Abstract
Kin selection could provide an explanation for social behavior in bacteria. The production of public goods such as extracellular molecules is metabolically costly for bacteria but could help them to exploit nutrients or invade a host. Some bacterial cells called social cheaters do not produce public goods; however, they take advantage of these extracellular molecules. In this review, the relationships between social behavior, cooperation, and evolution of bacterial pathogenicity are analyzed. This paper also examines the role of horizontal transfer of genes encoding for virulence factors and how the movement of mobile genetic elements would influence the pathogenicity and social relationships. Moreover, the link between ecological relationships and evolution in entomopathogenic bacteria, focusing on Bacillus thuringiensis is considered. Finally, the findings obtained with B. thuringiensis are extrapolated on Bacillus pumilus 15.1, an entomopathogenic strain whose pathogenicity is not understood yet.
Similar content being viewed by others
Introduction
The social relationships of microorganisms are based on a wide range of extracellular actions produced by individual cells, which can affect the reproductive efficiency of other nearby cells. The production of molecules such as enzymes or chelants, which allow for the exploitation of nutrients that would not otherwise be accessible, or the formation of multicellular structures, are examples of this kind of action (Buckling and Rainey [2002]; Griffin et al. [2004]; West et al. [2006]). Extracellular products and other resources are public goods that may be used by the individual that produces them or by all individuals of the group or population (West et al. [2006], [2007]). For example, bacteria produce numerous factors that are considered to be public goods and are released into the environment, such as quorum-sensing molecules (Daniels et al. [2003]; Diggle et al. [2007a]; Williams et al. [2007]), membrane vesicles (Schooling and Beveridge [2006]), microbial repellents (Burgess et al. [2003]), host manipulation factors (Brown [1999]), adhesive polymers (Rainey and Rainey [2003]), or siderophores (West and Buckling [2003]), amongst others. This social behavior is, from a metabolic point of view, costly for the individual cells, although it is beneficial for the group. However, certain cells, known as ‘cheaters’ can decide not to produce these beneficial molecules for the group and instead take advantage of the rewards of social actions without paying any cost (West et al. [2006]). Thus, in microorganisms, social cheaters that do not display cooperative behavior are considered mutants (West et al. [2007]; Wakano et al. [2009]; Foster [2010]; Smith et al. [2014]) and can evolve with relative speed if they are favorably selected (Velicer et al. [2000]; Rainey and Rainey [2003]; Foster et al. [2004]; Griffin et al. [2004]; Dugatkin et al. [2005]; Harrison and Buckling [2005]).
Evolution experiments conducted on a wide range of microorganisms have demonstrated that this kind of cooperative behavior is most prevalent when there is a high degree of genetic closeness between the cells (West et al. [2006]; Mitri et al. [2011]). For example, the opportunistic pathogen Pseudomonas aeruginosa (Schroeter, 1872) produces extracellular iron-chelating molecules, known as siderophores (West and Buckling [2003]). These molecules are iron-scavenging agents that are released into the environment in response to a lack of this element (Ratledge and Dover [2000]). The siderophores high affinity for iron enables them to capture the metal from compounds such as ferrous hydroxide and host organism proteins such as transferrin or ferritin (Neilands [1995]; Drechsel and Jung [1998]). In addition, the relationship between siderophore production and bacterial growth rate suggests that the production of siderophores contributes to bacterial virulence (West and Buckling [2003]). Some cells have evolved as social cheaters since they produce small quantities of siderophores and yet they benefit from the action of siderophores produced by other cells (Ross-Gillespie et al. [2007]; Mitri et al. [2011]). Using this strategy, the cheats increase their frequency in comparison with the wild genotype (that does produce the molecule) (Mitri et al. [2011]; Smith et al. [2014]). However, in order for siderophore production to remain high, populations must be exposed to a strong genetic bottleneck, which ensures that the genetic relationship between the cells that produces the chelating agent and the one that is not is high. There are numerous examples of the role of kin selection in explaining the cooperation between microorganisms, such as the example of Bacillus subtilis (Ehrenberg, 1835), which forms biofilms in which closely related cells cooperate by becoming differentiated and sharing out functions (Foster [2010]). This same kind of social behavior has been reported in various other species (Wakano et al. [2009]).
This sacrificial phenomenon does not occur in multicellular organisms since the cells do not differ genetically from one another. No cell has an advantage over another, and if any cell should vary, it would be excluded from the cell line. For this reason, the deception of individual cells is restricted in the majority of multicellular organisms (Kessin [2000]). However, there is another route to multicellularity that is adopted by organisms such as Dictyostelium or Myxococus (a group of social bacteria). When nutrients become limited and cell density is high, Myxococcus xanthus (Beebe, 1941) activates various genes that break up the aggregation of local groups that exchange intercellular signals, allowing the formation of spore-producing fruiting bodies (Kadam and Velicer [2006]). These fruiting bodies contain approximately 50,000 cells, but only a fraction of the cells have differentiated latent myxospores (Curtis et al. [2007]) which apparently sacrifice themselves for the common good (Fiegna and Velicer [2006]; Fiegna et al. [2006]; Kadam and Velicer [2006]). M. xanthus cells also secrete toxins that can kill other cells within the fruiting body, or the species that feed on them; therefore, it is considered an example of group predation (Foster [2010]). In addition, there are mutant social cheaters that make up part of the local group and contribute less to the production of the non-spore-producing parts of the fruiting body (Fiegna et al. [2006]; West et al. [2007]).
Cooperation can be found at all levels of biological organization: genes cooperate in genomes, organelles cooperate to form eukaryotic cells, cells cooperate to form multicellular organisms, pathogenic bacteria cooperate to overcome the defenses of their hosts, animals feed cooperatively, and humans and some species of insect cooperate to construct societies (West et al. [2006], [2007]). With regard to the cooperation of pathogenic bacteria, reproduction within the host can be a cooperative activity that involves the secretion of virulent factors whose benefits can be shared by all of the cells, whether they secrete them or not (Smith [2001]). In this way, cooperation and success in overcoming the host's defenses will depend on the nature of the virulence factor (public good) as well as the proportion of social cheaters present in a population. For example, this could explain why the enterotoxigenic Escherichia coli (Migula, 1895) (producer of LT and ST toxins) requires a greater population density to cause an infection when compared with the enterohemorrhagic E. coli, a producer of Shiga toxin (STX) (Smith and Halls [1968]; O'brien et al. [1984]), which requires a smaller number of cells. Enterohemorrhagic E. coli populations would therefore include a lower proportion of cheater cells.
Although there is no doubt about cooperative behavior, there is controversy on how this kind of behavior might be interpreted, via kin selection or via group selection (West et al. [2007]; Wilson [2008]). As mentioned above, there are various pieces of evidence that support the idea that this kind of behavior in bacteria could be explained by the kin selection theory. For example, in bacterial species that form biofilms, cells that are genetically related are found close to one another (Griffin et al. [2004]; Diggle et al. [2007a],[b]). Nevertheless, the fact that all of the cells originate from a single mother cell in the majority of biofilms has been presented as an argument in favor of group selection (West et al. [2006]; Boyle et al. [2013]; Drescher et al. [2013]).
Biofilms are communities of microbial cells, commonly composed of various lineages or species, which grow on surfaces surrounded by polymers (Kolenbrander [2000]; Hall-Stoodley et al. [2004]). In this kind of community, the cellular groups have limited space and influence each other depending on the distance between them. These spatial relationships are of the utmost importance for the compression of cellular community cooperation evolution (Durrett and Levin [1994]; Drescher et al. [2013]). When different cellular lineages are separated in space, cooperative phenotypes are more likely to benefit other cells belonging to their phenotype (Mitteldorf and Wilson [2000]; Griffin and West [2002]; Axelrod et al. [2004]). However, when the different cellular lineages are intermingled, cells that exploit the resources of others are able to prosper more efficiently (Griffin et al. [2004]; Diggle et al. [2007a],[b]; Sandoz et al. [2007]; Chuang et al. [2009]). If the cells of a genotype are mixed with other genotypes, it is more probable that competitive characteristics that cause potential harm to neighboring cells will evolve than when the cells are surrounded by clones (Mitri et al. [2011]). Therefore, it has been suggested that the spatial structure of biofilms is a critical factor in microbial interactions (Boyle et al. [2013]). Intra-species cooperation could be favored by spatial segregation that keeps secreting cells away from those that do not secrete, while inter-species cooperation is favored by the presence of various species (Sandoz et al. [2007]; Nadell et al. [2009]). Cooperative phenotypes are subject to exploitation by non-cooperative cell lineages since they could be influencing the interaction between cooperative and non-cooperative cells (Nadell et al. [2009], [2010]). It is difficult for cooperative cells to be successful in competition against non-cooperative cells, which exploit public goods without paying the costs. However, if the cooperative cells are spatially segregated and prefer to interact amongst themselves, they can prevail. The spatial distribution of genetic lineages is related to physical and biological parameters, such as the availability and capacity for nutrient diffusion, cellular metabolic efficiency, cell growth rate, or biomass density. Drastic changes in these parameters can change the spatial layout of microbial populations within a community and determine if cells with cooperative phenotypes could compete locally or globally with social cheaters. The result would be the segregation of cellular lines that favor the evolution of cooperative phenotypes (Nadell et al. [2009]).
There is also abundant evidence that demonstrates the fact that the presence of different genotypes in biofilms limits cooperation across a wide range of bacterial characteristics (Greig and Travisano [2004]; Gore et al. [2009]), such as the secretion of enzymes (Griffin et al. [2004]); iron capture (Diggle et al. [2007a]), the secretion of quorum-sensing molecules and the formation of fruiting bodies (Foster et al. [2002]; Buttery et al. [2009]). Accordingly, it has been suggested that interactions between species can have a profound influence on intra-species cooperative behavior in spatially structured microbial groups like biofilms. It has been demonstrated that ecological competition with other species preferentially prejudices secreting cells more than non-secreting cells or social cheaters. This may be due to the fact that investing in secreting extracellular molecules can delay the growth of cellular lineages at critical stages. This initial investment leaves secreting cells vulnerable to competition with other lineages, particularly in nutrient-poor conditions where resources are limiting and the majority of lineages are eliminated through a strong genetic bottleneck. The potential for bottlenecks in growing microbial groups has been well documented (Gage [2002]; Hallatschek et al. [2007]; Boyle et al. [2013]). Bottlenecks have been interpreted as favorable for the evolution of cooperation because they promote genetic identity in emerging clonal groups (Brockhurst [2007]; Nadell et al. [2010]). However, Mitri et al. ([2011]) suggest that bottlenecks can also be indicative of the fact that strong ecological competition can eliminate cells with cooperative behavior before they have had the chance to establish themselves. For example, it has been demonstrated that P. aeruginosa lineages that secrete siderophores that capture iron are vulnerable to competition from lineages that do not secrete (but that use the siderophores without producing them) when Staphylococcus aureus (Rosenbach, 1884) is added (Harrison et al. [2008]). Population bottlenecks fulfill a fundamental role in the maintenance of social characteristics in microorganisms. Some ecological parameters such as colonization or disturbance may favor cooperation, causing population bottlenecks that increase genetic kinship. Moreover, the size of the population bottleneck fulfills a fundamental role in the success of cooperation. In this way, kinship increases as the size of the bottleneck decreases, favoring the evolution of cooperation. Brockhurst ([2007]) used Pseudomomas fluorescens (Migula, 1985) SBW25 to experimentally prove that the quantity of social cheaters increases as the size of the bottleneck increases, which suggests that the reduction in kinship caused by large genetic bottlenecks works against cooperation.
Other experiments have underlined the importance of ecological competition in favoring cooperation between species. The model developed by Rankin et al. ([2007]) demonstrated that intra-species competition can increase or decrease the ability to compete with other species. It has also been proposed that interactions with other species can promote the evolution of secreting genotypes (Mitri et al. [2011]; Drescher et al. [2013]). Social isolation allows the secreting cells to form patches in which they preferentially help their own genotype; therefore, the spatial structure of a biofilm may promote the evolution of cooperation (Nowak and May [1992]; Xavier et al. [2011]). The importance of the effects of social isolation in natural communities has not yet been clarified. However, it has been suggested that it could be more significant under conditions where nutrients are abundant in which various species can coexist. An interesting case study is that of the human microbiome, particularly that of the intestine, where the cells can form dense biofilms containing various species (Macfarlane and Dillon [2007]).
As mentioned above, the secretion of public goods in biofilms is affected by the availability of nutrients. When competition for nutrients is strong, the addition of new species can inhibit cooperation by eradicating secreting lineages before they can establish themselves. When nutrients are abundant and various species are found in the same habitat, the secreting lineages of any species are surrounded by other species. This ‘social isolation’ protects those cells that produce public goods from competition by those from the same species that do not produce public goods, and this can improve cooperation between species (Hol et al. [2013]). In addition, limitations in the interactions between species have been observed since it is difficult to find conditions that encourage cooperation between cells of the same species and of other species (Mitri et al. [2011]). On the other hand, it has been suggested that species with different metabolic needs show a greater tendency toward mutualistic behavior (Little et al. [2008]). In general, cooperation in microbial communities will be favored when there is a high level of competition between communities (Mitri et al. [2011]).
In conclusion, the appearance of biofilm organization could have arisen without active coordination. This implies that certain properties like phenotypic differentiation, species stratification, and the formation of channels do not necessarily require cells to communicate amongst themselves using specialized signal molecules. Also, although local cooperation between bacteria occurs frequently, the evolution of cooperation between cells belonging to a biofilm may be unlikely. Strong conflicts may arise between species and lineages in a biofilm, and spontaneous mutations may cause conflicts even if the communities were initiated by cells that were genetically identical (Nadell et al. [2009]; Hol et al. [2013]).
On the other hand, the appearance of cooperation via kin selection has also been described in more complex organisms such as vertebrates (Griffin et al. [2004]). Without a doubt, the best way to clarify what kind of selection is affecting the evolution of cooperative behavior is by means of experimentation. The evidence provided by experimental studies of evolution (Griffin et al. [2004]; Kummerli et al. [2009]) has been a fundamental pillar in the understanding of microorganism sociability.
Review
Horizontal gene transfer and the evolution of bacterial pathogenicity
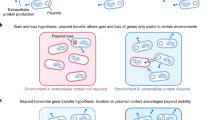
Bacteria transfer their genes vertically, from a mother cell to a daughter cell, similarly to what happens in more complex organisms, although they can also interchange their genes horizontally. Genes move quickly between genomes via mobile genetic elements such as plasmids, transposons, bacteriophages, and self-splicing molecular parasites (Nogueira et al. [2009]; Siefert [2009]). A vertically transmitted genome encodes for fundamental cellular processes, while a horizontally transmitted genome encodes for genes that allow for the exploitation of specialized niches or genes that provide resistance to toxic molecules (Hacker and Carniel [2001]). Many of the genes responsible for pathogenic bacterial virulence are found in mobile genetic elements that can be transmitted horizontally between different bacterial lineages. The horizontal transfer of virulence factor genes has played a fundamental role in the evolution of pathogenic bacteria. However, it is poorly understood why these genes are so mobile (Griffin et al. [2004]; West et al. [2006]; Nogueira et al. [2009]).
While plasmids benefit from the horizontal gene transfer as selfish DNA, this mobility implies a range of costs on host bacteria, especially in terms of the resources invested in conjugation (Nogueira et al. [2009]). On the other hand, bacteriophages are major contributors to the process of horizontal gene transfer given their environmental ubiquity, their great abundance in nature, and the functional effects that they cause in hosts (Hanage et al. [2005]). In this way, phages make a significant contribution to the diversity and evolution of bacteria since, in addition to acting as vectors for gene transfer, they promote a high coevolution rate and a high level of specialization between bacterial genotypes in natural microbial communities (Siefert [2009]). Lysogenic phages integrate their genome within the host genome until the prophage is excised from the host genome and cell lysis results. During this process, various segments of the host DNA can be introduced into the viral genome, and when lysis of the cell occurs, the host DNA is dispersed in the phages (Siefert [2009]). In this way, during lysogenic conversion, prophages can provide benefits to their hosts while they remain latent through the addition of new functions in the bacterial genome. For example, it is through this process that the harmless Vibrio cholerae (Pacini, 1854) lineage is transformed into the highly virulent lineage that causes cholera (Hanage et al. [2006]). Additionally, the insertion sequences (which can be autonomous or part of composite transposons) are efficient at moving themselves between bacterial genomes as they reshuffle and shape them (Chandler and Mahillon [2002]; Siguier et al. [2006]). It has been demonstrated that the massive expansion of insertion sequences has a positive correlation with the appearance of some pathogenic bacterial species (Siefert [2009]).
Numerous theories could explain why bacteria invest in gene mobility but none has been experimentally tested, which means that the evolutionary bases of the fundamental genome organization of bacteria remain unclear. The horizontal gene transfer is particularly important in the evolution of infectious diseases, in the spread of virulence genes, and in antibiotic resistance (Holden et al. [2004]; Frost et al. [2005]; Gogarten and Townsend [2005]; Murphy and Boyd [2008]). Key virulence genes, essential for infecting host organisms, are carried on mobile genetic elements in various clinically significant species such as S. aureus, Bacillus cereus (Frankland and Frankland, 1887), E. coli, and the agents that cause anthrax, cholera, and diphtheria (Smith [2001]).
Virulence, secretion, and horizontal mobility are closely related in bacterial genomes. Virulence factors are commonly secreted with the aim of reaching specialized tissue within the host (Smith [2001]). There is evidence that reveals that the E. coli genes responsible for secreted products tend to be more mobile than genes coding for intracellular products (Nogueira et al. [2009]). Secreted products are used as public goods by neighboring bacteria, independently of whether the neighbor secrets products for itself. In cooperative secretion, the individual bacteria that invest in secretion pay a cost in terms of growth, although the group benefits from the presence of virulence factors, for instance allowing an infection to become established in a host organism (West et al. [2006]; Raymond et al. [2010]).
Virulence factor genes would have evolved to increase the pathogenicity of bacteria, causing positive effects such as an increase in their transmission rate, which would help them to colonize new hosts, obtain new resources, evade the host defenses or disperse themselves to colonize new individuals (Ochman et al. [2000]; Nogueira et al. [2009]). However, extracellular virulence factors are potentially available to members of the pathogen population that do not produce the effector molecule. Thus, a social cheater cell could avoid the metabolic cost of producing the virulence factor, but it would increase its frequency during an infection due to the action of molecules produced by other cells in the population (Ross-Gillespie et al. [2007], [2009]).
If pathogens compete for resources within a host, the increase in cheats will reduce the transmission of the genotype that produces the virulence factor (Buckling and Rainey [2002]; Mitri et al. [2011]; Smith et al. [2014]). Nevertheless, it would be possible to reestablish the infectiveness by reducing the density of the cheater cells. The pathogenicity could also be restored by reintroducing functional versions of the genes coding for virulence factors in the cheats. As a result, it has been proposed that virulence factors are maintained in horizontally transmissible mobile genetic elements so that they can be reintroduced and prevent a high frequency of cheats (Smith [2001]; Wakano et al. [2009]). Accordingly, the existence of the tragedy of the commons in antibiotic-resistant plasmids was recently stated (Smith [2011]). Mobile genetic elements would be superinfecting bacteria that had already been previously infected, increasing the fitness of the plasmid. However, they would become victims of their own success since they reduce the density of their bacterial hosts (Smith [2011]).
The sociability of entomopathogenic bacteria and its ecology
Relatively few species of bacteria have been described as insect pathogens, but they have received a lot of attention as a result of their potential for pest control in agriculture and for the control of vector-transmitted diseases (van Emden and Service [2004]). Amongst these, most interest has been concentrated on Bacillus thuringiensis (Berliner, 1915), due to its specificity and its applicability as a biological control agent. B. thuringiensis is a strict aerobic, Gram-positive, flagellate, ubiquitous, sporulating bacteria that is morphologically and genetically related to B. cereus and B. anthracis (Cohn, 1872) and is widely used as a biological insecticide for insect control (Schnepf et al. [1998]; Crickmore et al. [2013]). B. thuringiensis produces protein crystals with insecticidal properties (Helgason et al. [2000]). The proteins of which they are composed are called δ-endotoxins and there are two main types: Cry toxins and Cyt toxins. δ-endotoxins are pore-forming proteins specifically active toward membranes from insects (Lepidoptera, Coleoptera, and Diptera), acari, nematodes, flat worms, and protozoa (Schnepf et al. [1998]; Lightwood et al. [2000]; Li et al. [2001]).
The mechanism of action of Cry toxins is a complex process that develops in various stages; however, the symptoms caused in the insect subjected to ingestion of the toxins can be summed up in the following steps: (1) ingestion ceases, (2) gut paralysis, (3) excretion of residues (vomiting and diarrhea), (4) total paralysis, and (5) death. A larva intoxicated with B. thuringiensis shows a characteristic black color due to tissue necrosis (Bravo et al. [1992]).
Cry insecticide toxins are the main virulence factors of B. thuringiensis and can be considered a public good. The sociability of these effector molecules has been studied under almost natural conditions using the larva of the diamondback moth, Plutella xyllostella (Linnaeus, 1758), as a host (Raymond et al. [2012]). As is the case with other bacteria that possess social behavior, certain B. thuringiensis genotypes do not produce Cry toxins. However, they are able to benefit from the production of the insecticidal proteins synthesized by other individuals in the population when infecting the host. Thus, non-toxin-producing cells can take advantage of the formation of pores in the epithelial cells of the insect gut to infect it. This would demonstrate that the reproductive efficiency of microorganisms can depend on the cooperation between cells, both in controlled conditions and in nature (Griffin et al. [2004]; Raymond et al. [2012]). Interestingly, the high densities reached by social cheaters could explain why B. thuringiensis does not cause epidemics on nature (Raymond et al. [2012]). This connection between cooperative virulence and epidemiology could also be relevant for species of bacteria that do not produce toxins, including some human pathogens.
The entomopathogenicity of Bacillus pumilus (Meyer and Gottheil, 1901) could also be explained by the social evolution of virulence factors. B. pumilus is a ubiquitous bacteria with a wide range of significant activities from a biotechnological point of view. Some strains of B. pumilus have fungicidal properties and have been used as biological control agents against phytopathogenic fungi (Bottone and Peluso [2000]; Lehman et al. [2001]; De-Bashan et al. [2010]), while others have shown powerful antibacterial activity (Aunpad and Na-Bangchang [2007]). In addition, B. pumilus has been reported as entomopathogenic bacteria. The first study that demonstrated the entomopathogenicity of B. pumilus is detailed in a patent that describes a strain active against the corn rootworm (e.g., Diabrotica undecimpunctata (Mannerheim, 1843), Diabotrica longicornis (Say, 1824)), the armyworm (Spodoptera exigua (Hubner, 1808)), and some species of nematodes (Heins et al. [1999]). The entomopathogenic activity of B. pumilus was confirmed in a study that described the isolation of the strain 15.1, a highly toxic strain against Mediterranean fruit fly larvae, Ceratitis capitata (Wiedemann, 1824) (Molina et al. [2010]). Since B. pumilus has never been considered as a classic entomopathogenic bacteria, the origin and the possible evolutionary route of the insecticidal activity of the B. pumilus 15.1 strain is a fairly interesting topic that would help to explain the process of pathogenicity. The specific case of B. thuringiensis, whose ecology is widely known, could represent the best analogous example for the discussion of this topic. Therefore, we will concentrate on B. thuringiensis strains active against leaf-eating insects (since it is the best case study), with the aim of explaining the possible origin of B. pumilus 15.1 pathogenicity.
The role of B. thuringiensis in the environment remains unclear due to, among other reasons, a lack of manipulative field experiments (Travers et al. [1987]; Raymond et al. [2010]). One of the many hypotheses that attempt to explain the role of B. thuringiensis suggests that the bacteria has evolved in order to provide symbiotic protection to plants since it forms part of the normal phylloplane microbiota (Smith and Couche [1991]; Elliot et al. [2000]). Another hypothesis proposes that B. thuringiensis is a natural soil inhabitant with incidental insecticidal activity (Martin and Travers [1989]). This hypothesis states that except from the fact that B. thuringiensis has previously been found in association with insects, there is no reason to believe that this association is vital. The ubiquity of B. thuringiensis in soil is consistent with this idea and further supports the hypothesis of Dulmage and Aizawa ([1982]), which proposes that the soil is the normal environment of B. thuringiensis. However, this bacteria is not usually toxic against insect larvae that live in the soil, but it is against insects with aerial or water-borne larvae.
On the other hand, Jensen et al. ([2003]) suggested that B. thuringiensis could be part of the commensal gut microbiota of various insect species without causing obvious disease or death. However, Raymond et al. ([2010]) demonstrated that B. thuringiensis behaves as a specialized insect pathogen in the field. This study suggests that the normal habitat of B. thuringiensis is the soil, and that movement from the soil (which acts as a reservoir) toward the aerial parts of the plant, where susceptible hosts are present, is a key feature of its ecology.
Similarly to B. thuringiensis, B. pumilus is an ubiquitous bacteria whose typical habitat is the soil. The B. pumilus 15.1 strain was isolated from a partially decomposed reed plant (Molina et al. [2010]), suggesting that plants can also form part of the natural habitat of this bacterium. Besides, as it has been mentioned above, other B. pumilus strains have been reported playing many other roles: first, as a growth-promoting rhizobacteria; second, providing protection to the plants as a result of its antifungal properties against phytopathogenic fungi; and finally playing a role in the plant defense reactions (Benhamou et al. [1996]).
It is a fact that B. pumilus interacts with plants and there is evidence to suggest that this ecological relationship is beneficial to both organisms. Smith and Couche ([1991]) proposed an explanation for this type of interaction between a plant and an entomopathogenic bacteria. This study proposed that B. thuringiensis populations found in the phylloplanes of plants could inhibit the feeding of insect larvae. This situation could be a symbiotic relationship in which B. thuringiensis also benefits from being a phylloplane epiphyte. In such a way, the plant could provide nutrients from leaf exudates and associated microflora and also provide a niche free from competition with other soil-borne spore-forming bacteria. On the phylloplane, B. thuringiensis is accessible to leaf-feeding insect larvae. If the larvae ingest sufficient toxin spore and crystals to inhibit their feeding, defoliation would be reduced, and as a result, the plant would be protected. This proposed hypothesis for plant-entomopathogenic bacteria association could also explain the relationship between B. pumilus and plants. Furthermore, this hypothesis does not exclude other concepts about the ecological role of B. pumilus, for instance, that it could act as an antifungal or an antibacterial agent in the soil microcosm (Elliot et al. [2000]).
Elliot et al. ([2000]) extended the plant hypothesis (originally described as predators and parasitoids) to entomopathogens and essentially agrees with Smith and Couche ([1991]). In order for a plant to employ an entomopathogen as a bodyguard, this relationship must represent a good return on investment (the benefits of the relationship must outweigh the costs) (Price et al. [1980], Elliot et al. [2000]). The ‘bodyguard’ hypothesis in which a bacteria acts mutualistically to a plant could explain why B. thuringiensis is maintained in the phylloplane and on the ground. The plant benefits from the entomopathogenic activity of the bacteria before its population reaches high densities (Elliot et al. [2000]; Buckling and Rainey [2002]).
Based on the aforementioned case of B. thuringiensis, on the fact that B. pumilus is a ubiquitous bacteria with the ground as its typical habitat, and on its ecological relationship with plants, it is suggested that the bodyguard hypothesis could also explain the entomopathogenicity of B. pumilus 15.1. C. capitata larvae are not leaf-feeding, but they are considered as phytophages. For this reason, and because of the relationship that B. pumilus maintains with plants, the application of the bodyguard theory could be feasible. This hypothesis may represent the best explanation for B. pumilus insecticidal activity. On the other hand, in spite of the fact that B. pumilus is a different strain, it has been reported as a pathogen for S. exigua (Heins et al. [1999]), a species with defoliating larvae.
In addition, the presence of crystals has been described in sporulating cultures of B. pumilus 15.1 that are similar to the Cry proteins of B. thuringiensis (Molina et al. [2009]). The role of these structures has not yet been elucidated. However, it could be suggested that they are involved in the entomopathogenicity of B. pumilus 15.1 due to their structural similarity with the insecticidal toxins of B. thuringiensis (Molina et al. [2009]). The molecules synthesized and excreted by B. pumilus 15.1 would be considered as the bacteria public goods; therefore, it is possible that they would have the same social behavior as that which has been widely reported. If so, the presence of social cheater cells could also explain why B. pumilus 15.1 does not cause epidemics in nature, from where it was isolated.
Conclusions
The production of public goods such as protein crystals that are toxic to insects by entomopathogenic bacteria could constitute an excellent model for the study of the relationship between the sociability of microorganisms and the evolution of their pathogenicity. In addition, the model would be robust due to the ability of sporulating microorganisms to resist adverse conditions and to continue producing public goods for the next generation. Similarly, it would be expected that due to the selective pressure applied by insects on bacteria with insecticidal activity, their virulence would increase through the production of a greater quantity of protein crystals after each pass through the insect. For all the forgoing reasons, understanding the evolution of pathogenicity and its relationship to sociability should focus on the study of bacteria that are pathogenic for insects.
Authors' information
CAM is affiliated to Centro Internacional de Zoonosis; Facultad de Medicina Veterinaria y Zootecnia, Universidad Central del Ecuador (UC), PO Box.17-03-100, Quito, Ecuador and Facultad de Ciencias Ambientales, Universidad Internacional SEK, Calle Alberto Einstein y 5ta. Transversal, Campus Miguel de Cervantes – Carcelén, Quito, Ecuador. SV is affiliated to Departamento de Bioquímica y Biología Molecular I, Facultad de Ciencias, Universidad de Granada, Campus Universitario Fuentenueva, 18071 Granada, España.
References
Aunpad R, Na-bangchang K: Pumilicin 4, a novel bacteriocin with anti-MRSA and anti-VRE activity produced by newly isolated bacteria Bacillus pumilus strain WAPB4. Curr Microbiol 2007, 55: 308–313. 10.1007/s00284-006-0632-2
Axelrod R, Hammond RA, Grafen A: Altruism via kin-selection strategies that rely on arbitrary tags with which they coevolve. Evolution 2004, 58: 1833–1838. 10.1111/j.0014-3820.2004.tb00465.x
Benhamou N, Kloepper JW, Quadt-Hallman A, Tuzun S: Induction of defense-related ultrastructural modifications in pea root tissues inoculated with endophytic bacteria. Plant Physiol 1996, 112: 919–929.
Bottone EJ, Peluso R (2000) Bacillus pumilus strain. US Patent 6,090,613, 18 Jul 2000
Boyle KE, Heilmann S, Van Ditmarsch D, Xavier JB: Exploiting social evolution in biofilms. Current Opin Microbiol 2013, 16: 207–212. 10.1016/j.mib.2013.01.003
Bravo A, Hendrickx K, Jansens S, Peferoen M: Immunocytochemical analysis of specific binding of Bacillus thuringiensis insecticidal crystal proteins to lepidopteran and coleopteran midgut membranes. J Invertebr Pathol 1992, 60: 247–253. 10.1016/0022-2011(92)90005-O
Brockhurst MA: Population bottlenecks promote cooperation in bacterial biofilms. PLoS One 2007, 2: e634. 10.1371/journal.pone.0000634
Brown SP: Cooperation and conflict in host-manipulating parasites. Proc R Soc Lond B 1999, 266: 1899–1904. 10.1098/rspb.1999.0864
Buckling A, Rainey PB: Antagonistic coevolution between a bacterium and a bacteriophage. Proc R Soc Lond B 2002, 269: 931–936. 10.1098/rspb.2001.1945
Burgess JG, Boyd KG, Armstrong E, Jiang Z, Yan L, Berggren M, May U, Pisacane T, Grammo A, Adams DR: The development of a marine natural product-based antifouling paint. Biofouling 2003,19(Suppl):197–205. 10.1080/0892701031000061778
Buttery NJ, Rozen D, Wolf J, Thompson CRL: Quantification of social behavior in D. discoideum reveals complex fixed and facultative strategies. Curr Biol 2009, 19: 1373–1377. 10.1016/j.cub.2009.06.058
Chandler M, Mahillon J: Insertion sequences revisited. In Mobile DNA, vol II. Edited by: Craig N, Craigie R, Gellernt M, Lambowitz M. ASM Press, Washington DC; 2002.
Chuang JS, Rivoire O, Leibler S: Simpson's paradox in a synthetic microbial system. Science 2009, 323: 272–275. 10.1126/science.1166739
Crickmore N, Baum J, Bravo A, Lereclus D, Narva K, Sampson K, Schnepf E, Sun M, Zeigler DR (2013) Bacillus thuringiensis toxin nomenclature. Accesed 16 March 2013, [http://www.btnomenclature.info/]
Curtis PD, Taylor RG, Welch RD, Shimkets LJ: Spatial organization of Myxococus xanthus during fruiting body formation. J Bacteriol 2007, 189: 9126–9130. 10.1128/JB.01008-07
Daniels R, Vanderleyden J, Michiels J: Quorum sensing and swarming migration in bacteria. FEMS Microbiol Rev 2003, 28: 261–289. 10.1016/j.femsre.2003.09.004
De-Bashan LE, Hernandez JP, Bashan Y, Maier RM: Bacillus pumilus ES4: candidate plant growth-promoting bacterium to enhance establishment of plants in mine tailings. Environ Exp Bot 2010, 69: 343–352. 10.1016/j.envexpbot.2010.04.014
Diggle SP, Gardner A, West SA, Griffin AS: Evolutionary theory of bacterial quorum sensing: when is a signal not a signal? Philos Trans R Soc B 2007, 362: 1241–1249. 10.1098/rstb.2007.2049
Diggle SP, Griffin AS, Campbell GS, West SA: Cooperation and conflict in quorum-sensing bacterial populations. Nature 2007, 450: 411–414. 10.1038/nature06279
Drechsel H, Jung G: Peptide siderophores. J Pept Sci 1998, 4: 147–181. 10.1002/(SICI)1099-1387(199805)4:3<147::AID-PSC136>3.0.CO;2-C
Drescher K, Nadell CD, Stone HA, Wingreen NS, Bassler BL (2013) Solutions to the public goods dilemma in bacterial biofilms. Curr Biol:S0960–S9822. doi:10.1016/j.cub.2013.10.030
Dugatkin LA, Perlin M, Lucas JS, Atlas R: Group-beneficial traits, frequency dependent selection and genotypic diversity: an antibiotic resistance paradigm. Proc R Soc Lond B 2005, 272: 79–83. 10.1098/rspb.2004.2916
Dulmage H, Aizawa K (1982) Distribution of Bacillus thuringiensis in nature. In: Kurstak E (ed) Microbial and Viral Pesticides. Marcel Dekker, Inc., New York
Durrett R, Levin S: The importance of being discrete (and spatial). Theor Popul Biol 1994, 46: 363–394. 10.1006/tpbi.1994.1032
Elliot SL, Sabelis MW, Janssen A, Van Der Geest LPS, Beerling AEM, Fransen J: Can plants use entomopathogens as bodyguards? Ecol Lett 2000, 3: 228–235. 10.1046/j.1461-0248.2000.00137.x
Fiegna F, Velicer GJ: Exploitative and hierarchical antagonism in a cooperative bacteria. PLoS Biol 2006, 3: e370. 10.1371/journal.pbio.0030370
Fiegna F, Yu Y, Kadam S, Velicer GJ: Evolution of an obligate social cheater to a superior cooperator. Nature 2006, 441: 310–314. 10.1038/nature04677
Foster KR: Social behaviour in microorganisms. In Social behaviour: genes, ecology and evolution. Edited by: Szekely T, Moore AJ, Komdeur J. Cambridge University Press, Cambridge; 2010.
Foster KR, Fortunato A, Strassmann JE, Queller DC: The costs and benefits of being a chimera. Proc R Soc Lond B 2002, 269: 2357–2362. 10.1098/rspb.2002.2163
Foster KR, Shaulsky G, Strassmann JE, Queller DC, Thompson CRL: Pleiotropy as a mechanism to stabilize cooperation. Nature 2004, 431: 693–696. 10.1038/nature02894
Frost LS, Leplae R, Summers AO, Toussaint A: Mobile genetic elements: the agents of open source evolution. Nat Rev Microbiol 2005, 3: 722–732. 10.1038/nrmicro1235
Gage DJ: Analysis of infection thread development using Gfp- and DsRedexpressing Sinorhizobium meliloti . J Bacteriol 2002, 184: 7042–7046. 10.1128/JB.184.24.7042-7046.2002
Gogarten JP, Townsend JP: Horizontal gene transfer, genome innovation and evolution. Nat Rev Microbiol 2005, 3: 679–687. 10.1038/nrmicro1204
Gore J, Youk H, Van Oudenaarden A: Snowdrift game dynamics and facultative cheating in yeast. Nature 2009, 459: 253–256. 10.1038/nature07921
Greig D, Travisano M: The Prisoner’s Dilemma and polymorphism in yeast SUC genes. Proc R Soc Lond B 2004, 271: S25-S26. 10.1098/rsbl.2003.0083
Griffin AS, West SA: Kin selection: fact and fiction. Trends Ecol Evol 2002, 17: 15–21. 10.1016/S0169-5347(01)02355-2
Griffin AS, West SA, Buckling A: Cooperation and competition in pathogenic bacteria. Nature 2004, 430: 1024–1027. 10.1038/nature02744
Hacker J, Carniel E: Ecological fitness, genomic islands and bacterial pathogenicity. A Darwinian view of the evolution of microbes. EMBO Rep 2001, 2: 376–381. 10.1093/embo-reports/kve097
Hallatschek O, Hersen P, Ramanathan S, Nelson DR: Genetic drift at expanding frontiers promotes gene segregation. Proc Natl Acad Sci USA 2007, 104: 19926–19930. 10.1073/pnas.0710150104
Hall-Stoodley L, Costerton JW, Stoodley P: Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol 2004, 2: 95–108. 10.1038/nrmicro821
Hanage WP, Fraser C, Spratt BG: Fuzzy species among recombinogenic bacteria. BMC Biol 2005, 3: 6. 10.1186/1741-7007-3-6
Hanage WP, Fraser C, Spratt BG: Sequences, sequence clusters and bacterial speciation. Philos Trans R Soc B 2006, 361: 1917–1927. 10.1098/rstb.2006.1917
Harrison F, Buckling A: Hypermutability impedes cooperation in pathogenic bacteria. Curr Biol 2005, 15: 1968–1971. 10.1016/j.cub.2005.09.048
Harrison F, Paul J, Massey RC, Buckling A: Interspecific competition and siderophore-mediated cooperation in Pseudomonas aeruginosa . ISME J 2008, 2: 49–55. 10.1038/ismej.2007.96
Heins SD, Manker DC, Jimenez DR, Marrone PG (1999) Bacillus pumilus strain for controlling corn rootworm, nematode and armyworm infestations. US Patent 6,001,637, 14 Dec 1999
Helgason E, Caugant DA, Olsen I, Kolsto AB: Genetic structure of population of Bacillus cereus and B. thuringiensis isolates associated with periodontitis and other human infections. J Clin Microbiol 2000, 38: 1615–1622.
Hol FJH, Galajda P, Nagy K, Woolthuis RG, Dekker C, Keymer JE: Spatial structure facilitates cooperation in a social dilemma: empirical evidence from a bacterial community. PLoS One 2013, 8: e77042. 10.1371/journal.pone.0077042
Holden MT, Feil EJ, Lindsay JA, Peacock SJ, Day NP, Enright MC, Foster TJ, Moore CE, Hurst L, Atkin R, Barron A, Bason N, Bentley SD, Chillingworth C, Chillingworth T, Churcher , Clark L, Corton C, Cronin A, Doggett J, Dowd L, Feltwell T, Hance Z, Harris B, Hauser H, Holroyd S, Jagels K, James KD, Lennard N, Line A, et al.: Complete genomes of two clinical Staphylococcus aureus strains: evidence for the rapid evolution of virulence and drug resistance. Proc Natl Acad Sci USA 2004, 101: 9786–9791. 10.1073/pnas.0402521101
Jensen GB, Hansen BM, Eilenberg J, Mahillon J: The hidden lifestyles of Bacillus cereus and relatives. Environ Microbiol 2003, 5: 631–640. 10.1046/j.1462-2920.2003.00461.x
Kadam SV, Velicer GJ: Variable patterns of density-dependent survival in social bacteria. Behav Ecol 2006, 17: 833–838. 10.1093/beheco/arl018
Kessin RH: Evolutionary biology: cooperation can be dangerous. Nature 2000, 408: 917–919. 10.1038/35050184
Kolenbrander PE: Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol 2000, 54: 413–437. 10.1146/annurev.micro.54.1.413
Kummerli R, Gardner A, West SA, Griffin AS: Limited dispersal, budding dispersal, and cooperation: an experimental study. Evolution 2009, 63: 939–949. 10.1111/j.1558-5646.2008.00548.x
Lehman LJ, Mccoy RJ, Messenger BJ, Manker DC, Orjala JE, Lindhard D, Marrone PG (2001) Strain of Bacillus pumilus for controlling plant diseases caused by fungi. US Patent 6,245,551 B1, 12 Jun 2001
Li J, Derbyshire DJ, Promdonkoy B, Ellar DJ: Structural implications for the transformation of the Bacillus thuringiensis delta-endotoxins from water soluble to membrane inserted form. Biochem Soc T 2001, 29: A54. 10.1042/BST0290571
Lightwood DJ, Ellar DJ, Jarrett P: Role of proteolysis in determining potency of Bacillus thuringiensis Cry1Ac delta -endotoxin. Appl Environ Microbiol 2000, 66: 5174–5181. 10.1128/AEM.66.12.5174-5181.2000
Little AEF, Robinson CJ, Peterson SB, Raffa KF, Handelsman J: Rules of engagement: interspecies interactions that regulate microbial communities. Annu Rev Microbiol 2008, 62: 375–401. 10.1146/annurev.micro.030608.101423
Macfarlane S, Dillon JF: Microbial biofilms in the human gastrointestinal tract. J Appl Microbiol 2007, 102: 1187–1196. 10.1111/j.1365-2672.2007.03287.x
Martin P, Travers R: Worldwide abundance and distribution of Bacillus thuringiensis isolates. Appl Environ Microbiol 1989, 55: 2437–2442.
Mitri S, Xavier J, Foster KR: Social evolution in multispecies biofilms. Proc Natl Acad Sci USA 2011, 108: 10839–10846. 10.1073/pnas.1100292108
Mitteldorf J, Wilson DS: Population viscosity and the evolution of altruism. J Theor Biol 2000, 204: 481–496. 10.1006/jtbi.2000.2007
Molina CA, Cana-Roca JF, Dominguez T, Osuna A, Vilchez S (2009) High activity of a Bacillus pumilus strain against Ceratitis capitata. In: Ehlers RU, Crickmore N, Enkerli J, Glazer I, Lopez-Ferber M, Tkaczuk C (eds) Insect pathogens and insect parasitic nematodes. 12th Meeting IOBC/WPRS and COST Action 862 “Bacterial Toxins for Insect Control”: Future research and development in the use of microbial agents and nematodes for biological insect control, vol 45. IOBC WPRS Bulletin, Pamplona, pp 191–194
Molina CA, Cana-Roca JF, Osuna A, Vilchez S: Selection of a Bacillus pumilus strain highly active against Ceratitis capitata (Wiedemann) larvae. Appl Environ Microbiol 2010, 76: 1320–1327. 10.1128/AEM.01624-09
Murphy RA, Boyd EF: Three pathogenicity islands of Vibrio cholerae can excise from the chromosome and form circular intermediates. J Bacteriol 2008, 190: 636–647. 10.1128/JB.00562-07
Nadell CD, Xavier JB, Foster KR: The sociobiology of biofilms. FEMS Microbiol Rev 2009, 33: 206–224. 10.1111/j.1574-6976.2008.00150.x
Nadell CD, Foster KR, Xavier JB: Emergence of spatial structure in cell groups and the evolution of cooperation. PLoS Comput Biol 2010, 6: e1000716. 10.1371/journal.pcbi.1000716
Neilands JB: Siderophores: structure and function of microbial iron transport compounds. J Biol Chem 1995, 270: 26723–26726. 10.1074/jbc.270.45.26723
Nogueira T, Rankin DJ, Touchon M, Taddei F, Brown SP, Rocha EPC: Horizontal gene transfer of the secretome drives the evolution of bacterial cooperation and virulence. Curr Biol 2009, 19: 1683–1691. 10.1016/j.cub.2009.08.056
Nowak MA, May RM: Evolutionary games and spatial chaos. Nature 1992, 359: 826–829. 10.1038/359826a0
O’Brien AD, Newland JW, Miller SF, Holmes RK, Smith HW, Formal SB: Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 1984, 266: 694–696. 10.1126/science.6387911
Ochman H, Lawrence JG, Groisman EA: Lateral gene transfer and the nature of bacterial innovation. Nature 2000, 405: 299–304. 10.1038/35012500
Price PW, Bouton CE, Gross P, Mcpheron BA, Thompson JN, Weis AE: Interactions among 3 trophic levels influence of plants on interactions between insect herbivores and natural enemies. Annu Rev Ecol Syst 1980, 11: 41–65. 10.1146/annurev.es.11.110180.000353
Rainey PB, Rainey K: Evolution of cooperation and conflict in experimental bacterial populations. Nature 2003, 425: 72–74. 10.1038/nature01906
Rankin DJ, López-Sepulcre A, Foster KR, Kokko H: Species-level selection reduces selfishness through competitive exclusion. J Evolution Biol 2007, 20: 1459–1468. 10.1111/j.1420-9101.2007.01337.x
Ratledge C, Dover LG: Iron metabolism in pathogenic bacteria. Annu Rev Microbiol 2000, 54: 881–941. 10.1146/annurev.micro.54.1.881
Raymond B, Wyres KL, Sheppard SK, Ellis RJ, Bonsall MB: Environmental factors determining the epidemiology and population genetic structure of the Bacillus cereus group in the field. PLoS Pathog 2010, 6: e1000905. 10.1371/journal.ppat.1000905
Raymond B, West SA, Griffin AS, Bonsall MB: The dynamics of cooperative bacterial virulence in the field. Science 2012, 337: 4. 10.1126/science.1218196
Ross-Gillespie A, Gardner A, West SA, Griffin AS: Frequency dependence and cooperation: theory and a test with bacteria. Am Nat 2007, 170: 331–342. 10.1086/519860
Ross-Gillespie A, Gardner A, Buckling A, West SA, Griffin AS: Density dependence and cooperation: theory and a test with bacteria. Evolution 2009, 63: 2315–2325. 10.1111/j.1558-5646.2009.00723.x
Sandoz KM, Mitzimberg SM, Schuster M: Social cheating in Pseudomonas aeruginosa quorum sensing. Proc Natl Acad Sci USA 2007, 104: 15876–15881. 10.1073/pnas.0705653104
Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH: Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol Mol Biol R 1998, 62: 775–806.
Schooling SR, Beveridge TJ: Membrane vesicles: an overlooked component of the matrices of biofilms. J Bacteriol 2006, 188: 5945–5957. 10.1128/JB.00257-06
Siefert JL: Defining the mobilome. In Horizontal gene transfer: genomes in flux. Edited by: Gogarten MB, Gogarten JP, Olendzenski L. Humana Press, New York; 2009.
Siguier P, Filee J, Chandler M: Insertion sequences in prokaryotic genomes. Curr Opin Microbiol 2006, 9: 526–531. 10.1016/j.mib.2006.08.005
Smith J: The social evolution of bacterial pathogenesis. Proc R Soc Lond B 2001, 268: 61–69. 10.1098/rspb.2000.1330
Smith J: Superinfection drives virulence evolution in experimental populations of bacteria and plasmids. Evolution 2011, 65: 831–841. 10.1111/j.1558-5646.2010.01178.x
Smith RA, Couche GA: The phylloplane as a source of Bacillus thuringiensis variants. Appl Environ Microbiol 1991, 57: 311–315.
Smith HW, Halls S: The transmissible nature of the genetic factor in Escherichia coli that controls exterotoxin production. J Gen Microbiol 1968, 52: 319–334. 10.1099/00221287-52-3-319
Smith J, Van Dyken JD, Velicer GJ (2014) Nonadaptive processes can create the appearance of facultative cheating in microbes. Evolution. doi:10.1111/evo.12306
Travers RS, Martin PA, Reichelderfer CF: Selective process for efficient isolation of soil Bacillus spp. Appl Environ Microbiol 1987, 53: 1263–1266.
Van Emden H, Service M (2004) Pest and vector control. Cambridge University Press, Cambridge
Velicer GJ, Kroos L, Lenski RE: Developmental cheating in the social bacterium Myxococcus xanthus . Nature 2000, 404: 598–601. 10.1038/35007066
Wakano JY, Nowak MA, Hauert C: Spatial dynamics of ecological public goods. Proc Natl Acad Sci USA 2009, 106: 7910–7914. 10.1073/pnas.0812644106
West SA, Buckling A: Cooperation, virulence and siderophore production in bacterial parasites. Proc R Soc Lond B 2003, 270: 37–44. 10.1098/rspb.2002.2209
West SA, Griffin AS, Gardner A, Diggle SP: Social evolution theory for microorganisms. Nat Rev Microbiol 2006, 4: 597–607. 10.1038/nrmicro1461
West SA, Griffin AS, Gardner A: Social semantics: altruism, cooperation, mutualism, strong reciprocity and group selection. J Evol Biol 2007, 20: 415–432. 10.1111/j.1420-9101.2006.01258.x
Williams P, Winzer K, Chan W, Camara M: Look who's talking: communication and quorum sensing in the bacterial world. Philos Trans R Soc B 2007, 362: 1119–1134. 10.1098/rstb.2007.2039
Wilson DS: Social semantics: toward a genuine pluralism in the study of social behaviour. J Evol Biol 2008, 21: 368–373. 10.1111/j.1420–9101.2007.01396.x 10.1111/j.1420-9101.2008.01500.x
Xavier JB, Kim W, Foster KR: A molecular mechanism that stabilizes cooperative secretions in Pseudomonas aeruginosa . Mol Microbiol 2011, 79: 166–179. 10.1111/j.1365-2958.2010.07436.x
Acknowledgements
The authors thank the three anonymous reviewers for their valuable comments and suggestions to improve the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
CAM reviewed the literature and wrote the manuscript. SV helped to draft the manuscript and provided comments on it. Both authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Molina, C.A., Vilchez, S. Cooperation and bacterial pathogenicity: an approach to social evolution. Rev. Chil. de Hist. Nat. 87, 14 (2014). https://doi.org/10.1186/s40693-014-0014-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40693-014-0014-2