Abstract
In the present study, Terminalia chebula (Myrobalan/Harda) natural dye extract was used for the development of eco-friendly shades on woolen yarn with different hues and tones. The effect of dye concentration on color strength (K/S) of woolen yarn dyed with T. chebula was assessed. Increasing the concentration of dye decreased lightness (L*) values of woolen yarn samples, indicating darker shades. Different metal salts such as alum, ferrous sulfate, and stannous chloride were used to enhance the fastness properties (light, wash, dry and wet rubs) of dyed woolen yarn. Pre-treatment of woolen yarn samples with metal salts has shown encouraging results with better fastness properties and enhanced color strength values. Five percent ferrous sulfate mordanted samples show greater saturation with increasing dye concentration from 0.5 to 15 % (o.w.f.). Fourier-transform infrared (FT-IR) analysis of T. chebula dye extract shows presence of carbonyl and hydroxyl functionalities.
Similar content being viewed by others
Background
Natural dyes have been used since time immemorial to color different textile materials. In the nineteenth century, synthetic dyes in view of their low cost, large variety of shades, and high dyeability overtook the use of natural dyes (Samantha and Agarwal 2009; Yusuf et al. 2013). Synthetic dyes and particularly azo-based dyes are recently discovered to pollute the environment. This has motivated textile researchers to reintroduce natural colorants from renewable resources once again into the dyeing industry. Natural dyes produced from plants, animals, insects, and minerals are mostly non-toxic, non-carcinogenic, biodegradable, and environment-friendly in nature with some biological activities (Khan et al. 2010; Shahid et al. 2013). To achieve the sophisticated demand of modern people, a lot of research has been undertaken in the field of natural dyes for obtaining colorful shades on textiles and evaluation of their tolerance to the light, wash, and rub effects (Khan et al. 2015; Islam and Mohammad 2015). Metallic mordants as well as bio-mordants can be used to enhance the color characteristics and fastness properties of natural dyes on a textile substrate (Dalby 1993; Vankar et al. 2008). It is also reported that natural dyes can not only be used to get natural shades but they can also provide functionalities to fabrics such as antibacterial activity (M. I. Khan et al. 2011; S. A. Khan et al. 2012; Shahid et al. 2012), antifungal activity (Yusuf et al. 2015), and ultraviolet protection (Zhou et al. 2015).
Tannin-based dyes are used for textile dyeing for their good substantivity towards them because of more numbers of auxochromic groups in coloring components. Terminalia chebula is a medicinal plant grown in India and Southeast Asia (Khan et al. 2005). It is one of the constituents of triphala used in India as medicine. Fruits of T. chebula are included in the Indian pharmacopeia under the category astringent. It possesses laxative, diuretic, cardiotonic, and hypoglycemic properties (Naik et al. 2004). The major phytoconstituents present in the fruits are hydrolysable tannins, gallic acid, chebulic acid, chebulic ellagitannins, and gallate esters (Pfundstein et al. 2010).
The present research paper is aimed to explore the dyeing potential of T. chebula on wool fiber. The effect of the dye concentration and different types of metal mordants such as alum, ferrous sulfate, and stannous chloride on color characteristics of developed shades and their fastness properties are also studied.
Materials
Wool yarn (100 % semi-worsted 60 counts) was purchased from MAMB Woollens Ltd. Bhadohi, UP, India. T. chebula dye extract in powder form was purchased from Sir Biotech India Ltd. Kanpur, UP, India. Metallic mordants potash alum (Al2K2(SO4)4·24H2O), iron sulfate (FeSO4·7H2O), and stannous chloride (SnCl2·2H2O) were used of laboratory grade.
Instrumentation
Fourier-transform infrared (FT-IR) spectra of T. chebula dye powder were obtained on a Perkin Elmer Spectrum RXI FT-IR system in order to investigate and observe auxochromic groups participating in wool-dye interactions (with the resolution of 4 cm−1). Bands in the FT-IR spectra were resolved in accordance with literature data. A Perkin Elmer Lambda-40 double beam UV-visible spectrophotometer was employed for recording absorbance values of dye solutions. A pH/mV meter (BD 1011) from Decibel digital technologies was used for measuring pH of dye solutions.
Methods
Mordanting
Pre-mordanting method was opted for which three eco-friendly mordants alum (Al2K2(SO4)4·24H2O), iron sulfate (FeSO4·7H2O), and tin chloride (SnCl2.2H2O) were selected. Woolen yarn samples were soaked in water before mordanting. The mordants were dissolved in water, and soaked woolen yarns were immersed into mordant solution at about 30 °C. Temperature of mordant solution was raised at a constant rate up to 91–93 °C and kept at this temperature for 60 min with constant stirring. Unused mordants on woolen yarn were removed by rinsing with tap water.
Optimization of mordants
Three different mordants such as alum (Al2K2(SO4)4·24H2O), iron sulfate (FeSO4·7H2O), and tin chloride (SnCl2·2H2O) used in this study were optimized for their better performance on wool in terms of achieving higher color strength and better fastness properties without sacrificing much of the mordant. Alum, iron sulfate, and tin chloride were optimized in the concentration range of 1.0–10, 1.0–5, and 0.1–1 % o.w.f (on weight of fiber). In view of the toxic nature of some metal salts, higher concentrations were not selected in this study.
Optimization was done on the basis of absorbance values (percentage exhaustion), recorded before and after dyeing with a UV-vis spectrophotometer, and percentage exhaustion was calculated by using the following equation.
Based on the absorption and visual appearance, each mordant was selected in a particular optimized concentration (Prabhavathi et al. 2014) and was evaluated for colorimetric and fastness characteristics.
Dyeing
Un-mordanted and mordanted woolen yarns were immersed into dyebaths of varying concentrations (15, 8, 3, 1, and 0.5 % o.w.f.) maintained at M:L (material to liquor) ratio of 1:40 at neutral pH conditions. Temperature of dyebath was raised at a constant rate up to 91–93 °C and kept at that temperature for 60 min with constant stirring to achieve uniform dyeing. Acid (HCl) (pH = 4.0) and alkali (Na2CO3) (pH = 9.0) after-treatment was performed to dyed samples to demonstrate the effect of pH on dyeing processes. Dyed and after-treated woolen yarn samples were washed with non-ionic detergent Safewash, Wipro (5 ml/L), and rinsed with tap water. The fibers were dried in shade at room temperature.
Color measurements
Color measurements of dyed woolen yarn were carried out by following standard procedures. Estimation of color parameter values in terms of K/S and CIE-L*a*b* values were recorded on Gretag Macbeth color-eye 7000A spectrophotometer connected to a computer with installed software of MiniScan XE Plus. Color strength (K/S) value was calculated by using Kubelka-Munk equation.
where K is the absorption coefficient, S is the scattering coefficient, and R is the reflectance of dyed samples.
Chroma (c*) and hue angles (h°) were calculated using the following equations:
Fastness determination
Light fastness of the dyed samples was conducted on Digi light Nx™, having water cooled by Mercury Blended Tungsten lamp, according to test method ISO 105-B02:1994 (Amd.2:2000). Wash fastness was measured in Digi wash SS™ (Laundrometer) as per the ISO 105-C06:1994 (2010) specifications. Dry and wet rub fastness of dyed samples were tested using Digi crock™ (Crockmeter) as per Indian standard IS 766:1988 (reaffirmed 2004) based on ISO 105-X12:2001 by mounting the fabric on a panel and giving ten strokes for both dry and wet rub fastness tests. The samples were assessed for staining on white adjacent fabric (cotton and wool).
Results and discussion
FT-IR spectra of Terminalia chebula dye
FT-IR analysis was used to identify the possible auxochromes responsible for the substantivity of dye. FT-IR spectra (Fig. 1) of extract powder of T. chebula were recorded which showed bands at 3240 and 2980 cm−1 due to O–H and aromatic C–H stretching vibrations, respectively; presence of bands at 1710 and 1595 cm−1 is due to C=O and C=C stretching vibrations, respectively. Stretching vibrations at 1201 and 1040 cm−1 are for C–O stretching peaks.
Optimization of mordant concentrations
Metal salt mordants have different interactions with wool and thereby may darken, brighten, or drastically alter the final color of the dyed wool samples. In this study, wool yarns were pre-treated with different types of metal salts (alum, ferrous sulfate, stannous chloride).
Figure 2a–c shows the effect of alum, iron sulfate, and tin chloride concentrations on the percentage exhaustion values of T. chebula-dyed woolen yarns. On increasing the concentration of alum (1–10 % o.w.f.), iron sulfate (1–5 % o.w.f.), and tin chloride (0.1–1.0 % o.w.f.) mordants, respectively, percentage exhaustion values increase. This increase may be attributed to the increasing interaction of respective metal ions or their higher coordinating ability with wool fiber and dye molecules.
From the experimental results, 10.0 % (o.w.f.) alum, 5.0 % (o.w.f.) iron sulfate, and 1.0 % (o.w.f.) tin chloride concentrations were found to give maximum exhaustion results and were taken as optimized mordant concentrations for subsequent dyeing experiments with T. chebula natural dye.
Color characteristics
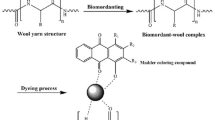
Colorimetric characteristics (K/S and CIE-L*a*b* values) of woolen yarn dyed with different concentrations of T. chebula (15.0, 8.0, 3.0, 1.0 and 0.5 % o.w.f.) with optimized mordant concentrations were analyzed (Fig. 3). Negatively charged dye molecules (anions) and metal (cations) have strong affinity for positively charged amino and negatively charged carboxyl groups (isoelectric nature of wool fiber), respectively. Electrostatic forces of attraction (ionic bonding) between coloring components and wool functional groups are responsible for the uptake of dye by wool in dyeing bath in addition to hydrophobic forces of attraction (van der Waals forces). From the dye-metal coordination complexes, the vacant sites are used to form coordinate bonds with the uncharged amino (–NH−) and carbonyl (C=O) groups of the amide group of wool (Fig. 4).
Effect of concentration of dye on color strength of dyed woolen yarn
With the increase in the concentration of dye from 0.5 to 15.0 % (o.w.f.), an increase in color strength (K/S) is observed resulting in deeper shades with increase in the dye adsorption. Due to increase in concentration of dye in dyebath, concentration gradient increases, which eventually results in an increase in the rate of diffusion of dye onto the surface of woolen yarn from the dyebath solution. A lower concentration of dye produces shades of gray-colored tones while a higher concentration of dye shifts color to the yellow region.
Effect of pH
Wool yarns were dyed with T. chebula in a dyebath maintained at neutral pH. To widen the shade range, dyed wool yarns were after-treated with acidic and alkaline solutions. After treatment of dyed wool yarn samples with acidic and alkaline media, appreciable changes in color parameters and fastness properties were observed. Acidic treatment lightens the shades as supported by low color strength values which may be due to hydrolysis of complexes and wool-dye interaction in acidic medium. Alkaline medium treatment improved the wash fastness (color change), but acidic medium treatment decreases the tolerance to washing. The different colors obtained on wool in neutral, acidic, and alkaline media are shown in Table 5.
Effects of mordants on colorimetric characteristics
Some transition metal ions and particularly iron salts in our case strongly bind with natural dye molecules due to their ability to form strong coordination complexes and thus produce deep color on the fabric (Uddin 2014). Better octahedral complexation property of iron leads to higher K/S values and the color change to grayish of iron-mordanted dyed woolen yarn than un-mordanted yarn (Mihalick and Donnelly 2006). Metal mordants highly affect the lightness and a* and b* values (Tables 1, 2, 3, and 4); as in the case of alum and tin, lightness increases up to 75 or more; and the a*-b* plot (Figs. 5 and 6) shifts towards the more yellow region owing to the lightening of shade property of alum and tin mordants. In the case of iron mordants, lightness decreases up to 60 or less, and the a*-b* plot (Fig. 7) shifts far away from the yellow coordinate in comparison to control-dyed (Fig. 8) woolen yarn owing to the saddening property of iron mordants. The chroma values (c*) were found minimum in the case of iron-mordanted samples in the range of 11–20 and maximum in alum-mordanted samples in the range of 18–24, whereas the values of tin-mordanted samples were found in the range of 18–22. The hue angles are in the range of 72° to 87°, and all the dyed woolen yarns were found in the yellow-red quadrant. Shade cards of all dyed samples are given in Table 5.
Fastness properties
Light, wash, and rub fastness properties (Tables 1, 2, 3, and 4) were evaluated on a grayscale and observed as average to excellent range.
Light fastness
For the light fastness, it was observed that all the woolen yarns dyed with T. chebula showed very good fastness results to light. The alum-mordanted dyed woolen yarns were found comparatively less tolerable towards light in comparison to control-, iron-, or tin-mordanted dyed woolen yarns (Maulik and Agarwal 2014). This is probably due to weak coordination bonding of alum with dye molecule, and the photolytic degradation happens, but in the case of iron, strong coordination bonding prevents photolytic degradation by protecting the chromophore with dissipation of energy of absorbed photons to the chelate structure formed with metal ions (Jothi 2008).
Wash fastness
From the wash fastness data in Tables 1, 2, 3, and 4, all the T. chebula-dyed woolen yarns (un-mordanted as well as mordanted) showed a good to excellent wash fastness rating of 3–5, and negligible staining on adjacent fabrics (cotton and wool) was observed. Relatively good fastness to washing for un-mordanted dyed woolen yarn is attributed to the affinity of coloring components to the yarn in the form of H bonding, ionic bonding, and van der Waals forces (Khan et al., 2015; Yusuf et al., 2015).
Rub fastness
Un-mordanted and mordanted dyed woolen yarns were tested for dry and wet rub fastness properties and were found more or less the same (4–5 on the grayscale) for both un-mordanted and mordanted dyed samples. Rub fastness of alum-mordanted samples were relatively found somewhat less than that of un-mordanted and mordanted with iron or tin which was due to weak coordination complexation of aluminum ions.
Conclusions
This study evaluated the color parameters (K/S, L*, a*, b*) and fastness properties of woolen yarns dyed with T. chebula natural dye. The use of different metal salt mordants produced a wide range of beautiful shades with good color and wash fastness (light, wash, and rub fastness) properties. It was found that the K/S values of dyed woolen yarn were found higher in the case of iron mordants and lightness was increased with alum and tin mordants. Furthermore, it was found that alum mordants affected negatively the light fastness. In the end, it can be said that T. chebula natural dye can provide bright hues with good color fastness properties with different types of mordants.
References
Dalby, G. (1993). Greener mordants for natural coloration. Journal of the Society of Dyers and Colourists, 109(1), 8–9.
Islam, S., & Mohammad, F. (2015). Natural colorants in the presence of anchors so-called mordants as promising coloring and antimicrobial agents for textile materials. ACS Sustainable Chemistry & Engineering, 3(10), 2361–2375.
Jothi, D. (2008). Extraction of natural dyes from African marigold flower (Tagetes erecta L) for textile coloration. Autex Research Journal, 8(2), 49–53.
Khan, M. A., Khan, M., Srivastava, P. K., & Mohammad, F. (2005). Extraction of natural dyes from myrobalan, gallnut and pomegranate, and their application on wool. Colourage, 52(12), 53–60.
Khan, M. I., Khan, S. A., Yusuf, M., Shahid, M., Mohammad, F., & Khan, M. A. (2010). Eco-friendly shades on wool using mixed mordants with Acacia catechu (Cutch). Colourage, 57(8), 81–88.
Khan, M. I., Ahmad, A., Khan, S. A., Yusuf, M., Shahid, M., Manzoor, N., et al. (2011). Assessment of antimicrobial activity of catechu and its dyed substrate. Journal of Cleaner Production, 19(12), 1385–1394.
Khan, S. A., Ahmad, A., Khan, M. I., Yusuf, M., Shahid, M., Manzoor, N., et al. (2012). Antimicrobial activity of wool yarn dyed with Rheum emodi L. (Indian Rhubarb). Dyes and Pigments, 95(2), 206–214.
Khan, S. A., Islam, S., Shahid, M., Khan, M. I., Yusuf, M., Rather, L. J., et al. (2015). Mixed metal mordant dyeing of wool using root extract of Rheum emodi (Indian Rhubarb/Dolu). Journal of Natural Fibers, 12(3), 243–255.
Maulik, S. R., & Agarwal, K. (2014). Painting on handloom cotton fabric with colourants extracted from natural sources. Indian Journal of Traditional Knowledge, 13(3), 589–595.
Mihalick, J. E., & Donnelly, K. M. (2006). Using metals to change the colors of natural dyes. Journal of Chemical Education, 83(10), 1550–1551.
Naik, G. H., Priyadarsini, K. I., Naik, D. B., Gangabhagirathi, R., & Mohan, H. (2004). Studies on the aqueous extract of Terminalia chebula as a potent antioxidant and a probable radioprotector. Phytomedicine, 11(6), 530–538.
Pfundstein, B., El Desouky, S. K., Hull, W. E., Haubner, R., Erben, G., & Owen, R. W. (2010). Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): characterization, quantitation and determination of antioxidant capacities. Phytochemistry, 71(10), 1132–1148.
Prabhavathi, R., Devi, A. S., & Anitha, D. (2014). Improving the colour fastness of the selected natural dyes on cotton. IOSR Journal of Polymer and Textile Engineering, 1(4), 21–26
Samantha, A.K., & Agarwal, P. (2009). Application of natural dyes on textiles, Indian Journal of Fibre & Textile Research, 34(December), 384–399.
Shahid, M., Ahmad, A., Yusuf, M., Khan, M. I., Khan, S. A., Manzoor, N., et al. (2012). Dyeing, fastness and antimicrobial properties of woolen yarns dyed with gallnut (Quercus infectoria Oliv.) extract. Dyes and Pigments, 95(1), 53–61.
Shahid, M., Islam, S., & Mohammad, F. (2013). Recent advancements in natural dye applications: a review. Journal of Cleaner Production, 53(August), 310–331.
Uddin, M. G. (2014). Effects of different mordants on silk fabric dyed with onion outer skin extracts. Journal of Textiles, 2014, 1–8.
Vankar, P. S., Shanker, R., Mahanta, D., & Tiwari, S. C. (2008). Ecofriendly sonicator dyeing of cotton with Rubia cordifolia Linn. using biomordant. Dyes and Pigments, 76(1), 207–212.
Yusuf, M., Shahid, M., Khan, S. A., Khan, M. I., Islam, S.-U., Mohammad, F., et al. (2013). Eco-dyeing of wool using aqueous extract of the roots of Indian Madder (Rubia cordifolia) as natural dye. Journal of Natural Fibers, 10(1), 14–28.
Yusuf, M., Shahid, M., Khan, M. I., Khan, S. A., Khan, M. A., & Mohammad, F. (2015). Dyeing studies with henna and madder: a research on effect of tin (II) chloride mordant. Journal of Saudi Chemical Society, 19(1), 64–72.
Zhou, Y., Zhang, J., Tang, R., & Zhang, J. (2015). Simultaneous dyeing and functionalization of silk with three natural yellow dyes. Industrial Crops and Products, 64(February), 224–232.
Acknowledgements
Financial support provided by the University Grants Commission, Govt. of India, through the Non-NET Fellowship for Ph.D. students (MS and MNB) and the BSR Research Fellowship in Sciences for Meritorious students (SI and LJR), is highly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MS and FM designed the experiment. MS conducted most of the experiments. SI interpreted the experimental data and drafted the manuscript. MNB helped MS in performing experiments on dyeing studies of the Harda plant. LJ helped to analyze the experiment. MAK provided technical help. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Shabbir, M., Islam, S.U., Bukhari, M.N. et al. Application of Terminalia chebula natural dye on wool fiber—evaluation of color and fastness properties. Text Cloth Sustain 2, 1 (2017). https://doi.org/10.1186/s40689-016-0011-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40689-016-0011-8