J Mater Civ Eng 29:1–7. https://doi.org/10.1061/(ASCE)MT.1943-5533.0001804
Whiffin VS, van Paassen LA, Harkes MP (2007) Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol J 24:417–423. https://doi.org/10.1080/01490450701436505
Martinez BC, DeJong JT, Ginn TR et al (2013) Experimental optimization of microbial-induced carbonate precipitation for soil improvement. J Geotech Geoenviron Eng 139:587–598. https://doi.org/10.1061/(asce)gt.1943-5606.0000787
Harkes MP, van Paassen LA, Booster JL et al (2010) Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol Eng 36:112–117. https://doi.org/10.1016/j.ecoleng.2009.01.004
van Paassen LA, Ghose R, van der Linden TJM et al (2010) Quantifying biomediated ground improvement by ureolysis: large-scale biogrout experiment. J Geotech Geoenviron Eng 136:1721–1728. https://doi.org/10.1061/(asce)gt.1943-5606.0000382
Nemati M, Voordouw G (2003) Modification of porous media permeability, using calcium carbonate produced enzymatically in situ. Enzyme Microb Technol 33:635–642. https://doi.org/10.1016/S0141-0229(03)00191-1
Neupane D, Yasuhara H, Kinoshita N, Putra H (2015) Distribution of grout material within 1-m sand column in insitu calcite precipitation technique. Soils Found 55:1512–1518. https://doi.org/10.1016/j.sandf.2015.10.015
Putra H, Yasuhara H, Kinoshita N, Hirata A (2017) Optimization of enzyme-mediated calcite precipitation as a soil-improvement technique: the effect of aragonite and gypsum on the mechanical properties of treated sand. Crystals. https://doi.org/10.3390/cryst7020059
Putra H, Yasuhara H, Kinoshita N et al (2018) Improving shear strength parameters of sandy soil using enzyme-mediated calcite precipitation technique. Civil Eng Dimens 20:91. https://doi.org/10.9744/ced.20.2.91-95
Neupane D, Yasuhara H, Putra H, Kinoshita N (2018) Inorganically precipitated phosphates and carbonates to improve porous material properties. EPI Int J Eng 1:1–6. https://doi.org/10.25042/epi-ije.022018.01
Almajed A, Tirkolaei HK, Kavazanjian E (2018) Baseline investigation on enzyme-induced calcium carbonate precipitation. J Geotech Geoenviron Eng 144:1–11. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001973
Almajed A, Tirkolaei HK, Kavazanjian E, Hamdan N (2019) Enzyme induced biocementated sand with high strength at low carbonate content. Sci Rep 9:1–7. https://doi.org/10.1038/s41598-018-38361-1
Putra H, Yasuhara H, Kinoshita N (2017) Optimum condition for the application of enzyme-mediated calcite precipitation technique as soil improvement method. Int J Adv Sci Eng Inform Technol 7:2145–2151. https://doi.org/10.18517/ijaseit.7.6.3425
Almajed AA, Kavazanjian E, Zapata C, Hamdan NM Enzyme Induced Carbonate Precipitation (EICP) for Soil Improvement. Enzyme Induced Carbonate Precipitation (EICP) for Soil Improvement
Arab MG (2019) Soil Stabilization using calcium carbonate precipitation via urea hydrolysis. Proceedings of the 4th World Congress on Civil, Structural, and Environmental Engineering. https://doi.org/10.11159/icgre19.149
Hamed Khodadadi T, Kavazanjian E, van Paassen L, DeJong J (2017) Bio-grout materials:a review. Grouting. https://doi.org/10.1061/9780784480793.001
Young J, Sup T (2018) Evaluation of soil improvement by carbonate precipitation with urease. J Korean Geotech Soc 33:61–69. https://doi.org/10.7843/kgs.2017.33.9.61
Putra H, Yasuhara H, Erizal et al (2020) Review of enzyme-induced calcite precipitation as a ground-improvement technique. Infrastructures. https://doi.org/10.3390/INFRASTRUCTURES5080066
Dilrukshi RAN, Nakashima K, Kawasaki S (2018) Soil improvement using plant-derived urease-induced calcium carbonate precipitation. Soils Found 58:894–910. https://doi.org/10.1016/j.sandf.2018.04.003
Lee S, Kim J (2020) An experimental study on enzymatic-induced carbonate precipitation using yellow soybeans for soil stabilization. KSCE J Civ Eng 24:2026–2037. https://doi.org/10.1007/s12205-020-1659-9
Khodadadi Tirkolaei H, Javadi N, Krishnan V et al (2020) Crude urease extract for biocementation. J Mater Civil Eng. https://doi.org/10.1061/(ASCE)MT.1943-5533.0003466
Pratama GBS, Yasuhara H, Kinoshita N, Putra H (2021) Application of soybean powder as urease enzyme replacement on EICP method for soil improvement technique. IOP Conf Ser Earth Environ Sci. https://doi.org/10.1088/1755-1315/622/1/012035
Gao Y, He J, Tang X, Chu J (2019) Calcium carbonate precipitation catalyzed by soybean urease as an improvement method for fine-grained soil. Soils Found 59:1631–1637. https://doi.org/10.1016/j.sandf.2019.03.014
Putra H, Erizal S et al (2021) Improvement of organic soil shear strength through calcite precipitation method using soybeans as bio-catalyst. Crystals 11:1044. https://doi.org/10.3390/cryst11091044
Das N, Kayastha AM, Srivastava PK (2002) Purification and characterization of urease from dehusked pigeonpea (Cajanus cajan L) seeds. Phytochemistry 61:513–521. https://doi.org/10.1016/s0031-9422(02)00270-4
Martin K, Tirkolaei HK, Kavazanjian E (2021) Enhancing the strength of granular material with a modified enzyme-induced carbonate precipitation (EICP) treatment solution. Constr Build Mater 271:121529. https://doi.org/10.1016/j.conbuildmat.2020.121529
ASTM A (2018) Standard Practice for Classification of Soils for Engineering Purposes (Unified Soil Classification System)-ASTM D2487-11. https://www.astm.org/d2487-11.html. Accessed 28 Feb 2022
Cuccurullo A, Gallipoli D, Bruno AW et al (2020) Earth stabilisation via carbonate precipitation by plant-derived urease for building applications. Geomech Energy Environ 30:100230. https://doi.org/10.1016/j.gete.2020.100230
Lofianda L, Putra H, Erizal E et al (2021) Potentially of soybean as bio-catalyst in calcite precipitation methods for improving the strength of sandy soil. Civil Eng Archit 9:2317–2325. https://doi.org/10.13189/cea.2021.090719
Altomare A, Cuocci C, Giacovazzo C et al (2008) QUALX: a computer program for qualitative analysis using powder diffraction data. J Appl Crystallogr 41:815–817. https://doi.org/10.1107/S0021889808016956
Ryu M, Ahn J, You K et al (2009) Synthesis of calcium carbonate in ethanol-ethylene glycol solvent. J Ceram Soc Japan 117:106–110. https://doi.org/10.2109/jcersj2.117.106
Mori Y, Enomae T, Isogai A (2009) Preparation of pure vaterite by simple mechanical mixing of two aqueous salt solutions. Mater Sci Eng, C 29:1409–1414. https://doi.org/10.1016/j.msec.2008.11.009
Svenskaya YuI, Fattah H, Zakharevich AM et al (2016) Ultrasonically assisted fabrication of vaterite submicron-sized carriers. Adv Powder Technol 27:618–624. https://doi.org/10.1016/j.apt.2016.02.014
Boyjoo Y, Pareek VK, Liu J (2014) Synthesis of micro and nano-sized calcium carbonate particles and their applications. J Mater Chem A 2:14270–14288. https://doi.org/10.1039/C4TA02070G
Konopacka-Łyskawa D (2019) Synthesis methods and favorable conditions for spherical vaterite precipitation: a review. Crystals 9:223. https://doi.org/10.3390/cryst9040223
Baiq HS, Yasuhara H, Kinoshita N et al (2020) Examination of calcite precipitation using plant-derived urease enzyme for soil improvement. Int J GEOMATE 19:231–237. https://doi.org/10.21660/2020.72.9481
Jimenez-Lopez C, Rodriguez-Navarro A, Dominguez-Vera JM, Garcia-Ruiz JM (2003) Influence of lysozyme on the precipitation of calcium carbonate: a kinetic and morphologic study. Geochim Cosmochim Acta 67:1667–1676. https://doi.org/10.1016/S0016-7037(02)01275-9
Tai CY, Chen FB (1998) Polymorphism of CaCO3 precipitated in a constant-composition environment. AIChE J 44:1790–1798. https://doi.org/10.1002/aic.690440810
Njegić-Džakula B, Falini G, Brečević L et al (2010) Effects of initial supersaturation on spontaneous precipitation of calcium carbonate in the presence of charged poly-l-amino acids. J Colloid Interface Sci 343:553–563. https://doi.org/10.1016/j.jcis.2009.12.010
Beck R, Andreassen J-P (2010) The onset of spherulitic growth in crystallization of calcium carbonate. J Cryst Growth 312:2226–2238. https://doi.org/10.1016/j.jcrysgro.2010.04.037
Baiq HS, Putra H, Almajed A, et al (2020) Efficacy of organic additive in EICP technique for soil improvement. The 2020 World Congress on Advances in Civil, Environmental, & Materials Research (ACEM20) 25–28
Walsh D, Lebeau B, Mann S (1999) Morphosynthesis of calcium carbonate (vaterite) microsponges. Adv Mater 11:324–328. https://doi.org/10.1002/(SICI)1521-4095(199903)11:4%3c324::AID-ADMA324%3e3.0.CO;2-A
Nooraiepour M, Masoudi M, Hellevang H (2021) Probabilistic nucleation governs time, amount, and location of mineral precipitation and geometry evolution in the porous medium. Sci Rep 11:1–17. https://doi.org/10.1038/s41598-021-95237-7
Funding
This work has been partly supported by a research grant from the Penta-Ocean Constructions Co., Ltd. Their support is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
GBSP contributed by defining the area of study, testing the specimens, conducting analysis, and drafting the manuscript. SF contributed by testing the specimens and collecting data. ZMI was involved in analyzing data, drafting the manuscript, and providing revised content for the manuscript. HY, NK, HP, and AA contributed by giving a critical review, revising the content of the manuscript, and giving final approval for submission. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pratama, G.B.S., Yasuhara, H., Kinoshita, N. et al. Efficacy of soybean-derived crude extract in enzyme-induced carbonate precipitation as soil-improvement technique. Geo-Engineering 15, 14 (2024). https://doi.org/10.1186/s40703-024-00204-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40703-024-00204-6