Abstract
Diabetic retinopathy (DR) is a form of microangiopathy. Reducing oxidative stress in the mitochondria and cell membranes decreases ischemic injury and end-organ damage to the retina. New approaches are needed, which reduce the risk and improve the outcomes of DR while complementing current therapeutic approaches. Homocysteine (Hcy) elevation and oxidative stress are potential therapeutic targets in DR.
Common genetic polymorphisms such as those of methylenetetrahydrofolate reductase (MTHFR), increase Hcy and DR risk and severity. Patients with DR have high incidences of deficiencies of crucial vitamins, minerals, and related compounds, which also lead to elevation of Hcy and oxidative stress. Addressing the effects of the MTHFR polymorphism and addressing comorbid deficiencies and insufficiencies reduce the impact and severity of the disease. This approach provides safe and simple strategies that support conventional care and improve outcomes.
Suboptimal vitamin co-factor availability also impairs the release of neurotrophic and neuroprotective growth factors. Collectively, this accounts for variability in presentation and response of DR to conventional therapy. Fortunately, there are straightforward recommendations for addressing these issues and supporting traditional treatment plans.
We have reviewed the literature for nutritional interventions that support conventional therapies to reduce disease risk and severity. Optimal combinations of vitamins B1, B2, B6, L-methylfolate, methylcobalamin (B12), C, D, natural vitamin E complex, lutein, zeaxanthin, alpha-lipoic acid, and n-acetylcysteine are identified for protecting the retina and choroid. Certain medical foods have been successfully used as therapy for retinopathy. Recommendations based on this review and our clinical experience are developed for clinicians to use to support conventional therapy for DR.
DR from both type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) have similar retinal findings and responses to nutritional therapies.
Similar content being viewed by others
Background
Food supplementation with vitamins, minerals, and nutraceuticals has been recommended by medical professionals for many decades [1]. It is a safe, simple, and inexpensive way to address risk factors and drivers of visual vascular disorders, including diabetic retinopathy (DR) [2,3,4,5,6,7,8]. DR is a form of microangiopathy. Elevated serum homocysteine (Hcy) increases microvascular damage [9, 10]. Reducing serum Hcy and oxidative stress of the mitochondria and cell membranes decreases ischemia and reduces end-organ damage to the visual system [11]. Though potential therapeutic targets are clear, the clinician is faced with a myriad of studies and single substance recommendations that are hard to grasp, explain to patients, or integrate with conventional diabetic and DR treatments.
This literature review summarizes the clinical benefits of nutritional supplements and medical foods for diabetes and DR, with emphasis on DR. We review the considerable literature supporting the vitamin and antioxidant interventions to reduce the risk and severity of vision loss. We review which forms of vitamins are optimal and the pitfalls of some synthetic vitamins. Finally, we distill these insights into simple, comprehensive recommendations for clinical practice. Time, usage, and future research will refine them, as clinicians gain experience with these new tools in their armamentarium to reduce the risk and severity of DR.
Current literature referencing supplementation was searched through PubMed using the search terms: vitamins, DR, hypertensive retinopathy, L-methylfolate, methylcobalamin, mitochondrial oxidative stress, MTHFR, and other closely related terms.
Scientific Basis of Vitamin and Nutraceutical Therapy for Diabetes and DR and Vision
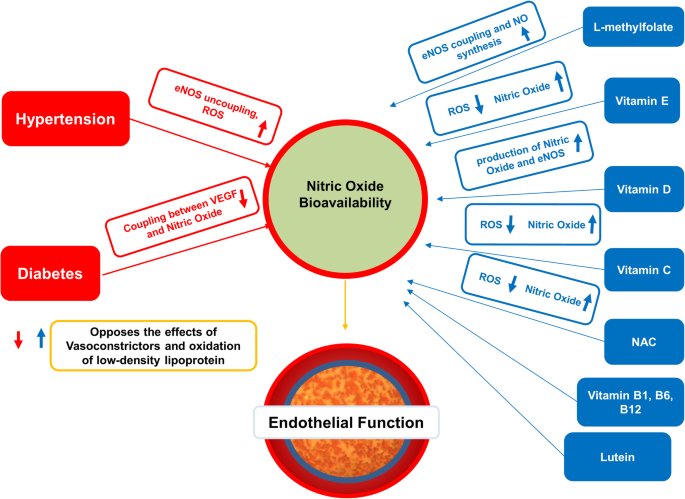
Vitamins and nutraceuticals have effects that directly increase the elasticity of blood vessels and metabolism [12,13,14]. Antioxidants are postulated to protect the body from free radicals and protect nitric oxide from inactivation [12, 13]. If the level of reactive oxidative species exceeds the capacity of antioxidant buffers, it creates oxidative stress. Measurements of oxidative stress can be an early indicator of hypertension, vascular disease, and diabetes [15].
Normally, nitric oxide promotes vascular health by controlling vascular tone (vasodilation), inhibiting platelet function, and preventing adhesion of leukocytes [16]. Reduced levels of nitric oxide result in endothelial dysfunction, causing inflammation, vasospasm, and thrombosis [17]. For example, vitamins C and E are antioxidants that limit oxidative stress by increasing nitric oxide. They also quench lipid peroxidation byproducts that injure cell membranes [18].
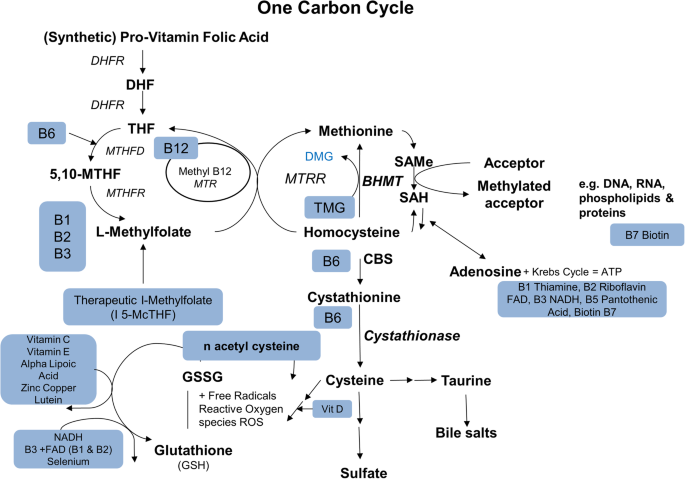
DR is impacted by deficiencies and reduced function genetic polymorphisms of the B vitamin cofactors of the One Carbon Cycle (Fig. 1) and the Citric Acid Cycle (Fig. 2) [19,20,21]. Polymorphisms of the methylenetetrahydrofolate reductase (MTHFR) gene lead to reduced methylation, elevation of Hcy, reduction of nitric oxide, microvascular disease- particularly capillary endothelial injury and apoptosis, microaneurysms, leakage, ischemia, retinal atrophy, neovascularization, and vision loss [22].
Vitamins and Cofactors of the One Carbon Cycle. DHF: Dihydrofolate; DHFR: Dihydrofolate reductase; THF: Tetrahydrofolate; MTHFD: Methylenetetrahydrofolate dehydrogenase; MTHFR: Methylenetetrahydrofolate reductase; MTR: 5-Methyltetrahydrofolate-Homocysteine Methyltransferase; MTRR: Methionine synthetase reductase; DMG: Dimethylglycine; TMG: Trimethylglycine; BHMT: Betaine-Homocysteine S-Methyltransferase; SAMe: S-adenosyl-L-methionine; SAH: S-adenosylhomocysteine; CBS: cystathionine β-synthase; GSSG: glutathione disulfide; NADH: nicotinamide adenine dinucleotide; FAD: flavin adenine dinucleotide; DNA: deoxyribonucleic acid; RNA: ribonucleic acid ; ATP: Adenosine triphosphate.
Multiple studies have shown that vitamins C, D, E, B1, folate, B12, lipoic acid, lutein, n-acetyl cysteine, and betaine can improve endothelial function, protect neurons, lower blood pressure, and improve visual acuity [8, 23,24,25,26,27,28,29,30,31,32,33,34,35]. The retina is the recipient of these benefits.
Main text
Vitamins
Vitamin A and Carotenoids
Vitamin A is a group of animal-derived fat-soluble retinoids essential for cell growth, cell differentiation, immunity, and vision. In the eye, vitamin A (aka retinol), is a component of rhodopsin, the light-sensitive pigment. It is also necessary for healthy corneal and conjunctival membranes. Deficiency is common where there is generalized malnutrition and is associated with night blindness, conjunctival xerosis, and corneal ulceration, particularly with concurrent measles infection [36, 37].
Vitamin A deficiency decreases maintenance levels of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), which ordinarily protect the retina from oxidative stress injury and stimulate repair. Repletion of vitamin A restores NGF and BDNF levels in the brain [38].
Lutein and Zeaxanthin are water-soluble plant-based carotenoids that easily cross the blood-brain and blood-retina barriers [39]. They are essential for vision but cannot be synthesized in the human body. Concentrated in the macula lutea, they act as powerful antioxidants stabilizing cell membranes and protecting from oxidative stress. They are believed to protect against age-related macular degeneration (AMD) and DR [40].
The Age-Related Eye Disease Studies (AREDS), are an important series of studies conducted by the National Eye Institute investigating how multi-vitamin antioxidant complexes affect AMD and other eye diseases. The first study, AREDS report 8, looked at the progression of moderate AMD [5].
Seddon et al. found that AMD protection was linear with lutein intake and that 6 mg/day or more of lutein was required [41]. Subsequently, the AREDS 2 study replaced beta carotene of the AREDS formula with 10 mg of lutein and 2 mg of zeaxanthin. AREDS2 found that patients with the lowest baseline intakes benefited the most [42].
These studies proved that optimal combinations of crucial nutrients, including lutein and zeaxanthin, could slow the progression of an inexorable degenerative disease like AMD. These findings were initially controversial but are cost-effective and have stood the test of time [43,44,45]. Evidence suggests that lutein supplementation also increases BDNF, preventing neurodegeneration, and preserving the electroretinograms [28, 46, 47].
Several studies extended the AREDS concepts to DR [48,49,50]. Brazionis et al. reported that similar to AMD, higher lutein, and zeaxanthin levels were associated with significantly lower odds of DR [51]. A randomized trial of several antioxidants showed lutein could delay the progression of DR over five years [48]. An interventional study reported that intake of 10 mg/day of lutein improved contrast sensitivity, glare, and visual acuity in patients with non-proliferative DR [52]. A two year trial of 10 mg lutein + 12 mg zeaxanthin in diabetic patients without DR showed improved retinal response density on multifocal electroretinography and a mild non-edematous increase in foveal thickness [53]
These two carotenoids show benefit for AMD and DR [29]. The optimal dosage of them may be higher than 10 mg of lutein and 2 mg of zeaxanthin. Many patients currently self-administer high doses of lutein and zeaxanthin, with no apparent harm. Toxicology studies are reassuring [54]. Long-term studies with larger sample sizes will help clarify optimal dosing.
B Vitamins: B1, B2, B3, B5, B6, Folate, B12
B vitamins are a group of essential water-soluble co-factors that regulate key cellular metabolic processes. These active metabolic pathways are critical to all cells (Figs. 1 and 2) [55].
Genetic polymorphisms and B vitamin insufficiencies wreak havoc on the homeostatic mechanisms of intermediate metabolism, particularly the One Carbon Cycle, which maintains healthy vascular endothelium by converting Hcy to methionine. Other functions include mitochondrial free radical quenching, blood pressure regulation (nitric oxide- norepinephrine), white matter tract signal conduction (synthesis of myelin), sleep (serotonin-melatonin diurnal cycles), attention and memory (dopamine and norepinephrine), and mood (serotonin). These are critical functions for neural, vascular, and visual function. They are at the root of many chronic illnesses as causative or aggravating conditions, which negatively impact health [19, 56,57,58,59]. A deficiency of any one B vitamin decreases the efficiency of all linked processes throughout the One Carbon pathway [19].
B-vitamin deficiencies, insufficiencies, and reduced function genetic polymorphisms first show their effects in the mitochondria of tissues with the highest metabolic activity [55].
The retina has the highest metabolic activity and metabolic stress in the human body. The inner retina has the highest metabolic vulnerability to ischemia [60,61,62,63,64]. The following is a review of the impact of individual B-vitamins on metabolic issues of DR.
Vitamin B1 (Thiamin)
Thiamin is a potent free radical scavenger that regulates intracellular glucose and prevents polyol pathway activation, which is induced by high intracellular glucose [65, 66]. Hyperglycemic-induced dysfunction of the polyol pathway is thought to induce DR in rats and humans [67, 68].
High serum levels of thiamin protect the vascular endothelium from advanced glycation end products injury [30, 69, 70].
Thiamin supplementation at high doses of 50-100 mg/day is safe and useful for neuroprotection as well as the treatment and prevention of end-organ vascular damage, including DR and diabetic nephropathy. Toxicity is so low that no Upper Limits (UL) have been proposed. Thiamin supplementation offers a very useful low-cost low-risk intervention for treating DR (Fig. 2) [71,72,73].
Vitamin B2 (Riboflavin)
Riboflavin is a flavonoid vitamin essential for intermediate metabolism, energy production, and mitochondrial function [74]. Riboflavin, as flavin adenine dinucleotide, is an essential cofactor for synthesizing L-methylfolate, the methyl source for methylcobalamin, which lowers Hcy [58].
The common C677T polymorphism of the MTHFR enzyme has impaired sensitivity to flavin adenine dinucleotide, reducing L-methylfolate synthesis. This causes elevated Hcy resulting in hypertension and vascular disease [40]. Increased serum Hcy in humans has been associated with loss of retinal thickness measured by optical coherence tomography and an increased incidence of DR (Fig. 1) [75].
Human supplementation with riboflavin increases L-methylfolate synthesis, lowers Hcy, and lowers blood pressure [58]. In a murine model, riboflavin supplementation increases glucose uptake and ameliorates oxidative stress. Riboflavin supplementation thus appears to protect the retina from oxidative stress, hyperglycemia, and Hcy-induced injury [59, 76].
Riboflavin supplementation also increases BDNF expression in a murine model [77]. Low levels of BDNF are associated with impaired glucose metabolism. BDNF is higher in prediabetic patients than in diabetic patients, suggesting neuroprotective benefits for patients with insulin resistance and pre-diabetes [78].
Optimal riboflavin dosages are not established for DR, but long-term treatment with 400 mg/day is common for migraine, and toxicity is so minimal that there is no UL established [39].
Vitamin B3 (Niacin)
Niacin is an essential water-soluble B-vitamin. High dose supplementation of niacin may cause or aggravate diabetes. Pharmacologic doses are used for lipid control 1000-3000 mg/day, with questionable benefit and an increased risk of impaired glucose tolerance and insulin resistance, hepatic toxicity, and all-cause mortality (Fig. 2) [79, 80].
Cystoid macular edema risk is increased with high dose niacin supplementation [81]. On the positive side, in a diabetic animal model, niacin supplementation increased endothelial growth factors, promoted migration, sprouting, and survival of endothelial cells and mediated vascular remodeling following occlusion of the middle cerebral artery [82].
Retinal vein occlusion (RVO) prevalence is increased in patients with diabetes and may present with DR [83]. Three small studies suggest that niacin supplementation hastens the resolution by vasodilation, and visual acuity may decrease when niacin is withdrawn [84].
The above studies suggest that niacin intake should remain between the recommended dietary allowances (RDA) and tolerable upper intake level (UL) (14-30 mg/day) unless the patient is monitored for an increased risk of diabetes [79]. There may be a benefit for high niacin for cholesterol where statins fail, in the acute post-stroke recovery phase, or for the treatment of recalcitrant cystoid macular edema, but the macula, blood sugar level, and liver enzymes must be monitored carefully. Except in these special conditions, we do not recommend long-term niacin supplementation above the UL.
Vitamin B5 (Pantothenic Acid)
Pantothenic acid is important for fatty acid metabolism, particularly in the Citric Acid Cycle. It is abundant naturally in foods and seldom requires supplementation (Fig. 2) [85]. In an animal model, 300 mg/kg supplementation of dexpanthenol restored endothelial function, improved antioxidant status, and decreased blood glucose level without side effects [86].
Vitamin B6 (Pyridoxal 5’ Phosphate, PLP, P5P)
Vitamin B6 is a cofactor for many key metabolic activities, including One Carbon Cycle methylation and Hcy metabolism (Fig. 2) [19, 21, 87]. The NHANES studies of B6 in the US population suggest that inadequate serum B6 levels occur in 10-40% of surveyed groups despite widespread use of multivitamins [88]. When MTHFR polymorphisms are present, deficiencies in B6 increase Hcy. Elevated Hcy is a risk factor for DR [10, 89, 90].
Vitamin B6 deficiency contributes to pancreatic islet cell autoimmunity resulting in type I diabetes [91, 92]. A large cohort of Japanese type 2 diabetics was followed for eight years monitoring vitamin B6 intake and the development of retinopathy. Lower intake, particularly in the lowest quartile, was associated with an increased incidence of DR [93].
There are several forms of B6. The natural active form is P5P. It appears to be safe in pharmacological doses. However, other forms of B6, such as the commonly encountered pyridoxine if given in pharmacological doses, may cause a peripheral neuropathy indistinguishable from B6 deficiency. This is caused by competitive inhibition of the enzyme that converts it into P5P. Thus, P5P is the form that is the safest and most efficient for Hcy intervention [94].
Supplementation with B6 optimally as P5P may reduce the risk of developing diabetes, and DR. Studies should be designed to verify this and optimize dosing because such intervention would be high yield, low risk, and affordable.
Vitamin B7 (Biotin)
Glucose metabolism and insulin resistance in T2DM cause dysregulation of glucose-6-phosphatase, hepatic enzymes phosphoenolpyruvate carboxykinase (PCK1, PCK2), and glucose kinase (GCK) metabolism resulting in excess glucose release into the blood. GCK activity is particularly reduced in T2DM, negatively correlating with HbA1c [95]. Animal models show that biotin regulates GCK and that supplemental biotin improves postprandial glucose response by affecting GCK and PCK1 [96, 97]. Biotin should be stopped 72 hours before testing for high-sensitivity troponin T, thyroid-stimulating hormone, follicle-stimulating hormone results, triiodothyronine, or vitamin D [98]. Biotin is safe and inexpensive, without an assigned UL (Fig. 2) [99].
Vitamin B9 (Folate)
Folates are essential water-soluble compounds that serve vital biochemical pathways in every cell of the body. L-methylfolate is the natural substrate for single-carbon methyl transfers in the synthesis of amino acids and nucleic acids (DNA, RNA). It indirectly regulates neurotransmitter and nitric oxide synthesis. L-methylfolate with methylcobalamin converts Hcy to methionine necessary for the synthesis of S-adenosyl-methionine, a key methyl donor for the synthesis and regulation of DNA required for protein synthesis and cell division (Fig. 2) [100].
At the cellular level, elevated Hcy disrupts the retinal blood barrier and increases pigment epithelial cell inflammatory cytokines, which cause retinal apoptosis [101]. Even mild hyperhomocysteinemia is a risk factor for insulin resistance in healthy subjects. The results of the Framingham Offspring Study and other similar studies suggest that it is a cause and marker for pre-diabetes [102,103,104].
The MTHFR enzyme is essential for adding the methyl group to upstream folates. Reduced activity MTHFR gene polymorphisms are common and impaired in their ability to generate L-methylfolate [105]. They are associated with elevated blood pressure and Hcy, as well as increased incidence and progression of DR [58, 106, 107].
L-methylfolate supplementation effectively restores impaired endothelial-dependent vasodilation and enhances endothelial health by converting Hcy to methionine, regardless of dietary deficiencies or genetic polymorphisms that inhibit folic acid processing or L-methylfolate synthesis [31, 104, 108,109,110,111].
L-methylfolate is the reduced natural bioactive form of folate. Folic acid is active only to the degree it has been converted to L-methylfolate [67]. The Institute of Medicine (IOM) reports no toxicity for the natural folates and has not issued tolerable ULs for natural folates. However, the IOM has set the UL for folic acid at 1.0 mg/day for adults [100, 108]. Reynolds has raised concerns that folic acid may cause neurologic injury when there is B12 deficiency [112]. Selhub and Rosenberg reviewed the evidence that a high intake of folic acid is linked to impaired cognition, memory, executive decision making, and retinoblastoma [113]. Folic acid poisoning can be fatal [114]. Any dosing above 1.0 m/day should be with L-methylfolate.
Elevated Hcy increases the risk of hypertension, hypertensive retinopathy, diabetes, and DR [115, 116]. L-methylfolate can be co-administered with vitamin B12 as methylcobalamin efficiently lowers Hcy [117]. Lowering Hcy with folate increases blood flow and perfusion [118]. The use of L-methylfolate with B2, B6 as P5P, and B12 to lower Hcy provides a safe, simple, and inexpensive to reduce and reverse DR and other diabetic end-organ diseases.
Vitamin B12 (Cobalamin)
Vitamin B12, cobalamin, is a complex water-soluble cofactor serving critical functions for cell synthesis, DNA regulation, Hcy metabolism, myelin synthesis, nerve growth, and neuron maintenance; all of which impact vision and DR. Methylcobalamin, one of two active forms, readily donates a methyl group to lower Hcy converting it to methionine [9, 57, 119,120,121,122,123]. Methylcobalamin, through methionine synthesis, regulates the synthesis of DNA, key amino acids, and proteins [124]. Elevated Hcy is associated with reduced cerebral blood flow, reduced retinal blood flow, and reduced caliber of the central retinal artery, vascular endothelial growth factor (VEGF) expression, and DR [125,126,127,128,129].
High dose methylcobalamin is efficient for reducing Hcy because it possesses a ready-to-donate methyl group and would have a beneficial effect on those markers (Fig. 1) [130].
Diabetes leads to small vessel disease of the brain and retina, with ischemia contributing to the pathogenesis of DR [131]. Initial mitochondrial dysfunction and Müller cell impairment are followed by structural loss of capillary endothelium, neurons, and photoreceptors. This process is visible in the eye as microaneurysms, exudates, cotton wool spots, capillary drop out, retinal edema, and retinal atrophy [132, 133].
Animal models suggest that treatment with NGF for retinal inflammation and neovascularization could be the next major therapeutic advance in tandem with anti-VEGF therapy. Supplemental B12 increases the release of NGF and BDNF [134]. Combined therapy increases retinal cell survival, rhodopsin expression, and neurite outgrowth in photoreceptors [135]. This may safely and inexpensively be accomplished by maintaining B12 levels in the high normal range. Raising neurotrophins by treating with B12 offers another opportunity for clinicians to reduce long-term vascular complications of DR.
B12 insufficiency and deficiency is common and has many causes [136]. Active transport B12 absorption from food requires intrinsic factor, secreted by the gastric parietal cells, in an acid milieu with intact small intestinal villi [137]. Metformin, Glipizide, and omeprazole, common medications for diabetic treatment impair B12 uptake. Passive transport uptake for B12 is about 1%; thus, high doses of active B12 bypass active transport mechanisms and are less prone to iatrogenic or disease state malabsorption [138,139,140]. Eussen et al. studied oral dosing of B12, concluding that satisfactory passive transport was dose-related and required at least 200 times greater than the RDA of 2-4 μg/day. Oral doses of 500-1000 μg/day, at least, were recommended [141].
B12 therapy, through enhanced reduction of Hcy and nerve growth factor release, is a neglected, inexpensive opportunity for clinicians to reduce vascular ischemia of the retina and to reduce the risk and severity of DR [9, 57, 120,121,122].
Recently, concerns have risen about the toxicity of cyanocobalamin, especially in people with diabetes. Methylcobalamin in high doses is non-toxic, even when advanced diabetic nephropathy is present. Methylcobalamin appears to be safer and more effective than cyanocobalamin in reducing Hcy, even with advanced renal disease [130, 142].
B-Vitamin Summary: Diabetes, DR, Hcy, Blood Flow Therapies
Monotherapy is the ordinary trend in medicine. However, in strategically formulated nutritional therapy, B-vitamin combinations at high dosages seem to have better outcomes than vitamin monotherapy. Martin et al. linked Hcy elevation to occlusive retinal vascular disease and proposed therapeutic intervention with vitamins B6, B9 (folate), and B12 [143]. Vitamins B2, B6, B9 (folate), and B12 are the primary cofactors for One Carbon Metabolism and are important for Hcy methylation and regulation [144]. Multivitamin complexes containing B1, B2, B6, L-methylfolate, and B12 have shown benefit for DR in human and animal trials [145,146,147].
Vitamin C (Ascorbic Acid)
Vitamin C is water-soluble and essential for regenerating other antioxidants such as vitamin E and glutathione [148, 149]. Vitamin C administration lowers blood pressure in patients with essential hypertension [150]. Human and diabetic animal trials have found that oral vitamin C reduces capillary endothelial dysfunction [32, 151]. Patients with proliferative DR have a 10-fold lower vitreous ascorbate concentration and an increased tendency to diabetic macular edema [152]. Vitamin C taken with statins reduces non-proliferative DR, in a dose-dependent fashion more than statins alone [153].
Vitamin D
Vitamin D is a group of fat-soluble vitamin secosteroids essential for calcium absorption, deposition, and regulation, which in turn regulate many important processes. The prevalence of vitamin D insufficiency in the US is above 40%, higher in the elderly, and in some ethnic groups [154]. Most supplements are vitamin D2 or D3, which are storage forms collectively referred to as "vitamin D." Serum testing for vitamin D 25 (OH) D2 + D3 measures body stores [155]. 1,25 (OH) D is the active vitamin synthesized by the kidneys as needed. It is seldom supplemented except in renal failure states. Vitamin D and calcium also regulate tear film mucin release and stability [156, 157].
Intestinal folate absorption and transport across the blood-brain barrier are upregulated by vitamin D [158, 159]. Since folates require sufficient available vitamin D to be effective, and vitamin D deficiency is so widespread, if folate is to be given therapeutically, it should be given with enough vitamin D to ensure optimal absorption and CNS transportation. Given widespread vitamin D deficiency, we suggest co-administration with the UL of 4000 IU vitamin D3 daily with any folate therapy unless serum vitamin D is known to be above the 50th percentile.
Vitamin D sufficiency is essential for insulin release, insulin sensitivity, reduction of inflammation, and reduction of arterial stiffness [155, 160,161,162,163,164,165]. Recently, optimal vitamin D levels have been shown to be important to reduce the risk and severity of DR [166]. Vitamin D plays a role in pancreatic β-cell function [167]. Deficiency reduces insulin sensitivity and increases the risk of atherosclerosis, CVD, T2DM, and hypertension [168,169,170]. 1,25–dihydroxy vitamin D triggers the secretion of insulin by stimulating pancreatic beta cells [164, 165]. Clinical trials had shown significant improvements in insulin sensitivity and HbA1c when patients were given vitamin D3 [171, 172].
Mutlu et al. reported that lower vitamin D was associated with retinal microvascular damage after studying the associations in 5675 participants with diabetes [173]. Vitamin D deficiency is linked to T1DM [174]. Vitamin D deficiency is also linked to T2DM and supplementation, which has been shown to decrease C-reactive protein, hs-CRP [175].
Serum levels of 25-hydroxy vitamin D above 30 ng/ml reduce the odds of DR [166, 176]. Vitamin D supplementation reduces intracellular reactive oxygen species decreasing VEGF expression [7].
Low serum vitamin D levels among patients with DM are associated with a higher risk and severity of DR for all the above reasons. Cumulatively, this suggests that vitamin D supplementation is beneficial to reduce the risk and severity of DR,
Vitamin E
Vitamin E is an amber lipid-soluble antioxidant associated with low-density lipoprotein. It primarily functions as a peroxyl radical scavenger involved in long-chain fatty acid stabilization of cell membranes [177, 178]. Vitamin E supplementation quenches free radicals and reduces retinal oxidative stress in the retina [178, 179].
Vitamin E supplementation reduces moderate blood pressure abnormalities, particularly systolic pressure [180,181,182]. Elevated blood pressure is a risk factor for both the incidence and severity of DR [183]. A careful randomized, double-masked, placebo-controlled crossover trial at the Joslin Institute established that for patients with T1DM of fewer than ten years duration, vitamin E supplementation of 1800 IU daily improved retinal blood flow [184]. Oxidative stress, which is elevated in DR, is decreased after treatment with vitamin E [185]. Vitamin E, when administered alone, has modest benefits on blood pressure and blood flow, which are salutary for diabetes and DR patients [186]. It appears vitamin E has a greater benefit when co-administered with vitamin C [187]. Studies suggest that natural vitamin E complex, not the racemic synthetic dl mixtures, is more potent and more likely to be beneficial [188].
Zinc
Zinc is an essential co-factor for cell division, DNA synthesis, immune function, as well as the metabolism of carbohydrates and proteins. Moderate zinc deficiency is common [189,190,191]. Zinc deficiency is associated with the progression of chronic disease states such as metabolic syndrome, diabetes, diabetic microvascular complications, and DR [192,193,194].
The Nurses' Health Study found a 20% difference in risk of diabetes between the highest and lowest zinc intake quintiles [195]. Low serum zinc levels are correlated with the duration of diabetes, elevated HbA1c, hypertension, and microvascular complications. Serum zinc levels fall progressively with increased duration of diabetes and severity of DR [194].
A trial of zinc supplementation in a murine T2DM analog improved glucose intolerance, insulin resistance, obesity, and hypertension [196]. This may be in part due to zinc protection against lipid peroxidation and pericyte protection. Increasing zinc also reduced ischemic inflammation while decreasing VEGF [192].
A rat model of DR was studied using an AREDS-based micronutrient supplementation, including zinc. Capillary deterioration over time was prevented with the AREDS formula despite similar hyperglycemia between treated and control groups [50]. A human trial should be done to establish optimum zinc levels for the treatment of DR.
Lipoic Acid (LA, Alpha Lipoic Acid, ALA, Thioctic Acid)
Lipoic acid also referred to as alpha-lipoic acid, is an important cofactor for mitochondrial metabolism. LA is needed to generate acetyl Co-A, the fuel of the Krebs Cycle, and the core of energy-generating metabolism [197, 198]. LA scavenges reactive oxygen species, enhancing the effects of such endogenous antioxidants as glutathione, vitamin C, and E by recycling. Lipoic acid supplementation increases available glutathione [199].
LA administration protects the retina, particularly the ganglion cells and pigment epithelial cells from ischemia and apoptosis [200, 201]. LA decreases hyperglycemia and hyperglycemic vascular endothelial dysfunction in T2DM patients [202, 203]. It reduces VEGF expression and is protective of the retinal ganglion cells and capillaries in animal models of DR [34, 204]. A small controlled study showed increased contrast sensitivity in patients with T1DM and T2DM who were supplemented with oral LA [205].
Supplementation is beneficial. It shows benefits for ischemia and oxidative stress. LA appears to be safe and well-tolerated at 600 mg/day as a dietary supplement. When used for diabetic polyneuropathy, it is effective and has fewer side effects than commonly prescribed medications [206]. LA represents a new opportunity for patients with diabetes, and DR. More research is strongly encouraged.
N-Acetyl Cysteine (NAC)
N-acetyl cysteine is a thiol antioxidant precursor to glutathione [207]. Glutathione is neuroprotective and retina protective [208, 209]. Cysteine availability is often the rate limiter for glutathione synthesis, and thus supplementing with NAC increases net glutathione levels, which is useful in disease states characterized by ischemia, increased oxidative stress, and reduced available glutathione [210, 211].
Hyperhomocysteinemia has been shown to increase reactive oxygen species in the retina, altering the blood-retinal barrier of human retinal endothelial cells [212]. Chronically elevated Hcy eventually causes vaso-occlusive retinopathy with retinal atrophy [75, 213].
Long term administration of NAC reduces reactive oxygen species in the retinal mitochondria, and decreases VEGF expression and proliferative retinopathy in diabetic animal models [214]. NAC restores the tight junctions reducing vascular leakage [212]. Ischemia-induced retinal pigment epithelial cell, ganglion cell, and photoreceptor apoptosis are reduced when NAC is present [35, 209, 215, 216]. It also reduces the expression of VEGF and Icariin-1, free radical species, ischemia, and structural changes of retinopathy [214]. Animal models suggest that it may decrease retinal detachment in proliferative vitreoretinopathy [217].
Therefore, NAC is beneficial at the cellular level to prevent retinal neuron and photoreceptor apoptosis and death while preserving the visual function and structure of the retina and optic nerve.
Trimethylglycine (TMG, Betaine)
TMG is a trimethylated dietary amino acid derivative primarily obtained from sugar beets. Betaine is not a vitamin because it can be synthesized in small amounts by methylating choline in the mitochondria in young adults. Though betaine has no RDA established, choline has an RDA. Diets are frequently low in both [218,219,220,221].
Homocysteine is at the crossroads of the One Carbon Cycle [221, 222]. The betaine-homocysteine methyltransferase pathway is an alternative pathway for converting homocysteine into methionine via the methylfolate-B12-homocysteine methylation pathway. They work together to keep serum Hcy low and methionine at an optimal level [223]. The main sources of the One Carbon Cycle methyl groups are choline, L-methylfolate, methylcobalamin, dimethylglycine, and TMG. Higher intake of betaine decreases inflammatory markers and serum Hcy [221, 224, 225]. Betaine naturally concentrates in the brain, liver, and kidneys, where it prevents the accumulation of Hcy and decreasing inflammation (Fig. 2) [218].
Betaine is used as a secondary treatment for elevated Hcy in patients who do not respond sufficiently to B6, folate, and B12 supplementation [226]. The human average dietary intake of betaine is 500-2000 mg/day. In a study of betaine reduction of Hcy, low dose additional supplementation with betaine anhydrous of 500-3000 mg/day lowered Hcy levels in healthy subjects, with a small further drop at 6000 mg/day [227].
Homocysteine elevation is associated with increased inflammation and decreased blood flow in the brain and retina, causing small vessel disease of the central nervous system (CNS), increased VEGF secretion, endothelial dysfunction, proliferative and non-proliferative retinopathy, as well as diabetic macular edema [126, 129, 228,229,230,231,232]. Betaine, along with vitamins B2, B6, folate, and B12, is very effective in reducing Hcy levels and reversing the triggers of hyperhomocysteinemia, a driver for DR [58, 233, 234].
Multivitamins and Nutraceuticals: Monotherapy or Polytherapy?
Drug-based therapies tend to be monotherapies with a single drug for a single target. Nutritional deficiencies are often multi-faceted. Genetic polymorphisms and nutritional deficiencies tend to have a broad impact on complex systems, such as the One Carbon Methylation Cycle or the Citric Acid Cycle, where there are many cofactors and substrates. Targeting more than one place in these cycles seems efficient for correcting cycle and functional imbalances (Figs. 1 and 2).
Multivitamins mineral complexes and B-complex multivitamins have long been used to treat and prevent multiple nutritional deficiencies because mono-deficiencies are rare [235,236,237]. In the central nervous system, where metabolic rates are high, there is a synergistic benefit to using several of the B-vitamins together [21].
For people with diabetes, B vitamin serum concentrations are lower, possibly due to higher renal clearance and lower reabsorption [238]. This leads to further deficiencies and the need for even higher B-vitamin levels.
Active transport uptake of vitamins and minerals have limits that may lead to a deficiency or prevent optimization [239]. However, with high doses, absorption and transport are passive, and dose-related [141, 240, 241]. Thus, there is precedence for this high dose multivitamin approach applied to conditions of the retina and central nervous system [19, 242]. Fortunately, long term experience has shown a few complications with this approach because of their low toxicity [243, 244].
The AREDS/AREDS2 studies showed powerful benefits and excellent safety for macular degeneration using a high dose combination of specific vitamins, minerals, and lutein plus zeaxanthin. No human trials have been done with the AREDS formulation for DR, however, Kowluru et al. studied diabetic rats given a diet that included the AREDS micronutrients. They found that the AREDS micronutrients decreased the accumulation of acellular retinal capillaries. Evidence of diabetic oxidative damage and retinal levels of manganese superoxide dismutase were decreased. Diabetes-induced increased nitric oxide synthetase was blocked. This mechanistically supports the use of such vitamin antioxidant combinations for DR [44, 50].
Folate and vitamin B12 intake and absorption decline with age and diabetes [238, 245, 246]. As discussed previously, they are essential to control homocysteine. There is also evidence that both natural aging and AMD are associated with homocysteine elevation and low folate and B12 serum levels [247]. Choriocapillaris endothelial loss slowly proceeds with age, increasing with AMD. This loss long predates visual detection [248, 249].
If the choriocapillaris endothelium in AMD is injured by hyperhomocysteinemia the same way as are the retinal capillaries in DR, this would suggest a unifying approach. There is such evidence. The Women's Antioxidant and Folic Acid Cardiovascular Study (WAFACS) trial was a large randomized, double-blind, and placebo-controlled study that examined the benefits of high dose B6, folate, and B12 in women at high risk of cardiovascular disease. With an average follow-up time of 7.3 years, after two years, a reduction of AMD emerged, reaching 40% by the end of the trial. This exceeds the benefit of the AREDS2 trial. The investigators proposed homocysteine reduction to account for this [250]. Adding high doses of B-vitamins to an AREDS2 type formula might further benefit macular degeneration.
The use of higher dose B complex multivitamins has also been shown to reduce the risk of all strokes and improve brain health in the Hope 2 trial [19, 251].
Medical Foods
Medical foods are a category of nutritional interventions to which ophthalmologists have had little exposure. They occupy a place between foods, dietary supplements, and prescription drugs. All ingredients of medical foods must be generally recognized as safe, Generally Recognized as Safe (GRAS), meaning that the FDA has approved them as safe for human consumption. FDA oversight for claims, purity, and manufacturing is more rigorous than dietary supplements. Labeling must be accurate, unlike the reality of dietary supplements [252,253,254,255,256].
The FDA restricts the use of medical foods to patients under the direct supervision of a physician. They are for situations in which diet alone is insufficient to obtain what is needed. Particularly, the FDA intends them to address inborn errors of metabolism caused by reduced function genetic polymorphisms resulting in or contributing to disease states. An example pertinent to DR is the elevation of blood pressure and serum Hcy due to MTHFR gene polymorphisms that impair the One Carbon Cycle [257].
Medical foods addressing Hcy elevation due to inborn errors of metabolism, do so by providing high doses of active forms of vitamins B6, L-methylfolate, methylcobalamin, or trimethylglycine. They work by donating a methyl group to homocysteine, thus converting it to methionine, reducing mitochondrial oxidative stress, and protecting the vascular endothelium. Over time, this reduces end-organ damage, such as retinopathy. Three medical foods are useful for treating elevated Hcy, reduced blood flow, and retinal ischemia.
Metanx® was developed to support neurovascular regeneration for diabetic peripheral neuropathy associated endothelial dysfunction in patients with inherited disorders of Hcy metabolism with elevated Hcy. Metanx® consists of 3.0 mg of L-methylfolate, 35 mg of pyridoxal 5’- phosphate P5P, and 2 mg of methylcobalamin [258]. Metanx® utilizes P5P, which is the non-toxic active form of vitamin B6 [94]. L-methylfolate is the natural, non-toxic active form of folate and methylcobalamin is the natural, non-toxic methylated active form of cobalamin. These are the most effective, least toxic vitamin forms for reducing Hcy, increasing blood flow, and raising brain-derived neurotropic factors for neuroprotection [117, 259, 260].
Cystadane® was developed for the long term management of elevated Hcy. Cystadane® is pure anhydrous betaine powder for the preparation of an oral solution [261]. Betaine (TMG), is another methyl source. Cystadane lowers homocysteine through the betaine-homocysteine methyltransferase pathway [223]. It is useful when B-vitamin therapy alone is insufficient to bring Hcy levels below 9 μmol/L.
Ocufolin® was developed to reduce retinal ischemia and retinopathy in patients with the common MTHFR polymorphisms. The ingredients collectively address critical metabolic pathways with vitamins and antioxidant co-factors, which lead to Hcy elevation, ischemia, and oxidative stress in those patients [262].
Studies of Medical foods for DR
Liu et al. found that MetanxTM inhibited ocular oxidative stress, inflammation, and protected against diabetic spatial frequency defects in a ten-month study of mice with DR [145]. In a six month observational study of human subjects, Smolek et al. found that MetanxTM reduced non-proliferative DR (NPDR), particularly foveal edema, retinal thickness, and improved the mean threshold retinal sensitivity [146]. The protective mechanism is most likely due to decreasing Hcy, which appears to constrict the central retinal artery [126, 232].
Schmidl et al. found that Ocufolin® reduced Hcy levels 30% in a trial of type 1 and 2 diabetics [263]. Wang et al. published a case series of eight patients with DR treated with OcufolinTM or a similar formulation (EyefolateTM). They showed visible improvement in retinopathy even in longstanding cases [262].
Based on published studies, it is possible to come to some recommendations for reducing the risk and severity of DR. It is helpful that the recommendations for DR mirror those for diabetes and macular degeneration generally, and for reducing Hcy, hypertension, and increasing BDNF and other nerve growth neurotropic factors so that many benefits flow from similar treatments.
Conclusions
We have identified vitamin deficiencies, antioxidant deficiencies, and the reduced function gene polymorphisms of MTHFR as common risk factors for hyperhomocysteinemia, neurotrophic factor depletion, and DR. We also note that their physiological mechanisms overlap and that the treatments are similar. Most chronic diseases are worsened by a deficiency of any essential nutrient. As we have seen, age, diet, and many factors further impair the absorption and utilization of these nutrients. This paper has identified several vitamins, minerals, and nutraceuticals, which are useful to address this situation. They include lutein, zeaxanthin, vitamin C, vitamin D, vitamin E, zinc, copper, alpha-lipoic acid, n-acetylcysteine, and complexes of B1, B2, B6, L-methylfolate, and methylB12. Some of these were also shown to be beneficial for AMD in the AREDS/AREDS2 trials. Addressing Hcy, raising BDNF and other neurotrophic factors, reducing oxidative stress and inflammation, while increasing blood flow is a low hanging fruit for the clinician who wishes to lower the burden and alter the course of disease in patients with DR.
It is possible for a patient and clinician to assemble all these individually. However, cost and logistics may be concerns. The AREDS2 formulation of the National Eye Institute was a major improvement, bringing together copper, zinc, lutein, zeaxanthin, vitamin C, and vitamin E for macular degeneration in a convenient, affordable, and effective format. AREDS2, however, does not address blood flow, ischemia, or Hcy reduction. It does not maximally increase glutathione, nor is it intended for retinal vascular diseases such as DR.
Similarly, medical foods that address common inborn errors of metabolism, homocysteine elevation, reduced blood flow, and ischemia makes it simpler for the patient and clinician to obtain these benefits. The FDA holds medical foods to higher standards of manufacturing, labeling, and safety than dietary supplements, resulting in precise, consistent dosing which physicians need for addressing serious health conditions.
These opportunities support without conflicting with conventional therapy for DR. While further studies are needed to determine optimal formulations and appropriate usage, clinicians should feel comfortable about the safety and utility of these modalities.
Availability of data and materials
Not applicable.
References
Dwyer JT, Coates PM, Smith MJ. Dietary supplements: regulatory challenges and research resources. Nutrients. 2018;10(1). pii: E41.
Deshmukh SV, Prabhakar B, Kulkarni YA. Water soluble vitamins and their role in diabetes and its complications. Curr Diabetes Rev. 2019. https://doi.org/10.2174/1573399815666190916114040.
Lei XW, Li Q, Zhang JZ, Zhang YM, Liu Y, Yang KH. The protective roles of folic acid in preventing diabetic retinopathy are potentially associated with suppressions on angiogenesis, inflammation, and oxidative stress. Ophthalmic Res. 2019;62(2):80–92.
Kim YS, Kim M, Choi MY, Lee DH, Roh GS, Kim HJ, et al. Alpha-lipoic acid reduces retinal cell death in diabetic mice. Biochem Biophys Res Commun. 2018;503(3):1307–4.
Age-Related Eye Disease Study Research G. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–36.
Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005–15.
Lu L, Lu Q, Chen W, Li J, Li C, Zheng Z. Vitamin D3 protects against diabetic retinopathy by inhibiting high-glucose-induced activation of the ROS/TXNIP/NLRP3 inflammasome pathway. J Diabetes Res. 2018;2018:8193523.
Kim YG, Lim HH, Lee SH, Shin MS, Kim CJ, Yang HJ. Betaine inhibits vascularization via suppression of Akt in the retinas of streptozotocin-induced hyperglycemic rats. Mol Med Rep. 2015;12(2):1639–44.
Satyanarayana A, Balakrishna N, Pitla S, Reddy PY, Mudili S, Lopamudra P, et al. Status of B-vitamins and homocysteine in diabetic retinopathy: association with vitamin-B12 deficiency and hyperhomocysteinemia. PLoS One. 2011;6(11):e26747.
Lei X, Zeng G, Zhang Y, Li Q, Zhang J, Bai Z, et al. Association between homocysteine level and the risk of diabetic retinopathy: a systematic review and meta-analysis. Diabetol Metab Syndr. 2018;10:61.
Calderon GD, Juarez OH, Hernandez GE, Punzo SM, De la Cruz ZD. Oxidative stress and diabetic retinopathy: development and treatment. Eye (Lond). 2017;31(8):1122–30.
Mozos I, Stoian D, Luca CT. Crosstalk between Vitamins A, B12, D, K, C, and E status and arterial stiffness. Dis Markers. 2017;2017:8784971.
Karwowski W, Naumnik B, Szczepański M, Myśliwiec M. The mechanism of vascular calcification - a systematic review. Med Sci Monit. 2012;18(1):RA1–11.
Plantinga Y, Ghiadoni L, Magagna A, Giannarelli C, Franzoni F, Taddei S, et al. Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am J Hypertens. 2007;20(4):392–7.
Cheung BM, Li C. Diabetes and hypertension: is there a common metabolic pathway? Curr Atheroscler Rep. 2012;14(2):160–6.
Loscalzo J, Welch G. Nitric oxide and its role in the cardiovascular system. Prog Cardiovasc Dis. 1995;38(2):87–104.
Förstermann U. Nitric oxide and oxidative stress in vascular disease. Pflugers Arch. 2010;459(6):923–39.
Carr A, Frei B. The role of natural antioxidants in preserving the biological activity of endothelium-derived nitric oxide. Free Radic Biol Med. 2000;28(12):1806–14.
Kennedy DO. B vitamins and the brain: mechanisms, dose and efficacy--a review. Nutrients. 2016;8(2):68.
Surendran S, Adaikalakoteswari A, Saravanan P, Shatwaan IA, Lovegrove JA, Vimaleswaran KS. An update on vitamin B12-related gene polymorphisms and B12 status. Genes Nutr. 2018;13:2.
Calderón -Ospina CA, Nava-Mesa MO. B vitamins in the nervous system: current knowledge of the biochemical modes of action and synergies of thiamine, pyridoxine, and cobalamin. CNS Neurosci Ther. 2020;26(1):5–13.
Maeda M, Yamamoto I, Fukuda M, Motomura T, Nishida M, Nonen S, et al. MTHFR gene polymorphism is susceptible to diabetic retinopathy but not to diabetic nephropathy in Japanese type 2 diabetic patients. J Diabetes Complications. 2008;22(2):119–25.
Meng Y, Li J, Chen X, She H, Zhao L, Peng Y, et al. Association between folic acid supplementation and retinal atherosclerosis in chinese adults with hypertension complicated by diabetes mellitus. Front Pharmacol. 2018;9:1159.
Stanhewicz AE, Alexander LM, Kenney WL. Folic acid supplementation improves microvascular function in older adults through nitric oxide-dependent mechanisms. Clin Sci (Lond). 2015;129(2):159–67.
Hoch AZ, Lynch SL, Jurva JW, Schimke JE, Gutterman DD. Folic acid supplementation improves vascular function in amenorrheic runners. Clin J Sport Med. 2010;20(3):205–10.
van Dijk RA, Rauwerda JA, Steyn M, Twisk JW, Stehouwer CD. Long-term homocysteine-lowering treatment with folic acid plus pyridoxine is associated with decreased blood pressure but not with improved brachial artery endothelium-dependent vasodilation or carotid artery stiffness: a 2-year, randomized, placebo-controlled trial. Arterioscler Thromb Vasc Biol. 2001;21(12):2072–9.
Grigoletti SS, Guindani G, Moraes RS, Ribeiro JP, Sprinz E. Short-term folinic acid supplementation improves vascular reactivity in HIV-infected individuals: a randomized trial. Nutrition. 2013;29(6):886–91.
Ozawa Y, Sasaki M, Takahashi N, Kamoshita M, Miyake S, Tsubota K. Neuroprotective effects of lutein in the retina. Curr Pharm Des. 2012;18(1):51–6.
Hu BJ, Hu YN, Lin S, Ma WJ, Li XR. Application of lutein and zeaxanthin in nonproliferative diabetic retinopathy. Int J Ophthalmol. 2011;4(3):303–6.
Beltramo E, Berrone E, Buttiglieri S, Porta M. Thiamine and benfotiamine prevent increased apoptosis in endothelial cells and pericytes cultured in high glucose. Diabetes Metab Res Rev. 2004;20(4):330–6.
Kalani A, Kamat PK, Givvimani S, Brown K, Metreveli N, Tyagi SC, et al. Nutri-epigenetics ameliorates blood-brain barrier damage and neurodegeneration in hyperhomocysteinemia: role of folic acid. J Mol Neurosci. 2014;52(2):202–15.
Jariyapongskul A, Rungjaroen T, Kasetsuwan N, Patumraj S, Seki J, Niimi H. Long-term effects of oral vitamin C supplementation on the endothelial dysfunction in the iris microvessels of diabetic rats. Microvasc Res. 2007;74(1):32–8.
Setola E, Monti LD, Galluccio E, Palloshi A, Fragasso G, Paroni R, et al. Insulin resistance and endothelial function are improved after folate and vitamin B12 therapy in patients with metabolic syndrome: relationship between homocysteine levels and hyperinsulinemia. Eur J Endocrinol. 2004;151(4):483–9.
Kowluru RA, Odenbach S. Effect of long-term administration of alpha-lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes. 2004;53(12):3233–8.
Gerona G, Lopez D, Palmero M, Maneu V. Antioxidant N-acetyl-cysteine protects retinal pigmented epithelial cells from long-term hypoxia changes in gene expression. J Ocul Pharmacol Ther. 2010;26(4):309–14.
Venkataswamy G. Ocular manifestations of vitamin A deficiency. Br J Ophthalmol. 1967;51(12):854–9.
Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ. 2011;343:d5094.
Kheirvari S, Uezu K, Yamamoto S, Nakaya Y. High-dose dietary supplementation of vitamin A induces brain-derived neurotrophic factor and nerve growth factor production in mice with simultaneous deficiency of vitamin A and zinc. Nutr Neurosci. 2008;11(5):228–34.
Mohn ES, Erdman JW Jr, Kuchan MJ, Neuringer M, Johnson EJ. Lutein accumulates in subcellular membranes of brain regions in adult rhesus macaques: Relationship to DHA oxidation products. PLoS One. 2017;12(10):e0186767.
Neelam K, Goenadi CJ, Lun K, Yip CC, Au Eong KG. Putative protective role of lutein and zeaxanthin in diabetic retinopathy. Br J Ophthalmol. 2017;101(5):551–8.
Seddon JM, Ajani UA, Sperduto RD, Hiller R, Blair N, Burton TC, et al. Dietary carotenoids, vitamins A, C, and E, and advanced age-related macular degeneration. Eye Dis Case-Control Study Group. JAMA. 1994;272(18):1413–20.
Chew EY, Clemons TE, Sangiovanni JP, Danis RP, Ferris FL III, Elman MJ, et al. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014;132(2):142–9.
Jia YP, Sun L, Yu HS, Liang LP, Li W, Ding H, et al. The pharmacological effects of lutein and zeaxanthin on visual disorders and cognition diseases. Molecules. 2017;22(4). pii: E610.
Chew EY. Nutrition, genes, and age-related macular degeneration: what have we learned from the trials? Ophthalmologica. 2017;238(1-2):1–5.
Lee AY, Butt T, Chew E, Agron E, Clemons TE, Egan CA, et al. Cost-effectiveness of age-related macular degeneration study supplements in the UK: combined trial and real-world outcomes data. Br J Ophthalmol. 2018;102(4):465–72.
Sasaki M, Ozawa Y, Kurihara T, Kubota S, Yuki K, Noda K, et al. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia. 2010;53(5):971–9.
Li SY, Fung FK, Fu ZJ, Wong D, Chan HH, Lo AC. Anti-inflammatory effects of lutein in retinal ischemic/hypoxic injury: in vivo and in vitro studies. Invest Ophthalmol Vis Sci. 2012;53(10):5976–84.
Garcia-Medina JJ, Pinazo-Duran MD, Garcia-Medina M, Zanon-Moreno V, Pons-Vazquez S. A 5-year follow-up of antioxidant supplementation in type 2 diabetic retinopathy. Eur J Ophthalmol. 2011;21(5):637–43.
Sahli MW, Mares JA, Meyers KJ, Klein R, Brady WE, Klein BE, et al. Dietary intake of lutein and diabetic retinopathy in the Atherosclerosis Risk in Communities Study (ARIC). Ophthalmic Epidemiol. 2016;23(2):99–108.
Kowluru RA, Kanwar M, Chan PS, Zhang JP. Inhibition of retinopathy and retinal metabolic abnormalities in diabetic rats with AREDS-based micronutrients. Arch Ophthalmol. 2008;126(9):1266–72.
Brazionis L, Rowley K, Itsiopoulos C, O'Dea K. Plasma carotenoids and diabetic retinopathy. Br J Nutr. 2009;101(2):270–7.
Zhang PC, Wu CR, Wang ZL, Wang LY, Han Y, Sun SL, et al. Effect of lutein supplementation on visual function in nonproliferative diabetic retinopathy. Asia Pac J Clin Nutr. 2017;26(3):406–11.
Moschos MM, Dettoraki M, Tsatsos M, Kitsos G, Kalogeropoulos C. Effect of carotenoids dietary supplementation on macular function in diabetic patients. Eye Vis (Lond). 2017;4:23.
Harikumar KB, Nimita CV, Preethi KC, Kuttan R, Shankaranarayana ML, Deshpande J. Toxicity profile of lutein and lutein ester isolated from marigold flowers (Tagetes erecta). Int J Toxicol. 2008;27(1):1–9.
Depeint F, Bruce WR, Shangari N, Mehta R, O'Brien PJ. Mitochondrial function and toxicity: role of the B vitamin family on mitochondrial energy metabolism. Chem Biol Interact. 2006;163(1-2):94–112.
Banka S, Blom HJ, Walter J, Aziz M, Urquhart J, Clouthier CM, et al. Identification and characterization of an inborn error of metabolism caused by dihydrofolate reductase deficiency. Am J Hum Genet. 2011;88(2):216–25.
Grober U, Kisters K, Schmidt J. Neuroenhancement with vitamin B12-underestimated neurological significance. Nutrients. 2013;5(12):5031–45.
McNulty H, Strain JJ, Hughes CF, Ward M. Riboflavin, MTHFR genotype and blood pressure: A personalized approach to prevention and treatment of hypertension. Mol Aspects Med. 2017;53:2–9.
Moat SJ, Ashfield-Watt PA, Powers HJ, Newcombe RG, McDowell IF. Effect of riboflavin status on the homocysteine-lowering effect of folate in relation to the MTHFR (C677T) genotype. Clin Chem. 2003;49(2):295–302.
Raichle ME, Gusnard DA. Appraising the brain's energy budget. Proc Natl Acad Sci U S A. 2002;99(16):10237–9.
Yu DY, Cringle SJ. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog Retin Eye Res. 2001;20(2):176–205.
Kurihara T, Westenskow PD, Gantner ML, Usui Y, Schultz A, Bravo S, et al. Hypoxia-induced metabolic stress in retinal pigment epithelial cells is sufficient to induce photoreceptor degeneration. Elife. 2016;5. pii: e14319. doi: 10.7554/eLife.14319.
Hughes WF. Quantitation of ischemic damage in the rat retina. Exp Eye Res. 1991;53(5):573–82.
Ames A 3rd. CNS energy metabolism as related to function. Brain Res Brain Res Rev. 2000;34(1-2):42–68.
Okai Y, Higashi-Okai K. F Sato E, Konaka R, Inoue M. Potent radical-scavenging activities of thiamin and thiamin diphosphate. J Clin Biochem Nutr. 2007;40(1):42–8.
Berrone E, Beltramo E, Solimine C, Ape AU, Porta M. Regulation of intracellular glucose and polyol pathway by thiamine and benfotiamine in vascular cells cultured in high glucose. J Biol Chem. 2006;281(14):9307–13.
Dagher Z, Park YS, Asnaghi V, Hoehn T, Gerhardinger C, Lorenzi M. Studies of rat and human retinas predict a role for the polyol pathway in human diabetic retinopathy. Diabetes. 2004;53(9):2404–11.
Luong KV, Nguyen LT. The impact of thiamine treatment in the diabetes mellitus. J Clin Med Res. 2012;4(3):153–60.
Pacal L, Kuricova K, Kankova K. Evidence for altered thiamine metabolism in diabetes: Is there a potential to oppose gluco- and lipotoxicity by rational supplementation? World J Diabetes. 2014;5(3):288–95.
Booth AA, Khalifah RG, Hudson BG. Thiamine pyrophosphate and pyridoxamine inhibit the formation of antigenic advanced glycation end-products: comparison with aminoguanidine. Biochem Biophys Res Commun. 1996;220(1):113–9.
Thiamin-Health Professional Fact Sheet. National Institutes of Health.2020. https://ods.od.nih.gov/factsheets/Thiamin-HealthProfessional/. Accessed 19 Feb 2020.
Raj V, Ojha S, Howarth FC, Belur PD, Subramanya SB. Therapeutic potential of benfotiamine and its molecular targets. Eur Rev Med Pharmacol Sci. 2018;22(10):3261–73.
Stirban A, Negrean M, Stratmann B, Gawlowski T, Horstmann T, Gotting C, et al. Benfotiamine prevents macro- and microvascular endothelial dysfunction and oxidative stress following a meal rich in advanced glycation end products in individuals with type 2 diabetes. Diabetes Care. 2006;29(9):2064–71.
Riboflavin-Health Professional Fact Sheet. National Institutes of Health.2020. https://ods.od.nih.gov/factsheets/Riboflavin-HealthProfessional/. Accessed 6 Mar 2020.
Srivastav K, Saxena S, Mahdi AA, Shukla RK, Meyer CH, Akduman L, et al. Increased serum level of homocysteine correlates with retinal nerve fiber layer thinning in diabetic retinopathy. Mol Vis. 2016;22:1352–60.
Alam MM, Iqbal S, Naseem I. Ameliorative effect of riboflavin on hyperglycemia, oxidative stress and DNA damage in type-2 diabetic mice: Mechanistic and therapeutic strategies. Arch Biochem Biophys. 2015;584:10–9.
Naghashpour M, Amani R, Sarkaki A, Ghadiri A, Samarbafzadeh A, Jafarirad S, et al. Brain-derived neurotrophic and immunologic factors: beneficial effects of riboflavin on motor disability in murine model of multiple sclerosis. Iran J Basic Med Sci. 2016;19(4):439–48.
Liu W, Han X, Zhou X, Zhang S, Cai X, Zhang L, et al. Brain derived neurotrophic factor in newly diagnosed diabetes and prediabetes. Mol Cell Endocrinol. 2016;429:106–13.
Niacin-Health Professional Fact Sheet. National Institutes of Health. 2020. https://ods.od.nih.gov/factsheets/Niacin-HealthProfessional/. Accessed 6 Mar 2020.
Kuvin JT, Ramet ME, Patel AR, Pandian NG, Mendelsohn ME, Karas RH. A novel mechanism for the beneficial vascular effects of high-density lipoprotein cholesterol: enhanced vasorelaxation and increased endothelial nitric oxide synthase expression. Am Heart J. 2002;144(1):165–72.
Domanico D, Verboschi F, Altimari S, Zompatori L, Vingolo EM. Ocular effects of niacin: a review of the literature. Med Hypothesis Discov Innov Ophthalmol. 2015;4(2):64–71.
Yan T, Chopp M, Ye X, Liu Z, Zacharek A, Cui Y, et al. Niaspan increases axonal remodeling after stroke in type 1 diabetes rats. Neurobiol Dis. 2012;46(1):157–64.
Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–41.
Gaynon MW, Paulus YM, Rahimy E, Alexander JL, Mansour SE. Effect of oral niacin on central retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2017;255(6):1085–92.
Pantothenic Acid-Health Professional Fact Sheet. 2020. https://ods.od.nih.gov/factsheets/PantothenicAcid-HealthProfessional/. Accessed 2 Mar 2020.
Demirci B, Demir O, Dost T, Birincioglu M. Protective effect of vitamin B5 (dexpanthenol) on cardiovascular damage induced by streptozocin in rats. Bratisl Lek Listy. 2014;115(4):190–6.
Vitamin B6-Health Professional Fact Sheet.2020. https://ods.od.nih.gov/factsheets/VitaminB6-HealthProfessional/. Accessed 24 Feb 2020.
Morris MS, Picciano MF, Jacques PF, Selhub J. Plasma pyridoxal 5'-phosphate in the US population: the National Health and Nutrition Examination Survey, 2003-2004. Am J Clin Nutr. 2008;87(5):1446–54.
Hoogeveen EK, Kostense PJ, Eysink PE, Polak BC, Beks PJ, Jakobs C, et al. Hyperhomocysteinemia is associated with the presence of retinopathy in type 2 diabetes mellitus: the Hoorn study. Arch Intern Med. 2000;160(19):2984–90.
Kowluru RA, Mohammad G, Sahajpal N. Faulty homocysteine recycling in diabetic retinopathy. Eye Vis (Lond). 2020;7:4.
Rubi B. Pyridoxal 5'-phosphate (PLP) deficiency might contribute to the onset of type I diabetes. Med Hypotheses. 2012;78(1):179–82.
Merigliano C, Mascolo E, Burla R, Saggio I, Verni F. The relationship between vitamin B6, diabetes and cancer. Front Genet. 2018;9:388.
Horikawa C, Aida R, Kamada C, Fujihara K, Tanaka S, Tanaka S, et al. Vitamin B6 intake and incidence of diabetic retinopathy in Japanese patients with type 2 diabetes: analysis of data from the Japan Diabetes Complications Study (JDCS). Eur J Nutr. 2019. https://doi.org/10.1007/s00394-019-02014-4.
Vrolijk MF, Opperhuizen A, Jansen EHJM, Hageman GJ, Bast A, Haenen GRMM. The vitamin B6 paradox: Supplementation with high concentrations of pyridoxine leads to decreased vitamin B6 function. Toxicol In Vitro. 2017;44:206–12.
Haeusler RA, Camastra S, Astiarraga B, Nannipieri M, Anselmino M, Ferrannini E. Decreased expression of hepatic glucokinase in type 2 diabetes. Mol Metab. 2015;4(3):222–6.
Xiang X, Liu Y, Zhang X, Zhang W, Wang Z. Effects of biotin on blood glucose regulation in type 2 diabetes rat model. Wei Sheng Yan Jiu. 2015;44(2):185–9, 195.
Sugita Y, Shirakawa H, Sugimoto R, Furukawa Y, Komai M. Effect of biotin treatment on hepatic gene expression in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem. 2008;72(5):1290–8.
Young MC, Saddoughi SA, Aho JM, Harmsen WS, Allen MS, Blackmon SH, et al. Comparison of laparoscopic versus open surgical management of Morgagni hernia. Ann Thorac Surg. 2019;107(1):257–61.
Biotin-Health Professional Fact Sheet. National Institutes of Health. 2020. https://ods.od.nih.gov/factsheets/Biotin-HealthProfessional/. Accessed 19 Feb 2020.
Folate-Health Professional Fact Sheet. National Institutes of Health. 2020. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/. Accessed 11 Mar 2020.
Singh M, Tyagi SC. Homocysteine mediates transcriptional changes of the inflammatory pathway signature genes in human retinal pigment epithelial cells. Int J Ophthalmol. 2017;10(5):696–704.
Rosolova H, Simon J, Mayer O Jr, Racek J, Dierze T, Jacobsen DW. Unexpected inverse relationship between insulin resistance and serum homocysteine in healthy subjects. Physiol Res. 2002;51(1):93–8.
Bar-On H, Kidron M, Friedlander Y, Ben-Yehuda A, Selhub J, Rosenberg IH, et al. Plasma total homocysteine levels in subjects with hyperinsulinemia. J Intern Med. 2000;247(2):287–94.
Meigs JB, Jacques PF, Selhub J, Singer DE, Nathan DM, Rifai N, et al. Fasting plasma homocysteine levels in the insulin resistance syndrome: the Framingham offspring study. Diabetes Care. 2001;24(8):1403–10.
van der Put NM, Gabreels F, Stevens EM, Smeitink JA, Trijbels FJ, Eskes TK, et al. A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet. 1998;62(5):1044–51.
Yigit S, Karakus N, Inanir A. Association of MTHFR gene C677T mutation with diabetic peripheral neuropathy and diabetic retinopathy. Mol Vis. 2013;19:1626–30.
Maeda M, Fujio Y, Azuma J. MTHFR gene polymorphism and diabetic retinopathy. Curr Diabetes Rev. 2006;2(4):467–76.
Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014;44(5):480–8.
Bozard BR, Ganapathy PS, Duplantier J, Mysona B, Ha Y, Roon P, et al. Molecular and biochemical characterization of folate transport proteins in retinal Muller cells. Invest Ophthalmol Vis Sci. 2010;51(6):3226–35.
Huang W, Prasad PD, Kekuda R, Leibach FH, Ganapathy V. Characterization of N5-methyltetrahydrofolate uptake in cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1997;38(8):1578–87.
Ibrahim AS, Mander S, Hussein KA, Elsherbiny NM, Smith SB, Al-Shabrawey M, et al. Hyperhomocysteinemia disrupts retinal pigment epithelial structure and function with features of age-related macular degeneration. Oncotarget. 2016;7(8):8532–45.
Reynolds EH. What is the safe upper intake level of folic acid for the nervous system? Implications for folic acid fortification policies. Eur J Clin Nutr. 2016;70(5):537–40.
Selhub J, Rosenberg IH. Excessive folic acid intake and relation to adverse health outcome. Biochimie. 2016;126:71–8.
Devnath GP, Kumaran S, Rajiv R, Shaha KK, Nagaraj A. Fatal folic acid toxicity in humans. J Forensic Sci. 2017;62(6):1668–70.
Levy BI, Schiffrin EL, Mourad JJ, Agostini D, Vicaut E, Safar ME, et al. Impaired tissue perfusion: a pathology common to hypertension, obesity, and diabetes mellitus. Circulation. 2008;118(9):968–76.
Muir ER, Renteria RC, Duong TQ. Reduced ocular blood flow as an early indicator of diabetic retinopathy in a mouse model of diabetes. Invest Ophthalmol Vis Sci. 2012;53(10):6488–94.
Fonseca VA, Lavery LA, Thethi TK, Daoud Y, DeSouza C, Ovalle F, et al. Metanx in type 2 diabetes with peripheral neuropathy: a randomized trial. Am J Med. 2013;126(2):141–9.
Woo KS, Chook P, Chan LL, Cheung AS, Fung WH, Qiao M, et al. Long-term improvement in homocysteine levels and arterial endothelial function after 1-year folic acid supplementation. Am J Med. 2002;112(7):535–9.
McMullin MF, Young PB, Bailie KE, Savage GA, Lappin TR, White R. Homocysteine and methylmalonic acid as indicators of folate and vitamin B12 deficiency in pregnancy. Clin Lab Haematol. 2001;23(3):161–5.
Weikert C, Dierkes J, Hoffmann K, Berger K, Drogan D, Klipstein-Grobusch K, et al. B vitamin plasma levels and the risk of ischemic stroke and transient ischemic attack in a German cohort. Stroke. 2007;38(11):2912–8.
Spence JD, Bang H, Chambless LE, Stampfer MJ. Vitamin Intervention For Stroke Prevention trial: an efficacy analysis. Stroke. 2005;36(11):2404–9.
Fotiou P, Raptis A, Apergis G, Dimitriadis G, Vergados I, Theodossiadis P. Vitamin status as a determinant of serum homocysteine concentration in type 2 diabetic retinopathy. J Diabetes Res. 2014;2014:807209.
Metz J. Cobalamin deficiency and the pathogenesis of nervous system disease. Annu Rev Nutr. 1992;12:59–79.
Ankar A, Kumar A. Vitamin B12 Deficiency (Cobalamin). Treasure Island: StatPearls; 2018.
Braun DJ, Abner E, Bakshi V, Goulding DS, Grau EM, Lin AL, et al. Blood flow deficits and cerebrovascular changes in a dietary model of hyperhomocysteinemia. ASN Neuro. 2019;11:1759091419865788. https://doi.org/10.1177/1759091419865788.
Gopinath B, Wang JJ, Flood VM, Burlutsky G, Wong TY, Mitchell P. The associations between blood levels of homocysteine, folate, vitamin B12, and retinal vascular caliber. Am J Ophthalmol. 2009;148(6):902–9.
Lee I, Lee H, Kim JM, Chae EH, Kim SJ, Chang N. Short-term hyperhomocysteinemia-induced oxidative stress activates retinal glial cells and increases vascular endothelial growth factor expression in rat retina. Biosci Biotechnol Biochem. 2007;71(5):1203–10.
Memisogullari R, Yuksel H, Coskun A, Yuksel HK, Yazgan O, Bilgin C. High serum homocysteine levels correlate with a decrease in the blood flow velocity of the ophthalmic artery in highway toll collectors. Tohoku J Exp Med. 2007;212(3):247–52.
Xu C, Wu Y, Liu G, Liu X, Wang F, Yu J. Relationship between homocysteine level and diabetic retinopathy: a systematic review and meta-analysis. Diagn Pathol. 2014;9:167.
Koyama K, Usami T, Takeuchi O, Morozumi K, Kimura G. Efficacy of methylcobalamin on lowering total homocysteine plasma concentrations in haemodialysis patients receiving high-dose folic acid supplementation. Nephrol Dial Transplant. 2002;17(5):916–22.
Wu MY, Yiang GT, Lai TT, Li CJ. The oxidative stress and mitochondrial dysfunction during the pathogenesis of diabetic retinopathy. Oxid Med Cell Longev. 2018;2018:3420187.
Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, et al. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25(4):397–424.
Nghiem AZ, Nderitu P, Lukic M, Khatun M, Largan R, Kortuem K, et al. Comparing diabetic retinopathy lesions in scanning laser ophthalmoscopy and colour fundus photography. Acta Ophthalmol. 2019;97(8):e1035–40.
Rathod RS, Khaire AA, Kale AA, Joshi SR. Effect of vitamin B12 and omega-3 fatty acid supplementation on brain neurotrophins and cognition in rats: a multigeneration study. Biochimie. 2016;128-129:201–8.
Rocco ML, Balzamino BO, Esposito G, Petrella C, Aloe L, Micera A. NGF/anti-VEGF combined exposure protects RCS retinal cells and photoreceptors that underwent a local worsening of inflammation. Graefes Arch Clin Exp Ophthalmol. 2017;255(3):567–74.
Allen LH. Causes of vitamin B12 and folate deficiency. Food Nutr Bull. 2008;29(2 Suppl):S20–34.
Vitamin B12-Health Professional Fact Sheet. National Institutes of Health. 2020. https://ods.od.nih.gov/factsheets/VitaminB12-HealthProfessional/. Accessed 30 Mar 2020.
Langan RC, Goodbred AJ. Vitamin B12 deficiency: recognition and management. Am Fam Physician. 2017;96(6):384–9.
Butler CC, Vidal-Alaball J, Cannings-John R, McCaddon A, Hood K, Papaioannou A, et al. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: a systematic review of randomized controlled trials. Fam Pract. 2006;23(3):279–85.
Chan CQ, Low LL, Lee KH. Oral vitamin B12 replacement for the treatment of pernicious anemia. Front Med (Lausanne). 2016;3:38.
Eussen SJ, de Groot LC, Clarke R, Schneede J, Ueland PM, Hoefnagels WH, et al. Oral cyanocobalamin supplementation in older people with vitamin B12 deficiency: a dose-finding trial. Arch Intern Med. 2005;165(10):1167–72.
Spence JD. Metabolic vitamin B12 deficiency: a missed opportunity to prevent dementia and stroke. Nutr Res. 2016;36(2):109–16.
Martin SC, Rauz S, Marr JE, Martin N, Jones AF, Dodson PM. Plasma total homocysteine and retinal vascular disease. Eye (Lond). 2000;14(Pt 4):590–3.
Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging. 2002;6(1):39–42.
Liu H, Tang J, Lee CA, Kern TS. Metanx and early stages of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2015;56(1):647–53.
Smolek MK, Notaroberto NF, Jaramillo AG, Pradillo LR. Intervention with vitamins in patients with nonproliferative diabetic retinopathy: a pilot study. Clin Ophthalmol. 2013;7:1451–8.
Wang J, Brown C, Shi C, Townsend J, Gameiro GR, Wang P. Improving diabetic and hypertensive retiopathy with a medical food containing L-Methylfolate: a preliminary report. Eye Vis (Lond). 2019;6:21.
Vitamin C-Health Professional Fact Sheet. National Institutes of Health. 2020. https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/. Accessed 27 Feb 2020.
Shang F, Lu M, Dudek E, Reddan J, Taylor A. Vitamin C and vitamin E restore the resistance of GSH-depleted lens cells to H2O2. Free Radic Biol Med. 2003;34(5):521–30.
Guan Y, Dai P, Wang H. Effects of vitamin C supplementation on essential hypertension: A systematic review and meta-analysis. Medicine (Baltimore). 2020;99(8):e19274.
Thosar SS, Bielko SL, Wiggins CC, Klaunig JE, Mather KJ, Wallace JP. Antioxidant vitamin C prevents decline in endothelial function during sitting. Med Sci Monit. 2015;21:1015–21.
Park SW, Ghim W, Oh S, Kim Y, Park UC, Kang J, et al. Association of vitreous vitamin C depletion with diabetic macular ischemia in proliferative diabetic retinopathy. PLoS One. 2019;14(6):e0218433.
Gurreri A, Pazzaglia A, Schiavi C. Role of statins and ascorbic acid in the natural history of diabetic retinopathy: a new, affordable therapy? Ophthalmic Surg Lasers Imaging Retina. 2019;50(5):S23–7.
Parva NR, Tadepalli S, Singh P, Qian A, Joshi R, Kandala H, et al. Prevalence of vitamin D deficiency and associated risk factors in the US population (2011-2012). Cureus. 2018;10(6):e2741.
Vitamin D-Health Professional Fact Sheet. National Institutes of Health. 2020. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/. Accessed 24 Mar 2020.
Jin KW, Ro JW, Shin YJ, Hyon JY, Wee WR, Park SG. Correlation of vitamin D levels with tear film stability and secretion in patients with dry eye syndrome. Acta Ophthalmol. 2017;95(3):e230–5.
Byun YS, Yoo YS, Kwon JY, Joo JS, Lim SA, Whang WJ, et al. Diquafosol promotes corneal epithelial healing via intracellular calcium-mediated ERK activation. Exp Eye Res. 2016;143:89–97.
Eloranta JJ, Zair ZM, Hiller C, Hausler S, Stieger B, Kullak-Ublick GA. Vitamin D3 and its nuclear receptor increase the expression and activity of the human proton-coupled folate transporter. Mol Pharmacol. 2009;76(5):1062–71.
Alam C, Aufreiter S, Georgiou CJ, Hoque MT, Finnell RH, O'Connor DL, et al. Upregulation of reduced folate carrier by vitamin D enhances brain folate uptake in mice lacking folate receptor alpha. Proc Natl Acad Sci U S A. 2019;116(35):17531–40.
Perez-Lopez FR. Vitamin D: the secosteroid hormone and human reproduction. Gynecol Endocrinol. 2007;23(1):13–24.
Carvalho JTG, Schneider M, Cuppari L, Grabulosa CC, Aoike T, BM QR, et al. Cholecalciferol decreases inflammation and improves vitamin D regulatory enzymes in lymphocytes in the uremic environment: A randomized controlled pilot trial. PLoS One. 2017;12(6):e0179540.
Dong Y, Stallmann-Jorgensen IS, Pollock NK, Harris RA, Keeton D, Huang Y, et al. A 16-week randomized clinical trial of 2000 international units daily vitamin D3 supplementation in black youth: 25-hydroxyvitamin D, adiposity, and arterial stiffness. J Clin Endocrinol Metab. 2010;95(10):4584–91.
Witham MD, Dove FJ, Dryburgh M, Sugden JA, Morris AD, Struthers AD. The effect of different doses of vitamin D(3) on markers of vascular health in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2010;53(10):2112–9.
Sergeev IN, Rhoten WB. 1,25-Dihydroxyvitamin D3 evokes oscillations of intracellular calcium in a pancreatic beta-cell line. Endocrinology. 1995;136(7):2852–61.
Kramer CK, Swaminathan B, Hanley AJ, Connelly PW, Sermer M, Zinman B, et al. Prospective associations of vitamin D status with beta-cell function, insulin sensitivity, and glycemia: the impact of parathyroid hormone status. Diabetes. 2014;63(11):3868–79.
Long M, Wang C, Liu D. Glycated hemoglobin A1C and vitamin D and their association with diabetic retinopathy severity. Nutr Diabetes. 2017;7(6):e281.
Rashidi B, Hoseini Z, Sahebkar A, Mirzaei H. Anti-Atherosclerotic Effects of Vitamins D and E in Suppression of Atherogenesis. J Cell Physiol. 2017;232(11):2968–76.
Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79(5):820–5.
Bonakdaran S, Rokni H. Diabetic CVD--focus on vitamin D. Cardiovasc Hematol Agents Med Chem. 2012;10(3):241–50.
Boucher BJ. Vitamin D insufficiency and diabetes risks. Curr Drug Targets. 2011;12(1):61–87.
Li X, Liu Y, Zheng Y, Wang P, Zhang Y. The effect of vitamin D supplementation on glycemic control in type 2 diabetes patients: a systematic review and meta-analysis. Nutrients. 2018;10(3). pii: E375. doi: https://doi.org/10.3390/nu10030375.
Lee CJ, Iyer G, Liu Y, Kalyani RR, Bamba N, Ligon CB, et al. The effect of vitamin D supplementation on glucose metabolism in type 2 diabetes mellitus: a systematic review and meta-analysis of intervention studies. J Diabetes Complications. 2017;31(7):1115–26.
Mutlu U, Ikram MA, Hofman A, de Jong PT, Uitterlinden AG, Klaver CC, et al. Vitamin D and retinal microvascular damage: The Rotterdam Study. Medicine (Baltimore). 2016;95(49):e5477.
Holick MF. Vitamin D deficiency. N Eng J Med. 2007;357(3):266–81.
Yu Y, Tian L, Xiao Y, Huang G, Zhang M. Effect of vitamin D supplementation on some inflammatory biomarkers in type 2 diabetes mellitus subjects: a systematic review and meta-analysis of randomized controlled trials. Ann Nutr Metab. 2018;73(1):62–73.
Millen AE, Sahli MW, Nie J, LaMonte MJ, Lutsey PL, Klein BE, et al. Adequate vitamin D status is associated with the reduced odds of prevalent diabetic retinopathy in African Americans and Caucasians. Cardiovasc Diabetol. 2016;15(1):128.
Vitamin E-Health Professional Fact Sheet. National Institutes of Health. 2020. https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/. Accessed 28 Feb 2020.
Sato K, Niki E, Shimasaki H. Free radical-mediated chain oxidation of low density lipoprotein and its synergistic inhibition by vitamin E and vitamin C. Arch Biochem Biophys. 1990;279(2):402–5.
Rozanowska M, Edge R, Land EJ, Navaratnam S, Sarna T, Truscott TG. Scavenging of retinoid cation radicals by Urate, Trolox, and alpha-, beta-, gamma-, and delta-tocopherols. Int J Mol Sci. 2019;20(11). pii: E2799. doi: https://doi.org/10.3390/ijms20112799.
Boshtam M, Rafiei M, Sadeghi K, Sarraf-Zadegan N. Vitamin E can reduce blood pressure in mild hypertensives. Int J Vitam Nutr Res. 2002;72(5):309–14.
Emami MR, Safabakhsh M, Alizadeh S, Asbaghi O, Khosroshahi MZ. Effect of vitamin E supplementation on blood pressure: a systematic review and meta-analysis. J Hum Hypertens. 2019;33(7):499–507.
Rafraf M, Bazyun B, Sarabchian MA, Safaeiyan A, Ghaemmaghami Hezaveh SJ. Impact of vitamin E supplementation on blood pressure and Hs-CRP in type 2 diabetic patients. Health Promot Perspect. 2012;2(1):72–9.
Liu L, Quang ND, Banu R, Kumar H, Tham YC, Cheng CY, et al. Hypertension, blood pressure control and diabetic retinopathy in a large population-based study. PLoS One. 2020;15(3):e0229665.
Bursell SE, Clermont AC, Aiello LP, Aiello LM, Schlossman DK, Feener EP, et al. High-dose vitamin E supplementation normalizes retinal blood flow and creatinine clearance in patients with type 1 diabetes. Diabetes Care. 1999;22(8):1245–51.
Chatziralli IP, Theodossiadis G, Dimitriadis P, Charalambidis M, Agorastos A, Migkos Z, et al. The effect of vitamin E on oxidative stress indicated by serum malondialdehyde in insulin-dependent type 2 diabetes mellitus patients with retinopathy. Open Ophthalmol J. 2017;11:51–8.
Kunisaki M, Bursell SE, Clermont AC, Ishii H, Ballas LM, Jirousek MR, et al. Vitamin E prevents diabetes-induced abnormal retinal blood flow via the diacylglycerol-protein kinase C pathway. Am J Physiol. 1995;269(2 Pt 1):E239–46.
Stoyanovsky DA, Goldman R, Darrow RM, Organisciak DT, Kagan VE. Endogenous ascorbate regenerates vitamin E in the retina directly and in combination with exogenous dihydrolipoic acid. Curr Eye Res. 1995;14(3):181–9.
Burton GW, Traber MG, Acuff RV, Walters DN, Kayden H, Hughes L, et al. Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am J Clin Nutr. 1998;67(4):669–84.
Derouiche S, Kechrid Z. Zinc supplementation overcomes effects of copper on zinc status, carbohydrate metabolism and some enzyme activities in diabetic and nondiabetic rats. Can J Diabetes. 2016;40(4):342–7.
Brand IA, Kleineke J. Intracellular zinc movement and its effect on the carbohydrate metabolism of isolated rat hepatocytes. J Biol Chem. 1996;271(4):1941–9.
Zinc-Health Professional Fact Sheet. National Institutes of Health. 2020. https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/. Accessed 6 Mar 2020.
Miao X, Sun W, Miao L, Fu Y, Wang Y, Su G, et al. Zinc and diabetic retinopathy. J Diabetes Res. 2013;2013:425854.
Prasad AS. Discovery of human zinc deficiency: its impact on human health and disease. Adv Nutr. 2013;4(2):176–90.
Luo YY, Zhao J, Han XY, Zhou XH, Wu J, Ji LN. Relationship between serum zinc level and microvascular complications in patients with type 2 diabetes. Chin Med J (Engl ). 2015;128(24):3276–82.
Sun Q, van Dam RM, Willett WC, Hu FB. Prospective study of zinc intake and risk of type 2 diabetes in women. Diabetes Care. 2009;32(4):629–34.
Adachi Y, Yoshida J, Kodera Y, Kiss T, Jakusch T, Enyedy EA, et al. Oral administration of a zinc complex improves type 2 diabetes and metabolic syndromes. Biochem Biophys Res Commun. 2006;351(1):165–70.
Solmonson A, DeBerardinis RJ. Lipoic acid metabolism and mitochondrial redox regulation. J Biol Chem. 2018;293(20):7522–30.
Berg JM, Tymoczko JL, Stryer L. Biochemistry. 5th ed. New York: WH Freeman & Co; 2002.
Park S, Karunakaran U, Jeoung NH, Jeon JH, Lee IK. Physiological effect and therapeutic application of alpha lipoic acid. Curr Med Chem. 2014;21(32):3636–45.
Voloboueva LA, Liu J, Suh JH, Ames BN, Miller SS. (R)-alpha-lipoic acid protects retinal pigment epithelial cells from oxidative damage. Invest Ophthalmol Vis Sci. 2005;46(11):4302–10.
Chidlow G, Schmidt KG, Wood JP, Melena J, Osborne NN. Alpha-lipoic acid protects the retina against ischemia-reperfusion. Neuropharmacology. 2002;43(6):1015–25.
Ansar H, Mazloom Z, Kazemi F, Hejazi N. Effect of alpha-lipoic acid on blood glucose, insulin resistance and glutathione peroxidase of type 2 diabetic patients. Saudi Med J. 2011;32(6):584–8.
Xiang GD, Sun HL, Zhao LS, Hou J, Yue L, Xu L. The antioxidant alpha-lipoic acid improves endothelial dysfunction induced by acute hyperglycaemia during OGTT in impaired glucose tolerance. Clin Endocrinol (Oxf). 2008;68(5):716–23.
Kan E, Alici O, Kan EK, Ayar A. Effects of alpha-lipoic acid on retinal ganglion cells, retinal thicknesses, and VEGF production in an experimental model of diabetes. Int Ophthalmol. 2017;37(6):1269–78.
Gebka A, Serkies-Minuth E, Raczynska D. Effect of the administration of alpha-lipoic acid on contrast sensitivity in patients with type 1 and type 2 diabetes. Mediators Inflamm. 2014;2014:131538.
Nguyen N, Takemoto JK. A case for alpha-lipoic acid as an alternative treatment for diabetic polyneuropathy. J Pharm Pharm Sci. 2018;21(1s):177s–91s.
Pei Y, Liu H, Yang Y, Yang Y, Jiao Y, Tay FR, et al. Biological Activities and Potential Oral Applications of N-Acetylcysteine: Progress and Prospects. Oxid Med Cell Longev. 2018;2018:2835787.
Aoyama K, Wang F, Matsumura N, Kiyonari H, Shioi G, Tanaka K, et al. Increased neuronal glutathione and neuroprotection in GTRAP3-18-deficient mice. Neurobiol Dis. 2012;45(3):973–82.
Schimel AM, Abraham L, Cox D, Sene A, Kraus C, Dace DS, et al. N-acetylcysteine amide (NACA) prevents retinal degeneration by up-regulating reduced glutathione production and reversing lipid peroxidation. Am J Pathol. 2011;178(5):2032–43.
Atkuri KR, Mantovani JJ, Herzenberg LA, Herzenberg LA. N-Acetylcysteine--a safe antidote for cysteine/glutathione deficiency. Curr Opin Pharmacol. 2007;7(4):355–9.
Kerksick C, Willoughby D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J Int Soc Sports Nutr. 2005;2:38–44.
Mohamed R, Sharma I, Ibrahim AS, Saleh H, Elsherbiny NM, Fulzele S, et al. Hyperhomocysteinemia alters retinal endothelial cells barrier function and angiogenic potential via activation of oxidative stress. Sci Rep. 2017;7(1):11952.
Loscalzo J. Homocysteine-mediated thrombosis and angiostasis in vascular pathobiology. J Clin Invest. 2009;119(11):3203–5.
Zhu Y, Zhang XL, Zhu BF, Ding YN. Effect of antioxidant N-acetylcysteine on diabetic retinopathy and expression of VEGF and ICAM-1 from retinal blood vessels of diabetic rats. Mol Biol Rep. 2012;39(4):3727–35.
Carver KA, Yang D. N-acetylcysteine amide protects against oxidative stress-induced microparticle release from human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2016;57(2):360–71.
Yang L, Tan P, Zhou W, Zhu X, Cui Y, Zhu L, et al. N-acetylcysteine protects against hypoxia mimetic-induced autophagy by targeting the HIF-1alpha pathway in retinal ganglion cells. Cell Mol Neurobiol. 2012;32(8):1275–85.
Lei H, Velez G, Cui J, Samad A, Maberley D, Matsubara J, et al. N-acetylcysteine suppresses retinal detachment in an experimental model of proliferative vitreoretinopathy. Am J Pathol. 2010;177(1):132–40.
Craig SA. Betaine in human nutrition. Am J Clin Nutr. 2004;80(3):539–49.
Choline-Health Professional Fact Sheet. National Institutes of Health. 2020. https://ods.od.nih.gov/factsheets/Choline-HealthProfessional/. Accessed 24 Feb 2020.
Drews K, Rozycka A, Barlik M, Klejewski A, Kurzawinska G, Wolski H, et al. Polymorphic variants of genes involved in choline pathway and the risk of intrauterine fetal death. Ginekol Pol. 2017;88(4):205–11.
Zhao G, He F, Wu C, Li P, Li N, Deng J, et al. Betaine in Inflammation: Mechanistic Aspects and Applications. Front Immunol. 2018;9:1070.
Barak AJ, Beckenhauer HC, Tuma DJ. Betaine, ethanol, and the liver: a review. Alcohol. 1996;13(4):395–8.
Obeid R. The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients. 2013;5(9):3481–95.
Chiuve SE, Giovannucci EL, Hankinson SE, Zeisel SH, Dougherty LW, Willett WC, et al. The association between betaine and choline intakes and the plasma concentrations of homocysteine in women. Am J Clin Nutr. 2007;86(4):1073–81.
Rajaie S, Esmaillzadeh A. Dietary choline and betaine intakes and risk of cardiovascular diseases: review of epidemiological evidence. ARYA Atheroscler. 2011;7(2):78–86.
Wilcken DE, Wilcken B, Dudman NP, Tyrrell PA. Homocystinuria--the effects of betaine in the treatment of patients not responsive to pyridoxine. N Engl J Med. 1983;309(8):448–53.
Olthof MR, van Vliet T, Boelsma E, Verhoef P. Low dose betaine supplementation leads to immediate and long term lowering of plasma homocysteine in healthy men and women. J Nutr. 2003;133(12):4135–8.
Elsherbiny NM, Sharma I, Kira D, Alhusban S, Samra YA, Jadeja R, et al. Homocysteine Induces inflammation in retina and brain. Biomolecules. 2020;10(3). pii: E393. doi: 10.3390/biom10030393.
Roybal CN, Yang S, Sun CW, Hurtado D, Vander Jagt DL, Townes TM, et al. Homocysteine increases the expression of vascular endothelial growth factor by a mechanism involving endoplasmic reticulum stress and transcription factor ATF4. J Biol Chem. 2004;279(15):14844–52.
Aydin E, Demir HD, Ozyurt H, Etikan I. Association of plasma homocysteine and macular edema in type 2 diabetes mellitus. Eur J Ophthalmol. 2008;18(2):226–32.
Brazionis L, Rowley K Sr, Itsiopoulos C, Harper CA, O'Dea K. Homocysteine and diabetic retinopathy. Diabetes Care. 2008;31(1):50–6.
Omae T, Nagaoka T, Tanano I, Yoshida A. Homocysteine inhibition of endothelium-dependent nitric oxide-mediated dilation of porcine retinal arterioles via enhanced superoxide production. Invest Ophthalmol Vis Sci. 2013;54(3):2288–95.
den Heijer M, Brouwer IA, Bos GM, Blom HJ, van der Put NM, Spaans AP, et al. Vitamin supplementation reduces blood homocysteine levels: a controlled trial in patients with venous thrombosis and healthy volunteers. Arterioscler Thromb Vasc Biol. 1998;18(3):356–61.
Ansari R, Mahta A, Mallack E, Luo JJ. Hyperhomocysteinemia and neurologic disorders: a review. J Clin Neurol. 2014;10(4):281–8.
ter Borg S, Verlaan S, Hemsworth J, Mijnarends DM, Schols JM, Luiking YC, et al. Micronutrient intakes and potential inadequacies of community-dwelling older adults: a systematic review. Br J Nutr. 2015;113(8):1195–206.
Bird JK, Murphy RA, Ciappio ED, McBurney MI. Risk of deficiency in multiple concurrent micronutrients in children and adults in the United States. Nutrients. 2017;9(7). pii: E655. doi: https://doi.org/10.3390/nu9070655.
Blumberg JB, Frei BB, Fulgoni VL, Weaver CM, Zeisel SH. Impact of frequency of multi-vitamin/multi-mineral supplement intake on nutritional adequacy and nutrient deficiencies in U.S. adults. Nutrients. 2017;9(8). pii: E849. doi: https://doi.org/10.3390/nu9080849.
Iwakawa H, Nakamura Y, Fukui T, Fukuwatari T, Ugi S, Maegawa H, et al. Concentrations of water-soluble vitamins in blood and urinary excretion in patients with diabetes mellitus. Nutr Metab Insights. 2016;9:85–92.
Said HM. Intestinal absorption of water-soluble vitamins in health and disease. Biochem J. 2011;437(3):357–72.
Kiela PR, Ghishan FK. Physiology of intestinal absorption and secretion. Best Pract Res Clin Gastroenterol. 2016;30(2):145–59.
Zielinska-Dawidziak M, Grajek K, Olejnik A, Czaczyk K, Grajek W. Transport of high concentration of thiamin, riboflavin and pyridoxine across intestinal epithelial cells Caco-2. J Nutr Sci Vitaminol (Tokyo). 2008;54(6):423–9.
Porter K, Hoey L, Hughes CF, Ward M, McNulty H. Causes, consequences and public health implications of low B-vitamin status in ageing. Nutrients. 2016;8(11). pii: E725. doi: https://doi.org/10.3390/nu8110725.
Kennedy DO, Veasey R, Watson A, Dodd F, Jones E, Maggini S, et al. Effects of high-dose B vitamin complex with vitamin C and minerals on subjective mood and performance in healthy males. Psychopharmacology (Berl). 2010;211(1):55–68.
Shibata K, Sugita C, Sano M, Fukuwatari T. Urinary excretion of B-group vitamins reflects the nutritional status of B-group vitamins in rats. J Nutr Sci. 2013;2:e12.
Clarke R, Grimley EJ, Schneede J, Nexo E, Bates C, Fletcher A, et al. Vitamin B12 and folate deficiency in later life. Age Ageing. 2004;33(1):34–41.
Hughes CF, Ward M, Hoey L, McNulty H. Vitamin B12 and ageing: current issues and interaction with folate. Ann Clin Biochem. 2013;50(Pt 4):315–29.
Huang P, Wang F, Sah BK, Jiang J, Ni Z, Wang J, et al. Homocysteine and the risk of age-related macular degeneration: a systematic review and meta-analysis. Sci Rep. 2015;5:10585.
Biesemeier A, Taubitz T, Julien S, Yoeruek E, Schraermeyer U. Choriocapillaris breakdown precedes retinal degeneration in age-related macular degeneration. Neurobiol Aging. 2014;35(11):2562–73.
Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex. Mol Aspects Med. 2012;33(4):295–317.
Christen WG, Glynn RJ, Chew EY, Albert CM, Manson JE. Folic acid, pyridoxine, and cyanocobalamin combination treatment and age-related macular degeneration in women: the Women's Antioxidant and Folic Acid Cardiovascular Study. Arch Intern Med. 2009;169(4):335–41.
Saposnik G, Ray JG, Sheridan P, McQueen M, Lonn E. Homocysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40(4):1365–72.
FAQs About Medical Foods-FDA. U.S. Food and Drug Administration. 2016. https://www.fda.gov/media/97726/download; Accessed May 2016.
Starr R. Should states and local governments regulate dietary supplements? Drug Test Anal. 2016;8(3-4):402–6.
Brown AC. An overview of herb and dietary supplement efficacy, safety and government regulations in the United States with suggested improvements. Part 1 of 5 series. Food Chem Toxicol. 2017;107(Pt A):449–71.
Marcus DM. Dietary supplements: What's in a name? What's in the bottle? Drug Test Anal. 2016;8(3-4):410–2.
Anahad O'connor. New York Attorney General Targets Supplements at Major Retailers. 2015. https://well.blogs.nytimes.com/2015/02/03/new-york-attorney-general-targets-supplements-at-major-retailers/. Accessed 3 Feb 2015.
Guidance for Industry: Frequently Asked Questions About Medical Foods; Second Edition. 2007. https://www.fda.gov/media/97726/download; Accessed 13 May 2007.
METANX® Capsules. 2017. https://www.metanx.com/pdf/METANXCapsulesPIStatement.pdf. Accessed Feb 2017.
Kemse N, Kale A, Chavan-Gautam P, Joshi S. Increased intake of vitamin B12, folate, and omega-3 fatty acids to improve cognitive performance in offspring born to rats with induced hypertension during pregnancy. Food Funct. 2018;9(7):3872–83.
Okada K, Tanaka H, Temporin K, Okamoto M, Kuroda Y, Moritomo H, et al. Methylcobalamin increases Erk1/2 and Akt activities through the methylation cycle and promotes nerve regeneration in a rat sciatic nerve injury model. Exp Neurol. 2010;222(2):191–203.
Label (PDF)-FDA. U.S. Food and Drug Administration. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020576s014lbl.pdf.; Accessed Oct 2018.
Wang J, Brown C, Shi C, Townsend J, Gameiro GR, Wang P, et al. Improving diabetic and hypertensive retinopathy with a medical food containing L-methylfolate: a preliminary report. Eye Vis (Lond). 2019;6:21.
Schmidl D, Werkmeister R, Szegedi S, Bata A, Stjepanek K, Puchner S, Garhofer G. The effect of folate supplementation on systemic homecysteine plasma concentration and ocular blood flow in patients with diabetes. In: Schweizerische Opthalmologische Gesellschaft (SOG) Annual conference. Friborg, Switzerland; 2018.
Acknowledgments
Not applicable.
Funding
Grant/financial support: The work was supported by the NIH Center Grant P30, EY014801, NINDS 1R01NS111115-01 (Wang), and a grant from Research to Prevent Blindness (RPB).
Author information
Authors and Affiliations
Contributions
CS, PW, SA, CB, ZL, JHT, JW and HJ searched and collected references, CS, PW, SA, CB, JW and HJ were the major contributors for writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Dr. Brown holds an ownership interest in Global Healthcare Focus, a small nutraceutical company concerned with developing products (including OcufolinTM) to improve health. Other authors have no proprietary interest in any materials or methods.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shi, C., Wang, P., Airen, S. et al. Nutritional and medical food therapies for diabetic retinopathy. Eye and Vis 7, 33 (2020). https://doi.org/10.1186/s40662-020-00199-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40662-020-00199-y