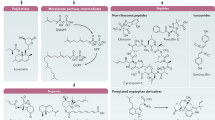
Abstract
Fungal specialized metabolites play an important role in the environment and have impacted human health and survival significantly. These specialized metabolites are often the end product of a series of sequential and collaborating biosynthetic enzymes that reside within different subcellular compartments. A wide variety of methods have been developed to understand fungal specialized metabolite biosynthesis in terms of the chemical conversions and the biosynthetic enzymes required, however there are far fewer studies elucidating the compartmentalization of the same enzymes. This review illustrates the biosynthesis of specialized metabolites where the localization of all, or some, of the biosynthetic enzymes have been determined and describes the methods used to identify the sub-cellular localization.
Similar content being viewed by others
Introduction
Specialized metabolites are small molecules found in plants, fungi, and bacteria, with a diverse array of molecular structures that vary considerably, even between closely related species. Specialized metabolites produced by fungi have specific ecological functions to aide in survival [1], and many have been adapted for medicinal or agricultural use [2] such as the antibiotic penicillin, the immunosuppressant mycophenolic acid, the cholesterol-lowering drug lovastatin, and the anti-fungal agent strobilurin (Fig. 1). Many fungal specialized metabolites, however, have a detrimental effect on human health and the environment [3] including the mycotoxins aflatoxin, fumonisin, deoxynivalenol, and patulin (Fig. 1).
Specialized metabolites are classified according to their biosynthetic origin and the major classes are: polyketides, terpenes, and non-ribosomal peptides; however molecules with mixed biosynthetic origin are common, including: meroterpenoids, polyketide/non-ribosomal peptides, and alkaloids. Polyketides are synthesized by polyketide synthases (PKS) from acyl-CoA substrates, most commonly acetyl-CoA and malonyl-CoA, although other small organic acids can be utilized [4]. In fungi, PKS enzymes can be further classified into non-reducing (NR) PKS, partially reducing (PR) PKS, and highly reducing (HR) PKS which vary according to the catalytic domains present, and the extent of reduction observed in the final polyketide product (Scheme 1A) [5, 6]. Terpenes are synthesized by terpene synthases (TS) (also known as terpene cyclases (TC)), from the head-to-tail condensation of isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) (Scheme 1B) [7]. Non-ribosomal peptides are synthesized by non-ribosomal peptide synthetases (NRPS) that condense proteinogenic and non-proteinogenic amino acids to generate diverse peptides (Scheme 1C) [8]. Polyketide/non-ribosomal peptides are often synthesized by PKS and NRPS hybrids known as polyketide synthases / non-ribosomal peptide synthetase (PKS-NRPS) [9].
Overview of major classes of biosynthetic enzymes in fungi. A Polyketide biosynthesis; B terpenoid biosynthesis; C non-ribosomal peptide biosynthesis. SAT : starter unit: acyl-carrier protein transacylase; KS: ketosynthase; AT: acyltransferase; PT: product template; ACP: acyl carrier protein; MT: methyltransferase; TD: terminal domain; TH: thiolation; KR: ketoreductase; ER: enoylreductase; TS / TC: terpene synthase / terpene cyclase; C: condensation; A: adenylaton; M: methylation; T: thiolation; E: epimerase. Catalytical domains that are shown faded may or may not be present
Typically, the final specialized metabolite observed has been modified or diversified by additional tailoring enzymes. The major classes of tailoring enzymes include: cytochrome P450 monooxygenases (P450), oxidases (OX), oxidoreductases (OXR), reductases (Red), hydrolases (HYD), dehydratases (DH), cyclases (CYC), methyltransferases (MT), and prenyltransferases (PT), although many additional types have been identified. Furthermore, there are increasing reports of protein-protein interactions between megasynth(et)ases (e.g. PKS and PKS-NRPS) and accessory and / or tailoring enzymes [10]. Additional, and often essential, enzymes required to efficiently regulate and excrete specialized metabolites include transcription factors (TF), transporters, and self-resistance proteins [1].
For most elucidated fungal biosynthetic pathways, the genes encoding the specialized metabolite are clustered together in the genome and co-regulated [1, 11]. Fungal biosynthetic gene clusters (BGCs) can readily be identified in sequenced genomes using bioinformatics tools such as fungiSMASH [12], and advances in molecular techniques such heterologous expression, gene disruption, and in vitro enzyme characterization enables the full elucidation of the pathway [13,14,15]. However, identifying the sub-cellular location of the individual enzymes within the native cell lags significantly behind. Understanding the sub-cellular localization, interactions, and transport of biosynthetic enzymes is useful for the efficient heterologous expression and engineering of BGCs in more amenable hosts for larger-scale production of medicinally or agriculturally important molecules [16]. This review highlights the progress that has been made in understanding where fungal specialized metabolites are synthesized within the cell and how the intermediates of these pathways are shuttled between locations and eventually exported.
Subcellular localization of biosynthetic enzymes
Fungi have a complex cellular organization with numerous sub-compartments [17]. Fungal cells contain a nucleus, mitochondria, the endoplasmic reticulum, Golgi apparatus, and small membrane-bound organelles (Fig. 2). These small organelles can be further distinguished as vesicles, endosomes, vacuoles, and peroxisomes, each with variety of specific functions. Investigations into the function of the different organelles pertaining to specialized metabolism has indicated that synthesis and transport of metabolites, and associated biosynthetic enzymes, is a highly dynamic process [18]. Often multiple organelles are used, and evidence suggests that different organelles are used depending on whether biosynthetic enzymes are associated with early, middle, or late-stage biosynthetic modifications [19]. These organelles may also be referred to as “toxisomes” indicating that they contain potentially toxic pathway molecules [20, 21].
An overview of the experimentally established location of fungal biosynthetic enzymes are shown in Table 1. Enzymes required for synthesis of the carbon backbone of the molecule (e.g., PKS, NRPS, TS/TC) are highlighted in bold and do not appear to occur in a single organelle. For example, PKS enzymes have been identified in the cytosol, peroxisomes, and unidentified organelles. Furthermore, the biosynthesis of the same specialized metabolite may occur in different organelles depending on which fungal species is investigated e.g., melanin biosynthesis. And finally, the dynamic nature of specialized metabolism means that some biosynthetic enzymes are located in more than one organelle (e.g., the enzymes highlighted in italic in Table 1). Although a highly complex and understudied area of fungal specialized metabolism, this review is intended to collate the progress on identifying the subcellular localization of fungal biosynthetic enzymes and highlight potential common themes. For simplicity each investigated metabolite will be discussed separately according to its biosynthetic origin.
Subcellular compartmentalization of polyketide biosynthesis
Aflatoxin biosynthesis
Aflatoxin is one of the most potent carcinogens known and is produced primarily by Aspergillus parasiticus and Aspergillus flavus [22]. The biosynthesis of aflatoxin requires over 25 genes which encode over 27 enzymatic activities. The BGC is regulated by the transcriptional regulator AflR and the transcriptional co-activator AflJ [23]. The first biosynthetic step is the synthesis of norsolorinic acid anthrone through the action of two fatty acid synthases (FAS; AflA and AflB) and a polyketide synthase (PKS; AflC), which form a protein complex (Scheme 2) [24, 25]. Norsolorinic acid anthrone is then oxidized into norsolorinic acid by HypC [26]. Further processing and modification of norsolorinic acid into aflatoxin is performed by a series of tailoring enzymes (Scheme 2). Modifications include: reduction by the NADPH-dependent ketoreductase (KR) AflD (also known as Nor-1) that converts norsolorinic acid to averantin [27]; oxidation by the alcohol dehydrogenase AflH that converts 5-hydroxy-averantin to 5’oxoaverantin [28]; and cyclization by the novel cyclase (CYC) AflK which converts 5’-oxoaverantin to averufin and subsequently versicolorin [28, 29]. In later steps, versicolorin A is converted to demethylsterigmatocystin by the NADPH-dependent reductase (Red) AflM (also known as Ver-1) [30] and sterigmatocystin is converted to O-methylsterigmatocystin by the methyltransferase (MT) AflP (also known as OmtA) [31].
The subcellular localization of many of the enzymes has been established using a variety of techniques including fluorescence protein fusions, transmission electron microscopy, and immunofluorescence microscopy. AflA, AflB, AflC and HypC are speculated to be located within peroxisomes due to the accumulation of norsolorinic acid in the peroxisomes [32]. Studying AflD fused to eGFP by confocal microscopy indicated that the enzyme is first synthesized in the cytoplasm and transported to vacuoles where it is active [33]. AflH was identified from the cytosolic fraction but its precise subcellular localization is unknown [28]. Similarly, AflK was identified from the cytosolic fragment of A. parasiticus initially and later shown by confocal laser scanning microscopy and immunogold transmission electron microscopy to be distributed in the cytoplasm and concentrated in structures that are ring-like and closely positioned on the outside surface of nuclei (Chiou et al. [34]). Examination of the protein sequence of AflK identified a signal peptide indicative of endoplasmic reticulum / Golgi localization [34]. Fusion of enhanced green fluorescence protein (eGFP) to AflM demonstrated that AflM is synthesized and localized in the cytoplasm and was found in the lumen of up to 80% of vacuoles [35]. Immunofluorescence microscopy determined that AflP is localized in the cytoplasm as well as vacuoles [36]. Using yellow fluorescence protein fused to AflJ indicated that AflJ localizes mostly in endosomes, and that AflR co-localizes with AflJ both in endosomes and nuclei [37].
Melanin biosynthesis
Fungal melanin is a pigmented biopolymer that protects its host from UV radiation, has an essential role in survival and infection, and is found as globular particles in fungal cell walls [38]. Most filamentous fungi synthesize melanin via a polyketide pathway, although the exact pathway has been demonstrated to differ between fungi. In Aspergillus fumigatus, biosynthesis of melanin requires at least six enzymes: the NRPKS Alb1 that synthesizes the polyketide YWA1 [39]; the chain shortening hydrolase (HYD) Ayg1 that converts YWA1 to 1,3,6,8-tetrahydroxynaphthalene (THN) [40, 41]; the reductase (Red) Arp2 that reduces 1,3,6,8-THN to scytalone; the scytalone dehydratase (DH) Arp1; the copper oxidase (OX) Abr1; and laccase (Lac) Abr2 (Scheme 3A). Arp1 dehydrates scytalone to produce 1,3,8-THN, and Arp2 acts a second time reducing 1,3,8-THN to vermelone. Vermelone is then oxidized by the copper oxidase Abr1 to 1,8-dihydroxynaphthalene and polymerized by the laccase Abr2 to melanin (Scheme 3A) [42, 43].
The localization of the melanin biosynthetic enzymes from Aspergillus fumigatus were investigated using fluorescence tags and specific organelle stains [19]. The PKS Alb1 was localized to endosomes, rather than being diffused through the cytoplasm as predicted bioinformatically. Furthermore, the additional three enzymes, Ayg1, Arp1, and Arp2, involved in early steps of the biosynthesis were also observed in endosomes, also in contrast to bioinformatics predictions. The two late-stage enzymes Abr1 and Abr2, predicted as secreted proteins, were found to accumulate at the cell wall [19].
Aspergillus nidulans also synthesizes melanin pigments utilizing a different pathway, where only two enzymes have been identified [44, 45]; the NRPKS WA and the laccase YA (Scheme 3B). Bioinformatics analysis predicts WA to be a cytosolic enzyme and YA a secreted enzyme, which indicates a clear separation of early-stage and late-stage melanin biosynthetic enzymes, common to many fungi [19]. However, their subcellular localization was determined to be in endosomes and at the cell wall respectively [19].
Investigation into the biosynthesis of melanin in Botrytis cinerea led to the observation that scytalone is secreted extracellularly and accumulation of this metabolite inhibits the growth of B. cinerea [46]. Similar to melanin biosynthesis in A. fumigatus, melanin biosynthesis in B. cinerea requires six biosynthetic enzymes: the PKS BcPKS12 (or BcPKS13 with the hydrolase BcYGH1); the reductases BcBRN1 and BcBRN2; and the dehydratase BcSCD1 (Scheme 3C) [47]. The subcellular location of the different melanin biosynthetic enzymes was determined via fusion with GFP in combination with organelle stains. The two PKS, BcPKS12 and BcPKS13, and the hydrolase BcYGH1 were demonstrated to be localized in peroxisomes, the two reductases BcBRN1 and BcBRN2 were detected within endosomes initially, and BcSCD1 is localized at the cell wall (Scheme 3C) [46]. The specific locations of BcBRN1 and BcBRN2 is further described in "Transient recruitment of enzymes and trafficking" section.
Patulin biosynthesis
Patulin is a polyketide-derived mycotoxin that is found in fruit and is produced mainly by Penicillium expansum [48]. The biosynthesis of patulin requires at least 10 steps and the BGC encodes 11 biosynthetic enzymes, a transcription factor, and three transporters [49]. PatK is a PRPKS that synthesizes 6-methylsalicylic acid that is then decarboxylated by the 6-methylsalicylic acid decarboxylase PatG, and sequentially hydroxylated by PatH and PatI, both P450s [50,51,52]. It is unknown how gentisyl alcohol is converted to isoepoxydon, however, the oxidase PatO and hypothetical protein (U) PatJ are proposed to be involved [53]. The dehydrogenase PatN converts isoepoxydon to phyllostine which is converted to neopatulin via the hypothetical protein PatF [53]. The dehydrogenase PatD converts neopatulin to E-ascladiol and then finally the action of the oxidoreductase PatE results in the synthesis of patulin (Scheme 4).
The localization of the enzymes responsible for biosynthesis for patulin were determined by fusing eGFP at their C-terminal [53]. Most biosynthetic enzymes (PatK, PatG, PatN, PatF, and PatD) were detected within the cytosol. PatB, a carboxyesterase, was also detected in the cytosol, however its precise biosynthetic role is unknown. The oxidoreductase PatE is localized to the cell wall and believed to be a secreted protein, whereas the two P450s PatH and PatI are localized to the endoplasmic reticulum. PatA, an acetate transporter, was also localized to the endoplasmic reticulum and the two transporters PatC and PatM were localized to the plasma membrane [53]. PatL is a Zn(II)2Cys6 binuclear cluster transcription factor and is localized in the cell nuclei [49].
Viriditoxin biosynthesis
Viriditoxin is a biaryl polyketide that exhibits antibiotic and cytotoxic bioactivities [54]. The biosynthesis was established in Paecilomyces variotti where the NRPKS VdtA synthesizes the polyketide backbone [55]. This is followed by methylation by VdtC, an O-methyltransferase, reduction by VdtF, oxygen insertion via VdtE, and dimerization catalyzed by the laccase VdtB. A transporter VdtG and regulator VdtR were also encoded by the biosynthetic gene cluster (Scheme 5). The subcellular localization of VdtA was investigated through fusion with GFP [55]. Fungal strains expressing this VdtA-GFP fusion protein displayed green fluorescence that was localized to small circular structures within the cells. These structures were deduced as not being peroxisomes, nuclei, or mitochondria [55]. The subcellular localization of the transporter VdtG was also investigated using GFP fusions and determined to be situated at the endoplasmic reticulum membrane, potentially within a toxisome.
AK-toxin biosynthesis
AK toxin is a polyketide-derived mycotoxin isolated from Alternaria alternata responsible for causing black spot of Japanese pears [56]. The polyketide portion, 9,10-epoxy-8-hydroxy-9-methyl-decatrienoic acid (EDA), present in several other toxins also isolated from A. alternata isolates, is proposed to arise from an unusual highly reducing polyketide synthase (HRPKS), a HMG-CoA synthase (HMGS) and a P450, at a minimum (Scheme 6) [57]. A BGC has been identified that encodes a PKS (AFT9h), a carboxyl-activating enzyme (AKT1), an α,β-hydrolase (AKT2), an enoyl-CoA hydratase (AKT3), a HMG-CoA synthase (AKT4), a P450 (AKT7) and a transcriptional regulator (AKTR), however the full sequence of biosynthetic steps remains to be elucidated [57,58,59,60].
Orthologs of the biosynthetic genes AKT1, AKT2, AKT3 are conserved in A. alternata strains that also produce toxins with an EDA core and therefore are believed to be required for the biosynthesis of this polyketide [61]. Analysis of the encoded enzyme sequence identified putative peroxisome tripeptide sequences in AKT1, AKT2 and AKT3. Localization of each enzyme to peroxisomes was confirmed by creating N-terminal GFP fusions that were expressed into A. alternata resulting in fluorescence localized to punctate organelles [61]. When the C-terminal tripeptide sequence was deleted, the resulting observed fluorescence was cytosolic [61].
Fumonisin biosynthesis
Fumonisins are polyketide-derived mycotoxins produced by Fusarium and Aspergillus sp. that contaminate corn [62]. Fumonisin B1 (FB1) is an inhibitor of sphingolipid biosynthesis, which leads to interference with eukaryotic cellular membranes, potentially leading to disease in humans and animals [63]. In Fusarium verticillioides the fumonisin BGC contains 16 genes including a HRPKS (Fum1) that synthesizes an octadecanoic acid precursor and the aminotransferase (Fum8) that condenses L-alanine with the polyketide (Scheme 7). Fum11, a tricarboxylate transporter; Fum10, an acyl-CoA synthetase; Fum7, a dehydrogenase; and Fum14 a bidomain NRPS; synthesize and transfer the sidechain to generate FB3 [64, 65]. Finally, the dioxygenase Fum3 adds a single hydroxyl group converting FB3 to FB1 [64].
Although the BGC has been extensively investigated, the functions of several genes (FUM15 – FUM19) remained unclear and the self-protection mechanism was unknown. Through gene disruption studies Fum19 was determined to be an ABC transporter that acts as repressor of the FUM BGC, and Fum17 and Fum18 were established as ceramide synthases which confer resistance to FB1 [66]. Furthermore, an additional three ceramide synthases (Cer1 – Cer3) were also found in F. verticillioides outside of the FUM BGC. To investigate the subcellular localization of Fum3, Fum8, Fum17, Fum18, and Cer1 were fused to different combinations of green, red, and blue fluorescent proteins. Fum17, Fum18 and Cer1 were found to colocalize in the perinuclear endoplasmic reticulum, whereas Fum8 and Fum3 were found in endoplasmic reticulum-derived vesicles and the cytosol respectively [66]. The conversion of FB3 into FB1 away from the ceramide synthases is proposed as preventing self-toxification [66].
Subcellular compartmentalization of terpenoid biosynthesis
Trichothecene biosynthesis
Trichothecenes are sesquiterpene mycotoxins that causes toxicity in plants, animals, and humans, synthesized by Fusarium sp. and other fungi. The mevalonate pathway enzymes Hms1 and Hmr1 are involved in the biosynthesis of mevalonate which is a precursor to farnesylpyrophosphate (FPP). FPP is converted to trichodiene by the terpene cyclase Tri5 (Scheme 8) [67]. Trichodiene is then converted to deoxynivalenol (DON) by a series of enzymes including three P450 Tri1, Tri4, and Tri11 [68]. Tri14 is an enzyme with unknown function that is also required for DON biosynthesis [69].
The subcellular localization of several enzymes required for DON biosynthesis have been established using fluorescent protein fusions. The two P450s Tri1 and Tri 4 co-localize to structures termed “toxisomes” and the HMG-CoA reductase Hmr1 co-localizes with Tri4 upon induction of mycotoxin production [20]. The toxisomes were later established as being associated with the endoplasmic reticulum and include Tri14, the enzyme with unknown function. The terpene synthase Tri5 is localized in the cytosol [70] and the HMG-CoA synthase (Hms1) is also [71]. The major facilitator superfamily (MFS) protein Tri12p is located within vesicles [20].
The composition of the toxisome was further investigated using transmission electron microscopy (TEM) [70]. The P450s Tri1, Tri4, and Tri11 are anchored to the endoplasmic reticulum membrane with the P450 reductase (P450R) and Hmr1 [72]. Although the terpene cyclase Tri5 is established as being cytosolic, inactivation of this enzyme prevented toxisome assembly at the endoplasmic reticulum [72]. Tri5 therefore was proposed to interact directly with the toxisome complex or potentially to be post-translationally modified and attach itself to the endoplasmic reticulum membrane [72].
Gibberellin biosynthesis
Gibberellic acids (GA) are a large family of diterpenoid compounds produced by fungi and plants that contain a gibberellan ring structure. In Fusarium fujikuroi the GGPS Ggs2 synthesizes GGPP which is converted to ent-kaurene by the diterpene cyclase Cps/Ks (Scheme 9) [73]. Two P450s, P450-4 and P450-1, convert ent-kaurene to ent-kaurenoic acid and GA14 respectively [74, 75], where GA14 is the precursor to the biologically active gibberellins used as plant phytohormones.
The subcellular localization of Ggs2, Csp/Ks, and HmgR were investigated using fluorescence protein fusion constructs. Cps/Ks was determined as having cytosolic localization but Ggs2 and HmgR were observed as small punctate structures [76]. Ggs2 contains a peroxisomal targeting signal but when co-expressed with a peroxisomal targeting fluorescence protein, the two did not co-localize, indicating that Ggs2 is contained within organelles that are not peroxisomes [76]. HmgR contains eight predicted transmembrane domains and its fluorescence pattern colocalized with the endoplasmic reticulum [76].
Paxilline biosynthesis
Paxilline is an indole-diterpene produced by Penicillium paxilli. The cyclic diterpene core arises from geranylgeranyl diphosphate (GGPP) synthesized by PaxG, a geranylgeranylpyrophosphate synthase [77]. The prenyltransferase PaxC transfers GGPP to indole 3-glycerol phosphate to form 3-geranylgeranyl indole. In an unusual sequential manner PaxM, an FAD-dependent monooxygenase, and PaxB, a novel terpene cyclase [78, 79], form paspaline which is a common carbon skeleton observed in many fungal indole-diterpene metabolites (Scheme 10). Finally, two P450s, PaxP and PaxQ, covert paspaline to paxilline.
Localization studies using both N-terminal and C-terminal eGFP fusions led to unstable transformants [77]. Bioinformatics analysis of PaxG revealed a tripeptide sequence indicative of peroxisomal targeting. Fusing this tripeptide sequence to eGFP led to green fluorescence in punctate organelles that were determined to be peroxisomes [77]. When a truncated PaxG, lacking the tripeptide sequence, was used to complement the function of an inactivated PaxG, paxilline biosynthesis could not be restored, demonstrating that the enzyme needs to be localized to the correct subcellular compartment [77].
Aphidicolin biosynthesis
Aphidicolin is a terpenoid that inhibits DNA polymerase [80]. In Phoma betae the biosynthesis of the terpene backbone arises via the geranylgeranyl diphosphate synthase (PbGGS) and the terpene synthase aphidicolin-16b-ol synthase (PbACS). Two P450s (PbP450-1 and PbP450-2) are required for two hydroxylations (Scheme 11) [81]. Investigation of aphidicolin biosynthesis using the heterologous host Aspergillus oryzae led to lower titers of production than expected so the subcellular localization of the individual biosynthetic enzymes were investigated in the heterologous host. Fusion of green fluorescence protein to each of the biosynthetic enzymes revealed that PbGGS and PbACS are localized to the cytoplasm whereas the two P450 enzymes are located at the endoplasmic reticulum membrane [82].
Subcellular compartmentalization of meroterpenoid biosynthesis
Mycophenolic acid biosynthesis
Mycophenolic acid is a meroterpenoid that inhibits inosine-5’-monophosphate dehydrogenase and is used commercially as an immunosuppressant drug [83, 84]. Recently, the biosynthesis of mycophenolic acid was established in Penicillium brevicompactum [85]. The NRPKS MpaC’ synthesizes 5-methylorsellinic acid, and the dual function P450/hydrolase MpaDE’ converts 5-methylorsellinic acid to 3,5-dihydroxy-7-(hydroxymethyl)-6-methylbenzoic acid and then 3,5-dihydroxy-6-methylphthalide. The prenyltransferase MpaA’ farnesylates 3,5-dihydroxy-6-methylphthalide to 4-farnesyl-3,5-dihydroxyphthalide (FDHMP) and the oxidase MpaB’ catalyzes the oxidative cleavage of FDHMP between the C19 and C20 double bond resulting in FDHMP-3 C. The O-methyltransferase MpaG’ methylates FDHMP-3 C to form MFDHMP-3 C. The acyl-CoA ligase PbACL891 converts MFDHMP-3 C to its acyl-CoA ester MFDHMP-3 C-CoA which is subsequently shortened via β-oxidation, controlled by the acyl-CoA hydrolase MpaH’, and finally released as mycophenolic acid (Scheme 12) [85].
MpaC’ and MpaG’ are reported as cytosolic enzymes and the subcellular localization of the membrane-associated enzymes was determined using N- or C-terminal GFP tags and specific organelle markers [85]. Both MpaB’ and MpaDE’ were detected localized with the endoplasmic reticulum while MpaA’ was determined to reside in ring like structures at the Golgi complex. MpaH’ contains a peroxisomal tripeptide sequence and, as expected, was found localized in peroxisomes; removal of the tripeptide sequence led to loss of localization [85].
Subcellular compartmentalization of peptide biosynthesis
Non-ribosomal peptides
Penicillin biosynthesis
Penicillin is a commonly used antibiotic used to treat bacterial infections. Due to the importance of penicillin for human health, the sub-cellular localization of the biosynthetic enzymes for are some of the most well investigated (Scheme 13). In penicillin biosynthesis the NRPS (ACVS) selects, activates, and condenses L−α-aminoadipic acid, L-cysteine and L-valine to synthesize α-aminoadipyl-L-cysteinyl-D-valine (ACV), the first enzyme-free intermediate in the pathway [86]. Isopenicillin N synthase (IPNS) then converts ACV to isopenicillin N [87]. Isopenicillin N is then converted to penicillin G through the action of isopenicillin N acyltransferase (IAT) [88].
The sub-cellular localization of penicillin has been studied extensively. The non-proteinogenic amino acid L−α-aminoadipic acid is stored in vacuoles [89] and it is speculated that this a deliberate self-protection mechanism [90]. The localization of the NRPS ACVS has reported as being associated with small organelles [91] later identified as vacuoles [89], however ACVS has also been identified in the cytosol [92]. IPNS was detected in the cytosol and IAT was detected in small organelles with a diameter of 200–800 nm [91], later identified as peroxisomes [93]. Phenylacetyl-CoA ligase (PCL), required for converting phenylacetic acid into phenylacetyl-CoA, was identified as containing a peroxisomal signal peptide and later confirmed in the peroxisomes [93,94,95].
Three transporters have been identified for shuttling intermediates between compartments (Scheme 13). The first, PenV, transports L-α-aminoadipic acid from the vacuole to the cytoplasm and is located at the vacuolar membrane [96]. PenM transports isopenicillin N from the cytoplasm to peroxisomes; fusion of PenM with DsRed protein confirmed its peroxisomal location and over-expression using a strong promoter increased production of penicillin G [97]. PaaT transports phenylacetic acid from the cytoplasm to peroxisomes, preventing self-toxification [90]. Fusion of PaaT to DsRed combined with organelle markers and fluorescent laser scanning microscopy demonstrated localization in the peroxisomal membrane [98]. Furthermore, over-expression of PaaT lead to significantly increased titers of penicillin production.
The final transport of penicillin G from peroxisomes to outside of the cell and into the growth media proceeds via an unknown mechanism. It is interesting to note that isopenicillin N is also secreted into the culture medium by an unknown mechanism [99], and it is speculated that the penicillin biosynthetic pathway encodes two different metabolites, isopenicillin N and penicillin G, that possess different biological functions [90].
Cephalosporin C biosynthesis
Cephalosporin C is an antibacterial agent produced by Acremonium chrysogenum that shares a similar biosynthesis to that of penicillin G, up to the formation of isopenicillin N (Scheme 14). In contrast to the penicillin pathway, the BGC encoding cephalosporin C contains eleven genes, split into two different clusters [90, 100]. The genes required for the early stages of biosynthesis (up to penicillin N) are located on one chromosome, however the genes encoding the later steps of the biosynthesis, cefEF and cefG, are located elsewhere on the genome [101]. In the divergent biosynthesis, isopenicillin N is epimerized to penicillin N by isopenicillin N-CoA ligase (CefD1) and isopenicillin N-CoA epimerase (CefD2), and then penicillin N is converted to deacetylcephalosporin C via deacetoxycephalosporin C (DAOC) by the bifunctional enzyme DAOC synthase / hydroxylase (CefEF) [102, 103]. In the final step deacetoxycephalosporin C is acetylated to form cephalosporin C [101].
There is less known about the sub-cellular localization of the biosynthetic enzymes that synthesize cephalosporin C compared to those required for penicillin biosynthesis. ACVS and IPNS are speculated to be cytosolic enzymes [90], analogous to the penicillin enzymes. CefD1 and CefD2 contain peroxisomal targeting sequences and homologs in P. chrysogenum were identified in the peroxisome [90, 95]. The transporters CefM and CefP also contain peroxisomal targeting sequences [95]; disruption of cefM led to an accumulation of penicillin N indicating a role in secretion from the peroxisomes [104], whereas disruption of cefP led to accumulation of isopenicillin N indicating a role in transporting isopenicillin N into the peroxisomes [105]. The sub-cellular localization of CefEF and CefG are speculated to be in the cytosol [90] and the final transporter CefT is indicated as being located both in the vacuoles and in the cell membrane, based on expression of CefT-GFP in the heterologous host P. chrysogenum [106]. Although inactivation of cefT did not prevent accumulation of cephalosporin C, overexpression led to almost twice as much production in both native and heterologous hosts [106, 107].
Fumiquinazoline biosynthesis
Fumiquinazolines are a family of peptidyl alkaloids produced by various fungi that have cytotoxic properties [108]. The biosynthesis requires the trimodular NRPS (FmqA) that condenses anthranilate, alanine, and tryptophan to produce fumiquinazoline F [109] this is then oxidized by the FAD-dependent monooxygenase (FMO) FmqB and condensed with alanine through the action of the monomodular NRPS (FmqC) to synthesize fumiquinazoline A [110]. Finally, the FMO FmqD converts fumiquinazoline A to fumiquinazoline C (Scheme 15) [111]. Also encoded by the BGC is an MFS transporter, FmqE; the BGC is regulated by the conidiation-specific transcription factor BrlA that is not encoded within the BGC [112].
Investigations of metabolite accumulation determined that fumiquinazoline F and fumiquinazoline A are found in all fungal tissues at comparable levels but fumiquinazoline C accumulates in conidial tissues [112]. FmqA is located with vesicles that differ in size but are uniformly spaced within hyphae, but are not vacuoles, mitochondria, or nuclei. FmqB and FmqC were distributed evenly throughout the cytoplasm. A conserved signal motif was identified in FmqD indicating that this enzyme is secreted [111]. When this signal peptide was deleted fumiquinazoline A production increased whereas production of fumiquinazoline C decreased. Over-expression of FmqD fused to eGFP determined that FmqD is localized to the cell well and the extent of localization depends on the growth stage of the fungal cells [112]. Further investigations revealed that localization of FmqD is actin-dependent and requires endoplasmic reticulum-Golgi transport to the cell wall, as expected for secretory proteins. FmqE was also detected in vesicles within the hyphae but in a different arrangement to FmqA [112].
Cyclosporin biosynthesis
Cyclosporin is a cyclic undecapeptide isolated from Tolypocladium inflatum that is used commercially as an immunosuppressant drug [113]. The biosynthetic gene cluster is proposed as consisting of 12 genes that encode: SimA, an 11 module NRPS; Sim B, an alanine racemase; SimE, a thioesterase; SimG, a highly reducing PKS; SimI, a P450; and SimJ, an aminotransferase [114]. SimG, SimI and SimJ collaborate to synthesize the unusual (4R)-4-[(E)-2-butenyl]-4-methyl-l-threonine and SimB racemizes D-alanine from L-alanine; these unnatural amino acids are incorporated by the NRPS SimA during biosynthesis and the terminal C domain releases the peptide by cyclization (Scheme 16). Also encoded in the gene cluster are: SimC, a cyclophilin; SimD, an ABC transporter; and SimL, a transcription factor [114].
The subcellular localization of SimA in T. inflatum was investigated using immunolocalization and electron microscopy. SimA and SimB were determined to be attached to the outside of the vacuolar membrane, in close proximity, and SimA was observed to exist as a globular complex consisting of 11 particles presumed to be the 11 amino-acid modules separated by linkers [115]. The final metabolite cyclosporin was found stored in high concentrations within vacuoles and can be released slowly via vacuolar / cytoplasmic membranes, or rapidly if the cell is lysed [115].
Triacetylfusarinine C and hydroxyferricrocin biosynthesis
Triacetylfusarinine C is a siderophore produced by various fungi that has an essential role in iron uptake and virulence [116]. Triacetylfusarinine C contains three N2-acetyl-N5-anhydromevalonyl-N5-hydroxyornithine residues joined through ester bonds. SidD is an iterative NRPS that synthesizes fusarinine C from N5-anhydromevalonyl-N5-hydroxyornithine (AMHO) [117]. AMHO is produced by: SidA, an ornithine monooxygenase; SidI, a mevalonyl-CoA ligase; SidH, a mevalonyl-CoA hydratase; and SidF, a anhydromevalonyl-CoA transferase [118, 119]. SidI and SidH synthesize anhydromevalonyl-CoA from mevalonate and SidF transfers this to N5-hydroxy-L-ornithine produced by SidA (Scheme 17). Finally, SidG, an acetyl transferase, catalyzes N2-acetylation of fusarinine C to produce triacetylfusarinine C.
Bioinformatic analysis of SidF, SidH, and SidI indicated C-termini peroxisomal targeting signals in SidF and SidH, and an N-terminal peroxisomal targeting signal in SidI [118]. Construction of GFP-tagged versions of the three enzymes in respective knock-out strains led to the detection of punctate structures observed in the cytoplasm and restoration of triacetylfusarinine C production. Peroxisomal location was confirmed though co-expression with peroxisomal targeting of red fluorescent protein. When the putative peroxisomal targeting signal was removed from SidH GFP was detected throughout the cytosol, however triacetylfusarinine C was not detected indicating that correct localization of SidH is required for biosynthesis [118]. The remaining biosynthetic enzymes are presumed to be localized in the cytosol but this is not known for certain. However, EstB, the esterase that hydrolyzes triacetylfusarinine C after binding iron, was detected in the cytoplasm of A. fumigatus particularly during iron starvation conditions [120].
A second siderophore pathway is also known in A. fumigatus where N5-hydroxy-L-ornithine is acetylated by the N-acetyltransferase SidL to generate N5-acetyl-N5-hydroxyornithine [121]. The NRPS SidC condenses N5-acetyl-N5-hydroxy-L-ornithine, serine and glycine to synthesize ferricrocin, which is hydroxylated to produce the siderophore hydroxyferricrocin (Scheme 17) [122]. eGFP fused SidL was used in complementation studies and epifluorescent microscopy determined that SidL is localized in the cytoplasm [121].
The subcellular localization of siderophore transporters has been investigated in Saccharomyces cerevisiae using GFP fusion proteins. The structures of the respective siderophores are shown in Fig. 3. Enb1 is an enterobactin transporter that is targeted to the plasma membrane whereas Sit1 is a ferrioxamine B transporter that is targeted to the vacuolar degradation pathway when no substrate is present [123]. Similarly, Arn1p is a transporter for the uptake of ferrichrome that is sorted from the trans-Golgi network to the vacuolar lumen when no siderophore is present [124].
Ribosomally synthesized peptides
Amanitin biosynthesis
Amatoxins are ribosomally synthesized and post-translationally modified peptides (RiPPs) produced by select mushroom species that are responsible for severe mushroom poisonings [125]. Amatoxins consist of eight peptide residues that are cyclized and contain tryptathione, sulfoxide, and hydroxyl functionalities, and the biological target is RNA polymerase II [126]. Two genes have been characterized in Amanita bisporigera that are required for amanitin biosynthesis (Fig. 4): AMA1 that encodes a propeptide of 35 amino acids [127] and AbPOPB that encodes a prolyl oligopeptidase that cleaves the proprotein (Luo et al., 2010). The additional enzymes required for post-translational modification of the core peptide are unknown [126].
The role of AbPOPB was confirmed from a distantly related mushroom Galerina marginate; recombinant GmPOPB was shown to catalyze hydrolysis and cyclization, two non-processive reactions in amanitin biosynthesis [128, 129]. To determine the subcellular localization of amanitin and POPB in A. bisporigera, immunolocalization techniques were used that indicate that amanitin and AbPOPB colocalize in vacuoles [130].
Enzyme trafficking and self-resistance to toxic intermediates
Transient recruitment of enzymes and trafficking
An emerging theme in several of the examples discussed above is the difficulties in precisely locating various biosynthetic enzymes, due to conducting experiments at different stages of fungal development, or conflicting results between bioinformatics predictions and microscopic/chemical observations. Subcellular localization of biosynthetic enzymes therefore appears to be a highly dynamic process and requires trafficking of various enzymes to specific locations.
For example, in A. fumigatus the four early-stage enzymes required for melanin biosynthesis are found localized in endosomes but lack signal peptide or transmembrane domains, and the two-late stage enzymes are found at the cell wall. Subsequent experiments using size-exclusion chromatography and coimmunoprecipitation assays determined several examples of protein-protein interactions and complex formation between the early- and late-stage enzymes [131]. However, it was not the formation of an enzyme complex that was responsible for the enzyme trafficking between sub-cellular locations; instead, post-translational lipid modification appears to be the major mechanism. By using a combination of chemical reporters and liquid chromatography – tandem mass spectrometry, three early-stage melanin enzymes, Alb1, Ayg1 and Arp2, were discovered to be palmitoylated [131]. When A. fumigatus Alb1-eGFP fusion strains were grown in the presence of the palmitoylation inhibitor 2-bromopalmitate, Alb1 was no longer localized and instead was observed diffused in the cytoplasm [131].
Melanin biosynthesis in B. cinerea is also proposed to involve enzyme trafficking since the subcellular localization of BcBRN1/2 is observed in the cytoplasm, endosomes, and the cell wall [46]. This correlates well with the observed toxicity of scytalone and the requirement of BcBRN1/2 in its synthesis and subsequent conversion (Scheme 3C).
Similar trafficking mechanisms have been observed or proposed in the biosynthesis of aflatoxin, melanin, patulin, penicillin, cephalosporin, and fumiquinazoline. The dynamic nature of protein translocation and subsequent trafficking to the target organelle may explain the difficulties of accurate bioinformatic predictions of some biosynthetic enzymes. However, this strategy of localizing specific biosynthetic enzymes and intermediates to different organelles enables some control over confining toxic intermediates and explains how intermediates are transported to subsequent organelles. Ultimately, fusion of different organelles leads to larger organelles, known as toxisomes, that contain intricate enzyme complexes and accumulate the increasingly toxic intermediates, thereby protecting the cell from deleterious molecules [18, 132].
Alternative mechanisms enabling self-resistance to toxic intermediates
It is well known that many fungal BGCs contain transporters which are proposed to provide fungi mechanisms to protect themselves, reviewed elsewhere [133]. Alternatively, BGCs may include an additional copy of the target protein that acts as a decoy, capturing the toxic specialized metabolite and preventing disastrous effects, also reviewed elsewhere [134]. However, for BGCs that encode neither transporters nor self-resistance proteins, other mechanisms must be available such as detoxifying enzymes, as first reported in gliotoxin biosynthesis [135].
Recently, a dual compartmentalized / detoxification enzyme strategy for self-resistance was reported during the biosynthesis of A26771B [136]. A26771B is a 16-membered macrolide antibiotic produced by Penicillium egyptiacum (Scheme 18). The BGC contains a HRPKS (BerkA), a trans-acting thiolesterase (TE; BerkB), a short-chain reductase/dehydrogenase (SDR; BerkC), a flavin-dependent monooxygenase (FMO; BerkD), a P450 (BerkE), and an acetyltransferase, (BerkF). The role of each enzyme was established through heterologous gene expression in Aspergillus nidulans, however co-expression of berkABCDEF lead to production of the inactive precursor; when berkC was omitted these precursors were no longer evident. The role of BerkC was established as reducing A26771B to the inactive form; BerkC therefore appears to function as a detoxification enzyme, inactivating the toxicity of A26771B inside P. egyptiacum [136].
The sub-cellular localization of BerkC and BerkD were investigated by individually fusing to super folder GFP (sfGFP) and expressing in A. nidulans [136]. BerkC was detected in the cytoplasm whereas BerkD was localized to the cell wall and septa, indicating that it is secreted extracellularly. The location of BerkF was also investigated through fusion with sfGFP and determined to be localized to intracellular organelles. It is proposed that the inactive precursor is exported out of the cell and the extracellular BerkD oxidizes this intermediate to the active A26771B [136]. As A26771B can be transported back into the cell, BerkC reduces A26771B to its inactive form which is exported outside of the cell in a redox cycle. It is currently unknown how this transport occurs, but it is proposed that BerkF and / or the acyl side chain introduced by BerkF facilitate the export [136].
The trafficking of redox enzymes to the cell wall / outside of the cell has been reported in the biosynthesis of melanin [19, 46], patulin [53], and fumiquinazoline [112], perhaps indicating a frequent self-resistance mechanism. Finally, the containment of specialized metabolites has been also reported within specific tissues e.g. hyphae and conidia [137], and spores [112, 132, 138].
Conclusions
This review collates examples of fungal specialized metabolite biosynthetic pathways where the subcellular localization of all, or some, of the biosynthetic pathways have been elucidated, revealing the complexity of these studies. Many pathways and associated enzymes are often individualized with the same enzyme classes being in different subcellular compartments in different fungi, or even the same metabolite being in different subcellular compartments in different fungi. Furthermore, subcellular localization of biosynthetic enzymes, intermediate trafficking, and transport is highly dynamic, demonstrating the challenges facing researchers studying this phenomenon.
To date single biosynthetic pathways are studied at a given time but as more investigations into these systems are conducted it will be interesting to observe how competing biosynthetic pathways are contained, or separated, within a cell. Future directions will need to uncover the strength, timescales, and kinetics of protein-protein interactions which in turn may require novel chemical and biophysical methods. However, this knowledge is crucial for metabolic engineers and for establishing new and robust heterologous hosts to generate larger-scale production of medicinally and agriculturally important fungal molecules.
Availability of data and materials
Not applicable.
Abbreviations
- BGC:
-
Biosynthetic gene clusters
- CS:
-
Ceramide synthase
- CYC:
-
Cyclase
- DH:
-
Dehydratase
- DMAPP:
-
Dimethylallyl pyrophosphate
- DON:
-
Deoxynivalenol
- EDA:
-
9,10-Epoxy-8-hydroxy-9-methyl-decatrienoic acid
- eGFP:
-
Enhanced green fluorescence protein
- FAS:
-
Fatty acid synthase
- FMO:
-
Flavin-dependent monooxygenase
- FPP:
-
Farnesylpyrophosphate
- GGPS:
-
Geranylgeranylphosphate synthase
- HMG-CoA:
-
β-Hydroxy-β-methylglutaryl-CoA
- HMGS:
-
β-Hydroxy-β-methylglutaryl synthase
- HRPKS:
-
Highly reducing polyketide synthase
- HYD:
-
Hydrolase
- IPP:
-
Isopentenyl pyrophosphate
- Lac:
-
Laccase
- MFS:
-
Major facilitator superfamily
- MT:
-
Methyltransferase
- NRPKS:
-
Non-reducing polyketide synthase
- NRPS:
-
Non-ribosomal peptide synthetase
- OX:
-
Oxidase
- OXR:
-
Oxidoreductase
- P450:
-
Cytochrome P450 monooxygenase
- PKS:
-
Polyketide synthases
- PKS/NRPS:
-
Polyketide synthase / non-ribosomal peptide synthetase
- PRPKS:
-
Partially reducing polyketide synthase
- PT:
-
Prenyltransferase
- Red:
-
Reductase
- RiPP:
-
Ribosomally synthesized and post-translationally modified peptide
- TC / TS:
-
Terpene cyclase / terpene synthase
- TEM:
-
Transmission electron microscopy
- TF:
-
Transcription factor
- THN:
-
1,3,6,8-Tetrahydroxynaphthalene
- Trans:
-
Transporter
- U:
-
Unknown / hypothetical protein
- UV:
-
Ultra-violet
References
Keller NP. Translating biosynthetic gene clusters into fungal armor and weaponry. Nat Chem Biol. 2015; 11: 671–677.
Aly AH, Debbab A, Proksch P. Fifty years of drug discovery from fungi. Fungal Diversity. 2011; 50: 3.
Braese S, Encinas A, Keck J, Nising CF. Chemistry and biology of mycotoxins and related fungal metabolites. Chem Rev. 2009; 109: 3903–3990.
Cox RJ, Skellam EJ. Fungal Non-Reducing Polyketide Synthases, Comprehensive Natural Products III: Chemistry and Biology (Editors-in-Chief: Hung-wen Liu, Tadhg Begley), 2020; 1: 266–309.
Cox RJ. Polyketides, proteins and genes in fungi: programmed nano-machines begin to reveal their secrets. Org Biomol Chem. 2007; 5: 2010–2026.
Chooi YH and Tang Y. Navigating the fungal polyketide chemical space: from genes to molecules. J Org Chem. 2012; 77: 9933–9953.
Quin MB, Flynn CM, Schmidt-Dannert C. Traversing the fungal terpenome. Nat Prod Rep. 2014; 31:1449–1473.
Bushley KE, Turgeon BG. Phylogenomics reveals subfamilies of fungal nonribosomal peptide synthetases and their evolutionary relationships. BMC Evol Biol. 2010;10:26.
Boettger D and Hertweck C. Molecular diversity sculpted by fungal PKS-NRPS hybrids. ChemBioChem. 2013; 14: 28–42.
Skellam E. Biosynthesis of fungal polyketides by collaborating and trans-acting enzymes. Nat Prod Rep. 2022; 39: 754–783.
Rokas A, Wisecaver JH, Lond AL. The birth, evolution and death of metabolic gene clusters in fungi. Nat Rev Microbiol., 2018; 16: 731–744.
Blin K, Shaw S, Klooseterman AM, Charlop-Powers Z, van Wezel GP, Medema MH, Weber T. antiSMASH 6.0: improving cluster detection and comparison capabilities. Nuc Acids Res. 2021; 49: W29-35.
Meng X, Fang Y, Ding M, Zhang Y, Jia K, Li Z, Collemare J, Liu W. Developing fungal heterologous expression platforms to explore and improve the production of natural products from fungal biodiversity. Biotechnol Adv. 2022; 54:107866.
Mei YZ, Zhu YL, Huang PW, Yang Q, Dai CC. Strategies for gene disruption and expression in filamentous fungi. Appl Microbiol Biotechnol. 2019; 103: 6041–6059.
Skellam E. Strategies for engineering natural product biosynthesis in fungi. Trends Biotechnol. 2019; 37: 416–427.
Awan AR, Blount BA, Bell DJ, Shaw WM, Ho JCH, McKiernan, Ellis T. Biosynthesis of the antibiotic nonribosomal peptide penicillin in baker’s yeast. Nat Commun. 2017; 8: 15202.
Read ND. Fungal cell structure and organization. In: Kibbler CC, Barton R, Gow NAR, Howell S, MacCallum DM, Manuel RJ, editors. Oxford Textbook of Medical Mycology. Oxford University Press. 2018. DOI: https://doi.org/10.1093/med/9780198755388.001.0001
Roze LV, Chanda A, Linz JE. Compartmentalization and molecular traffic in secondary metabolism: A new understanding of established cellular processes. Fung Genet Biol. 2011; 48: 35–48.
Upadhyay S, Xu X, Lowry D, Jackson JC, Roberson RW, Lin X. Subcellular compartmentalization and trafficking of the biosynthetic machinery for fungal melanin. Cell Rep. 2016; 14: 2511–2518.
Menke J, Weber J, Broz K, Kistler HC. Cellular development associated with induced mycotoxin synthesis in the filamentous fungus Fusarium graminearum. PLOS One. 2013; 8: e63077.
Chanda A, Roze LV, Kang S, Artymovich KA, Hicks GR, Raikhel NV, Calvo AM, Linz JE. A key role for vesicles in fungal secondary metabolism. Proc Nat Acad Sci. 2009; 106: 19533–19538.
Caceres I, Al Khoury A, El Khoury R, Lorber S, Oswald IP, El Khoury A, Atoui A, Puel O, Bailly JD. Aflatoxin biosynthesis and genetic regulation: A review. Toxins. 2020; 12: 150.
Chang PK. The Aspergillus parasiticus protein AFLJ interacts with the aflatoxin pathway-specific regulator AFLR. Mol Genet Genomics. 20303; 268: 711–719.
Watanabe C and Townsend C. Initial characterization of a type I fatty acid synthase and polyketide synthase multienzyme complex NorS in the biosynthesis of aflatoxin B1. Chem Biol. 2002; 9: 981–988.
Crawford JM, Dancy BCR, Hill EA, Udwary DW, Townsend CA. Identification of a starter unit acyl-carrier protein transacylase domain in an iterative type I polyketide synthase. Proc Nat Acad Sci. 2006; 103: 16728–16733.
Ehrlich KC, Li P, Scharfenstein L, Chang PK. HypC, the anthrone oxidase involved in aflatoxin biosynthesis. Appl Environ Microbiol. 2010; 76: 3374–3377.
Zhou R, Linz JE. Enzymatic function of the Nor-1 protein in aflatoxin biosynthesis in Aspergillus parasiticus. Appl Environ Microbiol. 1999; 65: 5639–41.
Sakuno E, Yabe K, Nakajima H. Involvement of two cytosolic enzymes and a novel intermediate, 5’-oxoaverantin, in the pathway from 5’-hydroxyaverantin to averufin in aflatoxin biosynthesis. Appl Environ Microbiol. 2003; 69: 6418–6426.
Sakuno E, Wen Y, Hatabayashi H, Arai H, Aoki C, Yabe K, Nakajima H. Aspergillus parasiticus cyclase catalyzes two dehydration steps in aflatoxin biosynthesis. Appl Environ Microbiol. 2005; 71: 2999–3006.
Henry KM and Townsend CA. Ordering the reductive and cytochrome P450 oxidative steps in demethylsterigmatocystin formation yields general insights into the biosynthesis of aflatoxin and related fungal metabolites. J Am Chem Soc. 2005; 127: 3724–3733.
Yu J, Cary JW, Bhatnagar D, Cleveland TE, Keller NP, Chu FS. Cloning and characterization of a cDNA from Aspergillus parasiticus encoding an O-methyltransferase involved in aflatoxin biosynthesis. Appl Environ Microbiol. 1993; 59:3564–3571.
Maggio-Hall LA, Wilson RA, Keller NP. Fundamental contribution of b-oxidation to polyketide mycotoxin production. Molecular Plant-Microbe Interactions. 2005; 18: 783–793.
Hong SY and Linz JE. Functional expression and sub-cellular localization of the early aflatoxin pathway enzyme Nor-1 in Aspergillus parasiticus. Mycol Res. 2009; 113: 591–601.
Chiou CH, Lee LW, Owens SA, Whallon JH, Klomparens KL, Townsend CA, Linz JE. Distribution and sub-cellular localization of the aflatoxin enzyme versicolorin B synthase in time-fractionated colonies of Aspergillus parasiticus. Arch Microbiol. 2004; 182: 67–79.
Hong SY and Linz YE. Functional expression and subcellular localization of the aflatoxin enzyme Ver-1 fused to enhanced green fluorescent protein. Appl Environ Microbiol. 2008; 74: 6385–6396.
Lee LW, Chiou CH, Klomparens KL, Cary JW, Linz JE. Subcellular localization of aflatoxin biosynthetic enzymes Nor-1, Ver-1, and OmtA in time-dependent fractionated colonies of Aspergillus parasiticus. Arch Microbiol. 2004; 181:204–214.
Ehrlich KC, Mack BM, Wei Q, Li P, Roze LV, Dazzo F, Cary JW, Bhatnagar D, Linz JE. Association with AflR in endosomes reveals new functions for AflJ in aflatoxin biosynthesis. Toxins. 2012; 4: 1582–1600.
Nosanchuk JD, Stark RE, Casadevall A. Fungal melanin: what do we know about structure? Front Microbiol. 2015. https://doi.org/10.3389/fmicb.2015.01463.
Watanabe A, Fujii I, Tsai HF, Chang YC, Kwon-Chung KJ, Ebizuka Y. Aspergillus fumigatus alb1 encodes naphthopyrone synthase when expressed in Aspergillus oryzae. FEMS Microbiol Lett. 2000; 192: 39–44.
Tsai HF, Fujii I, Watanabe A, Wheeler MH, Chang YC, Yasuoka Y, Ebizuka Y, Kwon-Chung KJ. Pentaketide melanin biosynthesis in Aspergillus fumigatus requires chain-length shortening of a heptaketide precursor. J Biol Chem. 2001; 276: 29292–29298.
Fujii I, Yasuoka Y, Tsai HF, Chang YC, Kwon-Chung KJ, Ebizuka Y. Hydrolytic polyketide shortening by Ayg1p, a novel enzyme involved in fungal melanin biosynthesis. J Biol Chem. 2004; 279: 44613–44620.
Tsai HF, Wheeler MH, Chang YC, Kwon-Chung KJ. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J Bacteriol. 1999; 181: 6469–77.
Sugareva V, Haertl A, Brock M, Huebner K, Rohde M, Heinekamp T, Brakhage AA. Characterization of the laccase-encoding abr2 of the dihydronaphthalene-like melanin gene cluster of Aspergillus fumigatus. Arch Microbiol. 2006; 186: 345–355.
Watanable A, Fujii I, Sankawa U, Mayorga ME, Timberlake WE, Ebizuka Y. Re-identification of Aspergillus nidulans wA gene to code for a polyketide synthase of naphthopyrone. Tet Lett. 2002; 43: 843–846.
Aramayo R and Timberlake WE. Sequence and molecular structure of the Aspergillus nidulans yA (laccase I) gene. Nuc Acids Res. 1990; 18: 3415.
Chen X, Zhu C, Na Y, Ren D, Zhang C, He Y, Wang Y, Xiang S, Ren W, Jiang Y, Xu L, Zhu P. Compartmentalization of melanin biosynthetic enzymes contributes to self-defense against intermediate compound scytalone in Botrytis cinerea. mBio. 2021; 12: e00007-21.
Schumacher J. DHN melanin biosynthesis in the plant pathogenic fungus Botrytis cinerea is based on two developmentally regulated key enzyme (PKS)-encoding genes. Mol Microbiol. 2016; 99: 729–748.
Moake MM, Padilla-Zakour OI, Worobo RW. Comprehensive review of patulin control methods in foods. Compr Rev Food Sci Food Saf. 2005; 4: 8–21.
Li B, Zong Y, Du Z, Chen Y, Zhang Z, Qin G, Zhao W, Tian S. Genomic characterization reveals insight into patulin biosynthesis and pathogenicity in Penicillium species. Molecular Plant-Microbe Interactions. 2015; 28: 635–647.
Artigot MP, Loiseau N, Laffitte J, Mas-Reguieg L, Tadrish S, Oswald IP, Puel O. Molecular cloning and functional characterization of two CYP619 cytochrome P450s involved in biosynthesis of patulin in Aspergillus clavatus. Microbiol. 2009; 155: 1738–1747.
Puel O, Galtier P, Oswald IP. Biosynthesis and toxicological effects of patulin. Toxins. 2010; 2: 613–631.
Snini SP, Tadrist S, Laffitte J, Jamin EL, Oswald IP, Puel O. The gene PatG involved in the biosynthesis pathway of patulin, a food-bourne mycotoxin, encodes a 6-methylsalicylic acid decarboxylase. Int J Food Microbiol. 2014; 171: 77–83.
Li B, Chen Y, Zong Y, Shang Y, Zhang Z, Xu X, Wang X, Long M, Tian S. Dissection of patulin biosynthesis, spatial control and regulation mechanism in Penicillium expansum. Environ Microbiol. 2019; 21: 1124–1139.
Liu Y, Kurtan T, Wang CY, Lin WH, Orfali R, Mueller WEG, Daletos G, Proksch P. Cladosporinone, a new viriditoxin derivative from the hypersaline lake derived fungus Cladosporium cladosporioides. J Antibiotics. 2016; 69: 702–706.
Urquhart AS, Hu J, Chooi YH, Idnurm A. The fungal gene cluster for biosynthesis of the antibacterial agent viriditoxin. Fungal Biol Biotechnol. 2019. https://doi.org/10.1186/s40694-019-0072-y
Meena M, Gupta SK, Swapnil P, Zehra A, Dubey MK, Upadhyay RS. Alternaria toxins: potential virulence factors and genes related to pathogenesis. Front Microbiol. 2017; https://doi.org/10.3389/fmicb.2017.01451
Witte TE, Villeneuve N, Boddy CN, Overy DP. Accessory chromosome-acquired secondary metabolism in plant pathogenic fungi: the evolution of biotrophs into host-specific pathogens. Front Microbiol. 2021; doi: https://doi.org/10.3389/fmicb.2021.664276
Tanaka A, Shiotani H, Yamamoto M, Tsuge T. Insertional mutagenesis and cloning of the genes required for biosynthesis of the host-specific AK-toxin in the Japanese pear pathotype of Alternaria alternata. Molecular Plant-Microbe Interactions. 1999; 12: 691–702.
Tanaka A, Tsuge T. Structural and functional complexity of the genomic region controlling AK-toxin biosynthesis and pathogenicity in the Japanese pear pathotype of Alternaria alternata. Molecular Plant-Microbe Interactions. 2000; 13: 975–986.
Takaoka S, Kurata M, Harimoto Y, Hatta R, Yamamoto M, Akimitsu K, Tsuge T. Complex regulation of secondary metabolism in the phytopathogenic fungus Alternaria alternata. New Phytologist. 2014; 202: 1297–1309.
Imazaki A, Tanaka A, Harimoto Y, Yamamoto M, Akimitsu K, Park P, Tsuge T. Contribution to peroxisomes to secondary metabolism and pathogenicity in the fungal plant pathogen Alternaria alternata. Eukaryotic Cell. 2010; 9: 682–694.
Kamle M, Mahato DK, Devi S, Lee KE, Kang SG, Kumar P. Fumonisins: impact on agriculture, food, and human health and their management strategies. Toxins. 2019; 11: 328.
Wang E, Norred WP, Bacon CW, Riley RT, Merrill AH, Jr. Inhibition of sphingolipid biosynthesis by fumonisins: implications for diseases associated with Fusarium moniliforme. J Biol Chem. 1991; 266: 14486–14490.
Butchko RAE, Plattner RD, Proctor RH. Deletion analysis of FUM genes involved in tricarballylic ester formation during fumonisin biosynthesis. J Agric Food Chem. 2006; 54: 9398–9404.
Proctor RH, Brown DW, Plattner RD, Desjardins AE. Co-expression of 15 contiguous genes delineates a fumonisin biosynthetic gene cluster in Gibberella moniliformis. Fungal Genetics and Biology. 2003; 38: 237–249.
Janevska S, Ferling I, Jojic K, Rautschek J, Hoefgen S, Proctor RH, Hillman F, Valiante V. Self-protection against the sphingolipid biosynthesis inhibitor fumonisin B1 is conferred by a FUM cluster-encoded ceramide synthase. mBio. 2020; 11: e00455-20.
Proctor RH, Hohn TM, McCormick SP. Reduced virulence of Gibberella zeae caused by disruption of trichothecene toxin biosynthetic gene. Molecular Plant-Microbe Interactions. 1995; 8: 593–601.
Proctor RH, McCormick SP, Kim HS, Cardoza RE, Stanley AM, Lindo L, Kelly A, Brown DW, Lee T, Vaughan MM, Alexander NJ, Busman M, Gutierrez S. Evolution of structural diversity of trichothecenes, a family of toxins produced by plant pathogenic and entomopathogenic fungi. PLOS Pathogens. 2018; 14: e1006946
Dyer RB, Plattner RD, Kendra DF, Brown DW. Fusarium graminearum TRI14 is required for high virulence and DON production on wheat but not for DON synthesis in vitro. J Agric Food Chem. 2005; 16: 9281–9287.
Boenisch MJ, Broz KL, Purvine SO, Chrisler WB, Nicora CD, Connolly LR, Freitag M, Baker SE, Kister HC. Structural reorganization of the fungal endoplasmic reticulum upon induction of mycotoxin biosynthesis. Sci Rep. 2017; 7: 44296.
Boenisch MJ, Blum A, Broz KL, Gardiner DM, Kistler HC. Nanoscale enrichment of the cytosolic enzyme trichodiene synthase near reorganized endoplasmic reticulum in Fusarium graminearum. Fungal Genetics and Biology. 2019; 124; 73–77.
Flynn CM, Broz KM, Jonkers W, Schmidt-Dannert, Kister HC. Expression of the Fusarium graminearum terpenome and involvement of the endoplasmic reticulum-derived toxisome. Fungal Genetics and Biology. 2019; 124: 78–87.
Tudzynski B, Hölter K. Gibberellin biosynthetic pathway in Gibberella fujikuroi: evidence for a gene cluster. Fungal Genet Biol. 1998; 25: 157–170
Tudzynski B, Hedden P, Carrera E, Gaskin P. The P450-4 gene of Gibberella fujikuroi encodes ent-kaurene oxidase in the gibberellin biosynthesis pathway. Appl. Environ. Microbiol. 2001; 67: 3514–3522.
Rojas MC, Hedden P, Gaskin P, Tudzynski B. The P450–1 gene of Gibberella fujikuroi encodes a multifunctional enzyme in gibberellin biosynthesis. Proc Natl Acad Sci. USA. 2001; 98; 5838–5843
Albermann S, Linnemannstoens P, Tudzynski B. Strategies for strain improvement in Fusarium fujikuroi: overexpression and localization of key enzymes of the isoprenoid pathway and their impact on gibberellin biosynthesis. Appl Microbiol Biotechnol. 2013; 97: 2979–2995.
Saikia S and Scott B. Functional analysis and subcellular localization of two geranylgeranyl diphosphate synthases from Penicillium paxillin. Mol Genet Genomics. 2009; 282: 257–271.
Tagami K, Liu C, Minami A, Moike M, Isaka T, Fueki S, Shichijo Y, Toshima H, Goma K, Daira T, Oikawa H. Reconstitution of biosynthetic machinery for indole-diterpene paxilline in Aspergillus oryzae. J Am Chem Soc. 2013; 135: 1260–1263.
Scott B, Young CA, Saikia S, McMillan LK, Monahan BJ, Koulman A, Astin J, Eaton CJ, Bryant A, Wrenn RE, Finch SC, Tapper BA, Parker EJ, Jameson GB. Deletion and gene expression analyses define the paxilline biosynthetic gene cluster in Penicillium paxillin. Toxins. 2013; 5: 1422–1446.
Ikegami S, Taguchi T, Ohashi M, et al. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978; 275: 458–460.
Fujii R, Minami A, Tsukagosh T, Sato N, Sahara T, Ohgiya S, Gomi K, Oikawa H. Total biosynthesis of diterpene aphidicolin, a specific inhibitor of DNA polymerase α: heterologous expression of four biosynthetic genes in Aspergillus oryzae. Biosci Biotechnol Biochem. 2011; 75: 1813–1817.
Ban A, Tanaka M, Fujii R, Minami A, Oikawa H, Shintani T, Gomi K. Subcellular localization of aphidicolin biosynthetic enzymes heterologously expressed in Aspergillus oryzae. Biosci Biotechnol Biochem. 2018; 82: 139–147.
Bentley R. Mycophenolic acid: A one hundred year odyssey from antibiotic to immunosuppressant. Chem Rev. 2000; 100: 3801–3826.
Jonsson CA and Carlsten H. Mycophenolic acid inhibits inosine 5’-monophosphate dehydrogenase and suppresses immunoglobulin and cytokine production of B cells. Int Immunopharmacol. 2003; 3: 31–37.
Zhang W, Du L, Qu Z, Zhang X, Li F, Li Z, Qi F, Wang X, Jiang Y, Men P, Sun J, Cao S, Geng C, Qi F, Wan X, Liu C, Li S. Compartmentalized biosynthesis of mycophenolic acid. Proc Nat Acad Sci USA. 2019; 116: 13305–13310.
Van Liempt H, von Doehren H, Kleinkauf H. d-(L-a-aminoadipyl)-L-cysteinyl-D-valine synthetase from Aspergillus nidulans. J Biol Chem. 1989; 264: 3680–3684.
Roach PL, Clifton IJ, Hensgens CMH, Shinata N, Schofield CJ, Hajdu J, Baldwin JE. Structure of isopenicillin N complexed with substrate and the mechanism of penicillin formation. Nature. 1997; 387: 827–830.
Alvarez E, Meesschaert B, Montenegro E, Gutierrez S, Diez B, Barredo JL, Martin JF. The isopenicillin N acyltransferase has isopenicillin N amidohydrolase, 6-aminopenicillanic acid acyltransferase and penicillin amidase activities, all of which are encoded by the single penDE gene. Eur J Biochem. 1993; 215: 323–332.
Ledenfeld T, Ghali D, Wolschek M, Kubicek-Pranz EM, Kubicek CP. Subcellular compartmentalization of penicillin biosynthesis in Penicillium chrysogenum. J Biol Chem. 1993; 268: 665–671.
Martin JF. Transport systems, intracellular traffic of intermediates and secretion of b-lactam antibiotics in fungi. Fungal Biol Biotechnol. 2020; 7: 6.
Mueller WH, van der Krift TP, Krouwer AJJ, Woesten HAB, van der Voort LHM, Smaal EB, Verkleij AJ. Localization of the pathway of the penicillin biosynthesis in Penicillium chrysogenum. EMBO J. 1991; 10: 489–495.
Van der Lende TR, van de Kamp M, van den Berg M, Sjollema K, Bovenberg RAL, Veenhuis M, Konings WN, Driessen AJM. d-(L-a-aminoadipyl)-L-cysteinyl-D-valine synthetase, that mediates the first committed step in penicillin biosynthesis, is a cytosolic enzyme. Fung Genet Biol. 2002; 37: 49–55.
Maijer WH, Gidijala L, Fekkens] S, Kiel JAKW, van den Berg MA, Lascaris R, Bovenberg RAL, van der Klei IJ. Peroxisomes are required for efficient penicillin biosynthesis in Penicillium chrysogenum. Appl Environ Microbiol. 2010. 76; 12: 5702–5709.
Lamas-Maceiras M, Vaca I, Rodriguez E, Casqueiro J, Martin JF. Amplification and disruption of the phenylacetyl-CoA ligase gene of Penicillium chrysogenum encoding an aryl-capping enzyme that supplies phenylacetic acid to the isopenicillin N-acyltransferase. Biochem J. 2006; 395: 147–155.
Kiel JAKW, van den Berg M, Fusetti F, Poolman B, Bovenberh RAL, Veenhuis M, van der Klei IJ. Matching the proteome to the genome: the microbody of penicillin-producing Penicillium chrysogenum cells. Funct Integr Genomics. 2009; 9: 167–184.
Fernandez-Aguado M, Teijeira F, Martin JF, Ullan RV. A vacuolar membrane protein affects drastically the biosynthesis of the ACV tripeptide and the beta-lactam pathway of Penicillium chrysogenum. Appl Microbiol Biotechnol. 2013; 97: 795–808.
Fernandez-Aguado M, Martin JF, Rodriguez-Castro R, Garcia-Estrada C, Albillos SM, Teijeira F, Ullam RV. New insights into the isopenicillin N transport in Penicillium chrysogenum. Metab Eng. 2014; 22: 89–103.
Fernandez-Aguado M, Ullan RV, Teijeira F, Rodriguez-Castro R, Martin JF. The transport of phenylacetic acid across the peroxisomal membrane is mediated by the PaaT protein in Penicillium chrysogenum. Appl Microbiol Biotechnol. 2013; 97: 3073–3084.
Garcia-Estrada C, Vaca I, Lamas-Maceiras M, Martin JF. In vivo transport of the intermediates of the penicillin biosynthetic pathways in tailored strains of Penicillium chrysogenum. Appl Microbiol Biotechnol. 2007; 76: 169–182.
Skatrud PL, Queener SW. An electrophoretic molecular karyotype for an industrial strain of Cephalosporium acremonium. Gene. 1989; 78: 331–338.
Gutierrez S, Velasco J, Fernandez FJ, Martin JF. The cefG gene of Cephalosporium acremonium is linked to the cefEF gene and encodes a deacetylcephalosporin C acetyltransferase closely related to homoserine O-acetyltransferase. J Bacteriol. 1992; 174: 3056–3064.
Ullan RV, Casqueiro, Naranjo L, Martin JF. Expression of cefD2 and the conversion of isopenicillin N into penicillin N by the two-component epimerase system are rate-limiting steps in cephalosporin biosynthesis. Mol Genet Genomics. 2004; 272: 562–570.
Samson SM, Dotzlaf JE, Slisz ML, Becker GW, van Frank RM, Veal LE, Yeh W, Miller JR, Queener SW, Ingolia TD. Cloning and expression of the fungal expandase / hydroxylase gene involved in cephalosporin biosynthesis. Biotechnology. 1987; 5: 1207–1214.
Teijeira F, Ullan RV, Guerra SM, Garcia-Estrada C, Vaca I, Martin JF. The transporter CefM involved in translocation of biosynthetic intermediates is essential for cephalosporin production. Biochem J 2009; 418: 113–124.
Ullan RV, Teijeira F, Guerra SM, Vaca I, Martin JF. Characterization of a novel peroxisome membrane protein essential for conversion of isopenicillin N into cephalosporin C. Biochem J. 2010; 432: 227–236.
Nijland JG, Kovalchuk A, van den Berg MA, Bovenberg RAL, Driessen AJM. Expression of the ransporter encoded by the cefT gene of Acremonium chrysogenum increases cephalosporin production in Pencillium chrysogenum. Fungal Genet Biol. 2008; 45: 1415–1421.
Ullan RV, Liu G, Casqueiro J, Gutierrez, O. Banuelos, Martin JF. The cefT gene of Acremonium chrysogenum C10 encodes a putative multidrug efflux pump protein that significantly increases cephalosporin C production. Mol Genet Genomics. 2002; 267: 673–683.
Resende DISP, Boonpothing P, Sousa E, Kijjoa A, Pinto M. Chemistry of the fumiquinazolines and structurally related alkaloids. Nat Prod Rep 2019; 26: 7–34.
Ames BD and Walsh CT. Anthranilate-activating modules from fungal nonribosomal peptide assembly lines. Biochem. 2010; 49: 3351–3365.
Ames BD, Liu X, Walsh CT. Enzymatic processing of fumiquinazoline F: a tandem oxidative-acylation strategy for the generation of multicyclic scaffolds in fungal indole alkaloid biosynthesis. Biochem. 2010: 49: 8564–8576.
Ames BD, Haynes SW, Gao X, Evans BS, Kelleher NL, Tang Y, Walsh CT. Complexity generation in fungal peptidyl alkaloid biosynthesis: oxidation of fumiquinazoline A to the heptacyclic hemiaminal fumiquinazoline C by the flavoenzyme Af12070 from Aspergillus fumigatus. Biochem. 2011; 50: 8756–8769.
Lim FY, Ames B, Walsh C, Keller NP. Co-ordination between BrlA regulation and secretion of the oxidoreductase FmqD directs selective accumulation of fumiquinazoline C to conidial tissues in Aspergillus fumigatus. Cellular Microbiol. 2014; 16: 1267–1283.
Survase SA, Kagliwal LD, Annapure US, Singhal RS. Cyclosporin A – a review on fermentative production, downstream processing and pharmacological applications. Biotechnol Advances. 2011; 29: 418–4435.
Yang X, Feng P, Yin Y, Bushley K, Spatafora JW, Wang C. Cyclosporine biosynthesis in Tolypocladium inflatum benefits fungal adaption to the environment. mBio. 2018; 9: e01211-18.
Hoppert M, Gentzsch C, Schoergendorfer K. Structure and localization of cyclosporin synthetase, the key enzyme of cyclosporin biosynthesis in Tolypocladium inflatum. Arch Microbiol. 2001; 176: 285–293.
Haas H. Fungal siderophore metabolism with a focus in Aspergillus fumigatus. Nat Prod Rep. 2014; 31: 1233–1490.
Hai Y, Jenner M, Tang Y. Fungal siderophore biosynthesis catalyzed by an iterative nonribosomal peptide synthetase. Chem Sci. 2020; 11: 11525.
Gruendlinger M, Yasmin S, Lechner BE, Geley S, Schretti M, Hynes M, Haas H. Fungal siderophore biosynthesis is partially localized in peroxisomes. Mol Microbiol. 2013; 88: 862–875.
Hissen AHT, Wan ANC, Warwas ML, Pinto LJ, Moore MM. The Aspergillus fumigatus siderophore biosynthetic gene sidA, encoding L-ornithine N5-oxygenase, is required for virulence. Infection and Immunity. 2005; 73: 5493–5503.
Kragl C, Schrettl M, Abt B, Sarg B, Lindner HH, Haas H. EstB-mediated hydrolysis of the siderophore triacetylfusarinine C optimizes iron uptake of Aspergillus fumigatus. Eukaryotic Cell. 2007; 6: 1278–1285.
Blatzer M, Schrettl M, Sarg B, Lindner HH, Pfaller K, Haas H. SidL, an Aspergillus fumigatus transacetylase involved in biosynthesis of the siderophores ferricrocin and hydroxferricrocin. Appl Environ Microbiol. 2011; 77: 4959–4966.
Schrettl M, Bignell E, Kragl C, Sabiha Y, Loss O, Eisendle M, Wallner A, Arst HN, Haynes K, Haas H. Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog. 2007; 3: e128.
Froissard M, Belgareh-Touze N, Dias M, Buisson N, Camadro JM, Haguenauer-Tsapis R, Lesuisse E. Trafficking of siderophore transporters in Saccharomyces cerevisiae and intracellular fate of ferrioxamine B conjugates. Traffic. 2007; 8: 1601–1616.
Deng Y, Guo Y, Watson H, Au WC, Shakoury-Elizeh M, Basrai MA, Bonifacino JS, Philpott CC. Gga2 mediates sequential ubiquitin-independent and ubiquitin-dependent steps in the trafficking of ARN1 from the trans-Golgi Network to the vacuole. J Biol Chem. 2009; 284: 23830–23841.
Walton JD, Hallen-Adams HE, Luo H. Ribosomal biosynthesis of the cyclic peptide toxins of Amanita mushrooms. Peptide Science. 2010; 94: 659–664.
Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G, Camarero JA, Campopiano DJ, Challis GL, Clardy J, Cotter PD, Craik DJ, Dawson M, Dittmann E, Donadio S, Dorrestein PC, Entian KD, Fischbach MA, Garavelli JS, Goeransson U, Gruber CW, Haft DH, Hemscheidt TK, Hertweck C, Hill C, Horswill AR, Jaspars M, Kelly WL, Klinman JP, Kuipers OP, Link AJ, Liu W, Marahiel MA, Mitchell DA, Moll GN, Moore BS, Mueller R, Nair SK, Nes IF, Norris GE, Olivera BM, Onaka H, Patchett ML, Piel J, Reaney MJT, Rebuffat S, Ross RP, Sahl HG, Schmidt EW, Selsted ME, Severinov K, Shen B, Sivonen K, Smith L, Stein T, Suessmuth RD, Tagg JR, Tang GL, Truman AW, Vederas JC, Walsh CT, Walton JD, Wenzel SC, Willey JM, van der Donk WA. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat Prod Rep. 2013; 30: 108–160
Hallen HE, Luo H, Scott-Craig JS, Walton JD. Gene family encoding the major toxins of lethal Amanita mushrooms. Proc Nat Acad Sci USA. 2007; 104: 19097–19101.
Luo H, Hallen-Adams HE, Scott-Craig JS, Walton JD. Ribosomal biosynthesis of a-amanitin in Galerina marginata. Fungal Genet Biol. 2012; 49: 123–129.
Luo H, Hong SY, Sgambelluri RM, Angelos E, Li X, Walton JD. Peptide macrocyclization catalyzed by a prolyl oligopeptidase involved in a-amanitin biosynthesis. Chem Biol. 2014; 21: 1610–1617.
Luo H, Hallen-Adams HE, Scott-Craig JS, Walton JD. Colocalization of amanitin and a candidate toxin-processing prolyl oligopeptidase in Amanita basidiocarps. Eukaryotic Cell. 2010; 9: 1891–1900.
Upadhyay S, Xu X, Lin X. Interactions between melanin enzymes and their atypical recruitment to the secretory pathway by palmitoylation. mBio. 2016; 7: e01925-16.
Lim FY and Keller NP. Spatial and temporal control of fungal natural product synthesis. Nat Prod Rep. 2014; 31: 1277.
Keller NP. Fungal secondary metabolism: regulation, function and drug discovery. Nat Rev Microbiol. 2019; 17: 167–180.
Yan Y, Liu N, Tang Y. Recent developments in self-resistance gene directed natural product discovery. Nat Prod Rep. 2020; 37: 879–892.
Scharf DH, Remme N, Heinekamp T, Hortschansky P, Brakhage AA, Hertweck C. Transannular disulfide formation in gliotoxin biosynthesis and its role in self-resistance of the human pathogen Aspergillus fumigatus. J Am Chem Soc. 2010; 132: 10136–10141.
Zhang Y, Bai J, Zhang L, Zhang C, Liu B, Hu Y. Self-resistance in the biosynthesis of fungal macrolides involving cycles of extracellular oxidative activation and intracellular reductive inactivation. Angew Chem Int Ed. 2021; 60: 6639–6645.
Guo CJ, Sun WW, Bruno KS, Oakley BR, Keller NP, Wang CCC. Spatial regulation of a common precursor from two distinct genes generates metabolic diversity. Chem Sci. 2015: 6: 5913–5921.
Berthier E, Lim FY, Deng Q, Guo CJ, Kontoyiannis DP, Wang CCC, Randy J, Beebe DJ, Huttenlocher A, Keller NP. Low-volume toolbox for the discovery of immunosuppressive fungal secondary metabolites. PLOS Pathogens. 2013; 9: e1003289.
Funding
This work was supported in part by the National Science Foundation (award number 2048347).
Author information
Authors and Affiliations
Contributions
The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Skellam, E. Subcellular localization of fungal specialized metabolites. Fungal Biol Biotechnol 9, 11 (2022). https://doi.org/10.1186/s40694-022-00140-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40694-022-00140-z