Abstract
Background
The accuracy of using total keratometry (TK) value in recent IOL power calculation formulas in highly myopic eyes remained unknown.
Methods
Highly myopic patients who underwent uneventful cataract surgery were prospectively enrolled in this prospective comparative study. At one month postoperatively, standard deviation (SD) of the prediction errors (PEs), mean and median absolute error (MedAE) of 103 highly myopic eyes were back-calculated and compared among ten formulas, including XGboost, RBF 3.0, Kane, Barrett Universal II, Emmetropia Verifying Optical 2.0, Cooke K6, Haigis, SRK/T, and Wang-Koch modifications of Haigis and SRK/T formulas, using either TK or standard keratometry (K) value.
Results
In highly myopic eyes, despite good agreement between TK and K (P > 0.05), larger differences between the two were associated with smaller central corneal thickness (P < 0.05). As to the refractive errors, TK method showed no differences compared to K method. The XGBoost, RBF 3.0 and Kane ranked top three when considering SDs of PEs. Using TK value, the XGboost calculator was comparable with the RBF 3.0 formula (P > 0.05), which both presented smaller MedAEs than others (all P < 0.05). As for the percentage of eyes within ± 0.50 D or ± 0.75 D of PE, the XGBoost TK showed comparable percentages with the RBF 3.0 TK formula (74.76% vs. 66.99%, or 90.29% vs. 87.38%, P > 0.05), and statistically larger percentages than the other eight formulas (P < 0.05).
Conclusions
Highly myopic eyes with thinner corneas tend to have larger differences between TK and K. The XGboost enhancement calculator and RBF 3.0 formula using TK showed the most promising outcomes in highly myopic eyes.
Similar content being viewed by others
Background
Nowadays, accurate refractive predictions are of great importance to refractive cataract procedures. Apart from improved surgical techniques, modern biometry and new generation of formulas are two other crucial determinants. Despite the promising outcomes reached in the intraocular lens (IOL) power calculation of non-myopes [1], making accurate IOL power predictions for highly myopic eyes remains quite challenging. It is especially difficult due to unreliable corneal power measurements of highly myopic eyes [2,3,4,5], and compounding effects of other scenarios, including previous refractive surgeries [6].
Accurate assessment of the corneal curvature is essential for IOL power prediction. Conventionally, the corneal power was estimated with a theoretical algorithm, using anterior corneal curvature only [7]. Now, total keratometry (TK) can be calculated using the newest IOLMaster 700, which measures anterior and posterior corneal curvatures, as well as corneal thickness. By replacing assumptions with actual measurements, the application of TK may provide additional benefits for IOL power calculations.
The TK might also improve the refractive prediction in highly myopic eyes. Traditional IOL power calculation formulas based on normal eyes might generate unexpected errors for highly myopic eyes [8]. Two vergence formulas, the SRK/T and Haigis, have shown acceptable outcomes in highly myopic eyes after Wang-Koch (WK) modifications for axial length (AL) [9]. Late generation formulas, represented by the Barrett Universal II (BUII) and Olsen formulas, were found to be more promising in long eyes [10]. More recently, formulas based on artificial intelligence (AI), such as the Kane, Radial Basis Function (RBF 3.0), and the XGBoost enhancement calculator we developed, have further improved outcomes [11,12,13,14]. Accuracies of other new formulas such as Cooke K6 formula, and Emmetropia Verifying Optical (EVO 2.0) formulas, remained unknown in highly myopic eyes. Rare previous studies have compared the performance of IOL calculation formulas when applying the new TK over standard keratometry (K) values in normal AL eyes [15, 16], or in all AL ranges [13, 17], but few in long AL eyes.
In this study, we hypothesized that TK may also fit well with current new formulas when dealing with highly myopic eyes and can somehow differ from the standard K method. Therefore, we conducted a prospective study to investigate the performance of ten IOL power calculation formulas, including the XGBoost, RBF 3.0, Kane, BUII, K6, EVO 2.0, Haigis, SRK/T, and WK modifications of Haigis and SRK/T formulas, using the new TK method in highly myopic eyes.
Methods
Ethics
In adherence with the Declaration of Helsinki and its amendments, this prospective comparative study was conducted with approval from the Ethics Committee of Eye and ENT Hospital of Fudan University (Shanghai, China, No. 2013021). Signed informed consent was obtained from all patients, who were informed of the usage of their clinical data. This study was affiliated with the Shanghai High Myopia Study, which was registered at www.clinicaltrials.gov (accession number NCT03062085).
Patients and eligibility
Patients who underwent uneventful cataract surgeries and IOL implantations in our hospital from December 2020 to August 2021 were enrolled in this one-year prospective study. The inclusion criteria were eyes with (1) AL ≥ 26.00 mm [10, 18, 19]; (2) successful biometry with the IOLMaster 700, including TK method; and (3) implanted HumanOptics MC X11 ASP IOL. Patients were excluded if they had previous ocular surgeries or traumas; had lid disorders, corneal opacity, glaucoma, zonular weakness, keratoconus, uveitis or other diseases that may have influenced the accuracy of manifest refraction; required premium IOL implants (such as multifocal or Toric IOLs); had severe intraoperative or postoperative complications, such as posterior capsular rupture, endophthalmitis, retinal detachment, etc.; had postoperative best-corrected distance visual acuity (BCVA) worse than 20/40 or were lost to one-month follow-up. Ultimately, 103 highly myopic eyes from 103 patients were included and further divided into three subgroups, according to AL, as follows: high myopia control (26.0–28.0 mm, n = 46), extreme myopia 1 (28.0–30.0 mm, n = 29) [20], and extreme myopia 2 (30.0 mm or more, n = 28) [21, 22].
Preoperative measurements
Routine preoperative examinations were performed, including BCVA (logarithm of the minimal angle of resolution [logMAR]), optical biometry (IOLMaster 700; Carl Zeiss Meditec, Jena, Germany, software version 1.80), corneal topography (Pentacam HR; Oculus Optikgeräte, Wetzlar, Germany, software version 1.22r05), optical coherence tomography (OCT) exam (Spectralis OCT; Heidelberg Engineering, Heidelberg, Germany, software version 6.12.4), and B-scan ultrasonography. Data for AL, anterior chamber depth (ACD) as measured from epithelium to anterior lens, lens thickness (LT), white-to-white distance (WTW), and central corneal thickness (CCT) were recorded. Both standard K and TK data were collected using the IOLMaster 700. An index of 1.3375 was used for standard K.
Surgical procedure
All surgeries were performed by the same experienced surgeon (YL). Standard topical anesthesia was administered in all cases. Through a 2.6 mm temporal clear corneal incision, a 5.0–5.5 mm capsulorhexis was made followed by phacoemulsification. An IOL was then implanted into the bag. No sutures were used in any of the eyes. After surgery, all patients were prescribed topical prednisolone acetate (Allergan Pharmaceutical Ireland, Westport, Ireland) and levofloxacin (Cravit, Santen Pharmaceutical) to be instilled four times a day for two weeks; and pranoprofen eye drops (Pranopulin, Senju Pharmaceutical, Osaka, Japan) to be instilled four times a day for 4 weeks.
Postoperative examinations
Ophthalmic examinations were carried out one month after surgery. uncorrected visual acuity (UCVA) and BCVA were recorded. Manifest refractions were performed by the same doctor (LW), with subjective methods, and presented as spherical equivalence (SE). The prediction error (PE) was defined as the actual postoperative SE minus predicted SE, which was back-calculated using the implanted IOL power. A negative PE indicates a postoperative refractive result that was more myopic than predicted by the individual formula. The A constants retrieved from the User Group for Laser Interference Biometry website (ocusoft.de/ulib/index.htm) and the lens factor of the BUII Formula provided by the APACRS website were used in the IOL power calculation. The accuracies of all formulas (XGBoost-based enhancement calculator optimized for highly myopic eyes [14], Hill-RBF 3.0 [13], Kane [http://www.iolformula.com], BUII [23], Cooke K6 [https://cookeformula.com/], EVO 2.0 [http://www.evoiolcalculator.com], Haigis [24] and SRK/T [25] formulas and those with WK modification of ALs [SRK/TWK and HaigisWK] [9] were compared. The mean absolute error (MAE), the median absolute errors (MedAEs), and the percentages of eyes within ± 0.25 D, ± 0.50 D, ± 0.75 D, and ± 1.00 D of the PE (± 0.25 D %, ± 0.50 D %, ± 0.75 D %, and ± 1.00 D %) were calculated and compared.
Statistical analysis
Based on ± 0.25 D %, a sample size of 52 eyes was needed to reach statistical significance using paired McNemar’s Chi-squared test, with an intended power of 80% and a significance level of 5% [15, 26]. Statistical analyses were performed with SPSS software (version 12.0, SPSS, Inc.). Continuous variables were described as the mean ± standard deviation (SD). The Bland-Altman method was used to visualize the agreement between the TK and K measurements. Intraclass correlation coefficient (ICC) analysis was calculated using the two-way mixed model and absolute agreement, and an ICC over 0.90 suggested high agreement and less variance between two measurements [15]. Pearson correlation analysis was applied for differences between TK and K methods and other biometric parameters, including AL, ACD, LT, WTW and CCT. One-sample student’s t test was applied to compare the mean PEs with zero in each formula. The Wilcoxon signed-rank test was used to evaluate the differences of absolute PEs between the K and TK methods. Friedman test with Bonferroni-Dunn’s post hoc correction were used to compare the absolute PEs generated by all formulas using either the TK or K method. Heteroscedastic tests for PE comparisons including F-test (for two groups) or H-test (for ≥ three groups) were applied. For ± 0.25 D %, ± 0.50 D %, ± 0.75 D %, and ± 1.00 D %, the paired McNemar’s Chi-squared test was used to compare TK and K performances, while Cochran’s Q test was used to compare the performances of all formulas. A P value of less than 0.05 was considered statistically significant.
Results
Demographic data
Demographic data and biometric parameters are presented in Table 1. The mean AL was 28.85 ± 2.34 mm, and ranged from 26.01 to 35.02 mm. The mean CCT was 546.80 ± 33.51 μm, and ranged from 474.86 to 636.41 μm.
Agreement between TK and K methods and influencing factors
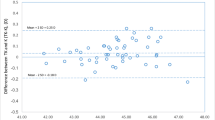
The agreement between TK and K methods was evaluated using the Bland-Altman test (Fig. 1). The mean difference was − 0.02 D, with 95% confidence interval (CI) range from − 0.23 to 0.18, demonstrating relatively good agreement (ICC = 0.997, P < 0.001). The agreements between flat TK and flat K, steep TK and steep K, and TK and K were demonstrated in Additional file 1: Table S1, all revealing high agreements (all ICCs > 0.900, P < 0.001). Pearson correlation analysis revealed that a larger difference between TK and K methods was associated with a thinner CCT (Fig. 2, r = − 0.212, P = 0.032), but was not associated with AL, WTW, LT or the corneal radius (all P > 0.05).
Prediction errors (PEs)
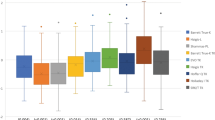
Mean PEs are presented in Fig. 3. The XGBoost enhancement calculator, RBF 3.0, and SRK/T formula showed emmetropic predictions (mean PE vs. 0, P > 0.05), the Haigis formula showed hyperopic predictions (mean PE > 0, P < 0.05), while other formulas showed myopic predictions (mean PE < 0, P < 0.05).
Mean prediction errors of ten formulas with total keratometry (TK) and standard keratometry (K). XGB, XGBoost enhancement calculator; RBF 3.0, Radial Basis Function formula version 3.0; BUII, Barrett Universal II formula; K6, Cooke K6 formula; EVO 2.0, Emmetropia Verifying Optical formula version 2.0. *Statistical significance (P < 0.05) using one-sample student’s t test to compare the mean prediction errors with zero
The absolute PEs were calculated for all highly myopic eyes (Table 2). No significant differences were found in the MedAEs between the TK and standard K methods (Wilcoxon signed rank test, P > 0.05 in all pairs). However, though without statistical significance, the TK method showed slightly smaller SD values of PE than the standard K method using newer generation formulas, including XGBoost enhancement calculator (0.518 vs. 0.572), RBF 3.0 formula (0.527 vs. 0.536), Kane formula (0.536 vs. 0.539), and BUII formula (0.604 vs. 0.630).
Amongst all ten formulas, the XGBoost enhancement calculator, RBF 3.0 and Kane formula ranked top three when considering SD of PEs in both TK and K groups (H test, P < 0.05). Particularly, the XGBoost enhancement calculator TK method demonstrated the lowest MAE (0.379 ± 0.351 D). In the TK group, the MedAE of the XGBoost enhancement formula was not significantly different from RBF 3.0 formula but was significantly lower than the other eight formulas; while the MedAE of the RBF 3.0 formula was not different statistically from both XGBoost enhancement calculator and Kane formula but was significantly lower than the other seven formulas (all P < 0.05 with Bonferroni correction). In the standard K group, the MedAE of the XGBoost enhancement formula was not statistically different with RBF 3.0, Kane, K6, and SRK/T formulas, but was statistically lower than five other formulas; while the MedAE of the RBF 3.0 formula was not statistically different with XGBoost enhancement calculator, Kane, BUII, K6, and SRK/T formulas, but was significantly lower than three other formulas (all P < 0.05 with Bonferroni correction).
The percentages of eyes within ± 0.25 D, ± 0.50 D, ± 0.75 D and ± 1.00 D of the PE were further compared in both TK and K groups (Fig. 4). No statistical difference was found in ± 0.2 5D % and ± 1.00 D %. In the TK group, the XGBoost enhancement calculator showed comparable ± 0.50 D % with RBF 3.0 formula (74.76% vs. 66.99%, P > 0.05), and significantly larger ± 0.50 D % than eight other formulas (Cochran's Q test, adjusted P value < 0.05); it also showed comparable ± 0.75 D % with both RBF 3.0 and BUII formula (90.29% vs. 87.38% and 73.79%, respectively, both P > 0.05), and was statistically larger ± 0.75 D % than seven other formulas (Cochran's Q test, adjusted P value < 0.05). In the K group, the XGBoost enhancement calculator showed significantly larger ± 0.50 D % and ± 0.75 D % than Kane, EVO 2.0, and Haigis formulas (Cochran's Q test, adjusted P value < 0.05), and was not significantly different from six other formulas.
Percentages of eyes within ± 0.25 D, ± 0.50 D, ± 0.75 D and ± 1.00 D of prediction errors using different formulas with total keratometry (TK) and standard keratometry (K). XGB, XGBoost enhancement calculator; RBF 3.0, Radial Basis Function formula 3.0; BUII, Barrett Universal II formula; K6, Cooke K6 formula; EVO 2.0, Emmetropia Verifying Optical formula version 2.0
Subgroup comparisons
A subgroup analysis was conducted (Additional file 1: Table S2), and no statistically significant differences were found between the TK and K methods for any subgroup (all P > 0.05). In the AL 26.00 to 28.00 mm group, the MedAE was significantly lower in XGboost compared to EVO 2.0 and HaigisWK formulas in both TK and K groups. In the AL 28.00 to 30.00 mm group, the MedAE was statistically lower in RBF 3.0 formula compared to K6 formula in the TK group. In the AL > 30.00 mm group, the MedAE were statistically lower in XGboost, and RBF 3.0 compared to Kane, Haigis, and SRK/T in TK group, or compared to Kane and Haigis in K group (all P < 0.05 with Bonferroni correction).
Discussion
Even with continuously advanced IOL power calculation formulas, cataract surgery on highly myopic eyes often results in unexpected refractive outcomes [12]. Recent improvements in TK biometry have demonstrated benefits in IOL calculation [16, 17, 27,28,29], but few studies have demonstrated its potential in highly myopic eyes, especially for those without prior refractive surgeries [13]. In this study, we demonstrated that highly myopic eyes with thinner CCTs tend to have larger differences between TK and K methods, while the XGBoost enhancement calculator and RBF 3.0 formula, with either TK or K method, seemed to be the most promising options for IOL power calculation for this special population.
The TK method concept is relatively new and its difference from standard K is worth investigating. We demonstrated relatively good agreement between the two methods in highly myopic eyes. Shajari et al. have also shown comparable astigmatism measurements between TK and standard K in normal AL eyes [30]. A greater difference between TK and K has previously been associated with flatter corneas in eyes that have undergone myopic refractive surgeries [27]. In this study, though there was good agreement, a larger difference between TK and K values was found in highly myopic eyes with thinner CCTs. It might be because the assessment of the posterior corneal surface with the IOLMaster 700 was done with consideration for the corneal pachymetry data. Therefore, for eyes with thinner corneal thicknesses, it might be that the TK measurement generates more accurate outcomes and should be recommended.
The improvement of K measurement in IOL power calculation began with the invention of ray tracing techniques using a rotating Scheimpflug camera [30]. Some studies have revealed that though Pentacam K readings (such as true net power and total corneal refractive power) differ from IOLMaster standard K readings, the PEs obtained with each machine are comparable for normal eyes [31,32,33]. The newer IOLMaster 700 obtains a reading of total corneal power, taking both corneal thickness and actual values for the radius of the posterior cornea into account, using telecentric 3-zone K and swept-source OCT technology. By replacing hypotheses and modeling with actual measurements, the IOLMaster 700 may provide reliable data on corneal power in some challenging cases, such as surgically modified [7, 28, 29, 34] and high astigmatic corneas [17, 35, 36]. Here, we found no significant difference between TK and standard K in highly myopic eyes within this certain range of corneal thickness. Tsessler et al. also found that the use of TK did not provide significant improvement to its prediction accuracy for all AL ranges [13]. However, Fabian et al. concluded that TK was better than standard K for normal eyes [17]. This can be attributed to the study population which included only astigmatic eyes (K ≥ 0.75 D) [17]. Here, in order to determine the agreement and fitness of TK in highly myopic eyes with a wide range of CCTs, we defined no restrictions on corneal astigmatism and even excluded highly astigmatic eyes needing Toric IOL implantations. Still, more attention on TK implementation should be paid to highly myopic eyes with thinner corneas. In addition, using intraoperative aberrometry to measure aphakic refraction was demonstrated to return more accurate results than preoperative biometry using standard K values for highly myopic eyes [37]. The accuracy comparison of intraoperative aberrometry and the new TK measurement may merit further investigation.
Many efforts have been made to determine the most accurate formula for highly myopic eyes [2, 12, 22, 38]. Intentional myopic overcorrection is typically planned for cataract surgeries on highly myopic patients [38], so that they may continue living with their familiar short-sightedness. Here, though most of the MedAEs and MAEs were relatively lower with the TK method than the K method, no significant difference between the two methods was observed. After applying the TK value, we still found different prediction accuracies for the ten formulas. The most promising in all highly myopic eyes might be two of the three AI formulas, the XGBoost enhancement calculator and RBF 3.0 formula. The XGBoost enhancement calculator was specifically designed to optimize the refractive prediction for highly myopic eyes using machine learning [14]. The RBF 3.0 formula, an improvement from the 2.0 version, employed pattern recognition and a sophisticated form of data interpolation [13]. The RBF 3.0 formula only accepts target refraction values from + 1.0 D to − 2.5 D. Therefore, the XGBoost enhancement calculator might be more useful when less than − 2.5 D myopic refractive targets were scheduled for extremely long eyes. The TK value seemed to fit the newer AI-based and traditional vergence formulas, but further optimization might be achieved by using more real-world TK measurements and prediction outcomes.
Although the separation into subgroups might lower the power for paired comparisons of TK and K methods, it is still interesting to note that K method for the 28–30 mm subgroup (as compared to the TK group) had lower MedAEs in nine formulas (XGBoost, RBF, Kane, BUII, EVO, K6, HaigisWK, SRK/T, and SRK/TWK). However, for the AL > 30 mm subgroup, the TK methods showed lower MedAEs for XGBoost, RBF and Kane, but not the others. These suggest that for eyes with AL > 30 mm, using new TK method with these fourth-generation formulas has more potential in terms of accuracy. Encouragingly, promising outcomes were still revealed when comparing performance of ten formulas in a subgroup analysis. Particularly, the XGBoost and RBF 3.0 formula seemed to work much better for eyes with AL > 30 mm, demonstrating lower MedAEs than the other eight formulas.
The limitation of this study would be that the formula comparison results are applicable to this model of IOL (HumanOptics MC X11 ASP). Since other IOL models have different geometries or optical zones, future studies will be needed to determine that these results are repeatable in other IOL models.
Conclusions
In conclusion, for highly myopic eyes, the TK method showed good agreement with the standard K, yet a larger difference between the TK and K methods was found in highly myopic eyes with thinner corneas. We also verified that TK can be incorporated into modern IOL power calculations, while the XGBoost enhancement calculator and RBF 3.0, using either the TK or K method, showed more significantly promising outcomes than other formulas in highly myopic eyes.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Savini G, Di Maita M, Hoffer KJ, Næser K, Schiano-Lomoriello D, Vagge A, et al. Comparison of 13 formulas for IOL power calculation with measurements from partial coherence interferometry. Br J Ophthalmol. 2021;105(4):484–9.
Zhu X, He W, Sun X, Dai J, Lu Y. Fixation stability and refractive error after cataract surgery in highly myopic eyes. Am J Ophthalmol. 2016;169:89–94.
Dong J, Tang M, Zhang Y, Jia Y, Zhang H, Jia Z, et al. Comparison of anterior segment biometric measurements between Pentacam HR and IOLMaster in normal and high myopic eyes. PLoS One. 2015;10(11):e0143110.
Guo XX, You R, Li SS, Yang XF, Zhao L, Zhang F, et al. Comparison of ocular parameters of two biometric measurement devices in highly myopic eyes. Int J Ophthalmol. 2019;12(10):1548–54.
Aksoy M, Asena L, Güngör SG, Küçüködük A, Akman A. Comparison of refractive outcomes using Scheimpflug Holladay equivalent keratometry or IOLMaster 700 keratometry for IOL power calculation. Int Ophthalmol. 2021;41(6):2205–12.
Rosa N, Cione F, Pepe A, Musto S, De Bernardo M. An advanced lens measurement approach (ALMA) in post refractive surgery IOL power calculation with unknown preoperative parameters. PLoS One. 2020;15(8):e0237990.
Abulafia A, Hill WE, Koch DD, Wang L, Barrett GD. Accuracy of the Barrett True-K formula for intraocular lens power prediction after laser in situ keratomileusis or photorefractive keratectomy for myopia. J Cataract Refract Surg. 2016;42(3):363–9.
Wang Q, Jiang W, Lin T, Zhu Y, Chen C, Lin H, et al. Accuracy of intraocular lens power calculation formulas in long eyes: a systematic review and meta-analysis. Clin Exp Ophthalmol. 2018;46(7):738–49.
Wang L, Shirayama M, Ma XJ, Kohnen T, Koch DD. Optimizing intraocular lens power calculations in eyes with axial lengths above 25.0 mm. J Cataract Refract Surg. 2011;37(11):2018–27.
Rong X, He W, Zhu Q, Qian D, Lu Y, Zhu X. Intraocular lens power calculation in eyes with extreme myopia: comparison of Barrett Universal II, Haigis, and Olsen formulas. J Cataract Refract Surg. 2019;45(6):732–7.
Connell BJ, Kane JX. Comparison of the Kane formula with existing formulas for intraocular lens power selection. BMJ Open Ophthalmol. 2019;4(1): e000251.
Liu J, Wang L, Chai F, Han Y, Qian S, Koch DD, et al. Comparison of intraocular lens power calculation formulas in Chinese eyes with axial myopia. J Cataract Refract Surg. 2019;45(6):725–31.
Tsessler M, Cohen S, Wang L, Koch DD, Zadok D, Abulafia A. Evaluating the prediction accuracy of the Hill-RBF 3.0 formula using a heteroscedastic statistical method. J Cataract Refract Surg. 2021;48(1):37–43.
Wei L, Song Y, He W, Chen X, Ma B, Lu Y, et al. Accuracy improvement of IOL power prediction for highly myopic eyes with an XGBoost machine learning-based calculator. Front Med (Lausanne). 2020;7:592663.
Srivannaboon S, Chirapapaisan C. Comparison of refractive outcomes using conventional keratometry or total keratometry for IOL power calculation in cataract surgery. Graefes Arch Clin Exp Ophthalmol. 2019;257(12):2677–82.
Hoshikawa R, Kamiya K, Fujimura F, Shoji N. Comparison of conventional keratometry and total keratometry in normal eyes. Biomed Res Int. 2020;2020:8075924.
Fabian E, Wehner W. Prediction accuracy of total keratometry compared to standard keratometry using different intraocular lens power formulas. J Refract Surg. 2019;35(6):362–8.
Abulafia A, Barrett GD, Rotenberg M, Kleinmann G, Levy A, Reitblat O, et al. Intraocular lens power calculation for eyes with an axial length greater than 26.0 mm: comparison of formulas and methods. J Cataract Refract Surg. 2015;41(3):548–56.
Cooke DL, Cooke TL. Comparison of 9 intraocular lens power calculation formulas. J Cataract Refract Surg. 2016;42(8):1157–64.
Liu H, Li FF, Xia HJ, Zhou J. Visual quality after implantation of trifocal intraocular lenses in highly myopic eyes with different axial lengths. Int J Ophthalmol. 2021;14(3):371–7.
Wu TT, Kung YH, Chang CY, Chang SP. Surgical outcomes in eyes with extremely high myopia for macular hole without retinal detachment. Retina. 2018;38(10):2051–5.
Cheng H, Wang L, Kane JX, Li J, Liu L, Wu M. Accuracy of artificial intelligence formulas and axial length adjustments for highly myopic eyes. Am J Ophthalmol. 2021;223:100–7.
Barrett G. Barrett universal II formula. http://calc.apacrs.org/barrett_universal2105/. 2018. Accessed 7 Sept 2021.
Haigis W, Lege B, Miller N, Schneider B. Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch Clin Exp Ophthalmol. 2000;238(9):765–73.
Retzlaff JA, Sanders DR, Kraff MC. Development of the SRK/T intraocular lens implant power calculation formula. J Cataract Refract Surg. 1990;16(3):333–40.
Lachin JM. Power and sample size evaluation for the McNemar test with application to matched case–control studies. Stat Med. 1992;11(9):1239–51.
Wang L, Spektor T, de Souza RG, Koch DD. Evaluation of total keratometry and its accuracy for intraocular lens power calculation in eyes after corneal refractive surgery. J Cataract Refract Surg. 2019;45(10):1416–21.
Lawless M, Jiang JY, Hodge C, Sutton G, Roberts TV, Barrett G. Total keratometry in intraocular lens power calculations in eyes with previous laser refractive surgery. Clin Exp Ophthalmol. 2020;48(6):749–56.
Yeo TK, Heng WJ, Pek D, Wong J, Fam HB. Accuracy of intraocular lens formulas using total keratometry in eyes with previous myopic laser refractive surgery. Eye (Lond). 2021;35(6):1705–11.
Shajari M, Sonntag R, Ramsauer M, Kreutzer T, Vounotrypidis E, Kohnen T, et al. Evaluation of total corneal power measurements with a new optical biometer. J Cataract Refract Surg. 2020;46(5):675–81.
Saad E, Shammas MC, Shammas HJ. Scheimpflug corneal power measurements for intraocular lens power calculation in cataract surgery. Am J Ophthalmol. 2013;156(3):460–7.e2.
Savini G, Hoffer KJ, Lomoriello DS, Ducoli P. Simulated keratometry versus total corneal power by ray tracing: a comparison in prediction accuracy of intraocular lens power. Cornea. 2017;36(11):1368–72.
Shammas HJ, Hoffer KJ, Shammas MC. Scheimpflug photography keratometry readings for routine intraocular lens power calculation. J Cataract Refract Surg. 2009;35(2):330–4.
Ryu S, Jun I, Kim TI, Seo KY, Kim EK. Prediction accuracy of conventional and total keratometry for intraocular lens power calculation in femtosecond laser-assisted cataract surgery. Sci Rep. 2021;11(1):12869.
Levron A, El Chehab H, Agard E, Chudzinski R, Billant J, Dot C. Impact of measured total keratometry versus anterior keratometry on the refractive outcomes of the AT TORBI 709-MP toric intraocular lens. Graefes Arch Clin Exp Ophthalmol. 2021;259(5):1199–207.
Savini G, Næser K, Schiano-Lomoriello D, Ducoli P. Optimized keratometry and total corneal astigmatism for toric intraocular lens calculation. J Cataract Refract Surg. 2017;43(9):1140–8.
Hill DC, Sudhakar S, Hill CS, King TS, Scott IU, Ernst BB, et al. Intraoperative aberrometry versus preoperative biometry for intraocular lens power selection in axial myopia. J Cataract Refract Surg. 2017;43(4):505–10.
Dalto RF, Ferreira MA, Queiroz W, Coelho RP, Paula JS, Messias A. Haigis and SRKT formulae accuracy for intentional myopic overcorrection. Int Ophthalmol. 2018;38(4):1459–63.
Acknowledgements
Not applicable.
Funding
This article was supported by research grants from the National Natural Science Foundation of China (Grant Nos. 82122017, 81870642, 81970780 and 81670835), Science and Technology Innovation Action Plan of Shanghai Science and Technology Commission (Grant Nos. 19441900700 and 21S31904900), Clinical Research Plan of Shanghai Shenkang Hospital Development Center (Grant Nos. SHDC2020CR4078 and SHDC12019X08), and the Fudan University “Outstanding 2025” Program.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The protocol and conduction of this study was approved by the Ethics Committee of Eye and ENT Hospital of Fudan University (Shanghai, China, [No. 2013021]) and adhered to the tenets of the Declaration of Helsinki. Signed informed consent was obtained from all participants, who were informed of the usage of their clinical data.
Consent for publication
Not applicable.
Competing interests
The authors have no proprietary or commercial interest in any materials discussed in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Agreement between total keratometry and standard keratometry in different central corneal thickness subgroups. Table S2. Absolute prediction errors of different IOL formulas using total keratometry or standard keratometry in highly myopic subgroups.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wei, L., Cheng, K., He, W. et al. Application of total keratometry in ten intraocular lens power calculation formulas in highly myopic eyes. Eye and Vis 9, 21 (2022). https://doi.org/10.1186/s40662-022-00293-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40662-022-00293-3