Abstract
Background
Research on prostate cancer is mostly performed using cell lines derived from metastatic disease, not reflecting stages of tumor initiation or early progression. Establishment of cancer cell lines derived from the primary tumor site has not been described so far. By definition, cancer cells are able to be cultured indefinitely, whereas normal epithelial cells undergo senescence in vitro. Epithelial cells can be immortalized, accomplished by using viral integration of immortalization factors. Viral approaches, however, might be impaired by regulatory and safety issues as well as random integration into regulatory genetic elements, modifying precise gene expression. We intend to use surgical specimen of prostate cancer patients to (i) prove for establishment of cancer cell lines, and (ii) perform non-viral, Sleeping Beauty (SB) transposase-based immortalization of prostate epithelial cells.
Methods
Radical prostatectomy samples of prostate cancer patients (n = 4) were dissociated and cultured in vitro. Cells were cultivated either without or after non-viral, Sleeping-Beauty transposase-based stable transfection with immortalization factors SV40LT and hTERT. Established cell lines were analyzed in vitro and in vivo for characteristics of prostate (cancer) cells.
Results
Initial cell cultures without genetic manipulation underwent senescence within ≤ 15 passages, demonstrating inability to successfully derive primary prostate cancer cell lines. By using SB transposase-based integration of immortalization factors, we were able to establish primary prostate cell lines. Three out of four cell lines displayed epithelial characteristics, however without expression of prostate (cancer) characteristics, e.g., androgen receptor. In vivo, one cell line exhibited tumorigenic potential, yet characteristics of prostate adenocarcinoma were absent.
Conclusion
Whereas no primary prostate cancer cell line could be established, we provide for the first-time immortalization of primary prostate cells using the SB transposase system, thereby preventing regulatory and molecular issues based on viral immortalization approaches. Although, none of the newly derived cell lines demonstrated prostate cancer characteristics, tumor formation was observed in one cell line. Given the non-prostate adenocarcinoma properties of the tumor, cells have presumably undergone oncogenic transformation rather than prostate cancer differentiation. Still, these cell lines might be used as a tool for research on prostate cancer initiation and early cancer progression.
Similar content being viewed by others
Background
With 1.4 million new cases in 2020, prostate cancer (PCa) is the second most common tumor among men worldwide, and is responsible for 6.8% of cancer-related deaths [1]. Although incidentally high, little is known about the process of tumor induction. A set of genetic alterations has been described to be involved in initiation of prostate adenocarcinomas, e.g., mutations of SPOP1 or FOXA1, loss of RB1, fusion of TMPRRS2 and ERG or aberrant activation of the androgen receptor (AR) [2,3,4,5]. Given the heterogeneity within primary prostate cancer in terms of genotype and differentiation, an orchestrated, yet not uniformly, process of mutational gene activation or inactivation is likely.
Research on cancer initiation has a long history of using cell lines derived from primary tumors. Whereas several cancer cell lines of other solid tumors like colon or breast cancer are implemented and widely deployed in carcinoma research, primary prostate cancer cell lines are not available [6,7,8]. However, research on primary prostate cancer requires models mirroring the early stage of disease, either right before tumor initiation or upon transformation into prostate cancer cells [9]. The lack of preclinical primary cancer models is a major drawback of prostate cancer research. Most cell lines used in prostate cancer research, e.g., LNCaP, VCaP, or PC-3 cell lines, are derived from metastatic tumor samples, not reflecting the initial stage of prostate cancer. Hence, research on early prostate cancer models is challenging.
One way to circumvent challenges of primary prostate cancer cell derivation might be genetic manipulation of healthy prostate epithelial cells to transform these cells into carcinogenic prostate cancer cells by using known driver or initiation mutations. However, healthy primary epithelial cells are unable to be cultured in vitro indefinitely. To establish long term culture of cell lines, primary cells need to undergo a process of immortalization, thereby preventing a limited lifespan and senescence of cells [10]. Most of these approaches rely on viral integration of immortalization factors, e.g., simian virus 40 large T antigen (SV40LT) or human telomerase reverse transcriptase (hTERT). Although efficient, viral integration approaches need certain organizational circumstances, including regulatory and safety issues. Additionally, viral integration is of risk of targeting either gene expression regulatory elements or coding regions of expressed genes. Both might lead to alteration of gene expression [11].
An alternative to viral integration is the application of non-viral transposon-based integration. The Sleeping Beauty (SB) transposable system originates from a reconstructed Tc1/mariner-type transposon [12]. This system relies on site specific DNA integration through transposase-transposon interaction followed by restriction and integration of a DNA fragment into cellular DNA. In comparison to viral integration, SB integrates into actively transcribed genes less frequently [13]. Furthermore, by standard transfection of two vectors, one coding the SB transposase and another coding the gene of interest, no advanced safety or security levels are needed for treatment of cells. Immortalization using the SB system has been described in primary pig fibroblasts [14]. Yet, primary epithelial cells have not been immortalized using the SB system.
Aim of this study was to establish primary prostate cell lines derived from radical prostatectomy specimen by two different methodological approaches: (i) direct derivation from primary human prostate cancer cells, or (ii) non-viral transposon-based immortalization of epithelial prostate cells.
Methods
Patients
Patient samples were obtained from n = 4 patients diagnosed with localized prostate adenocarcinomas undergoing prostatectomy in the Department of Urology, University Hospital Münster. The local ethical committee approved the study (2007-467-f-S), and all patients gave informed consent. Native prostatic tissue was examined and processed by a pathologist. The prostatic capsules were intact and seminal vesicles, apex and vesical parts of the prostates were identified. According to preoperative results of prostatic biopsies, samples of prostatic tissue were obtained from anatomical sites of carcinoma-positive biopsy and stored in HBSS buffer (Sigma-Aldrich, Pasching, Germany) at 4 °C until further processing.
Tissue dissociation and initial cell culture
Tissue samples were minced into small (> 2 mm3) pieces and incubated with Dispase/Collagenase IV (Stemcell, Vancouver, Canada) in DMEM/F12 (Sigma-Aldrich) with 5% FCS (Thermo Fisher Scientific, Waltham, MA, USA). On the next day, cells were centrifuged, and pellets were washed with DPBS (Sigma-Aldrich). Afterwards, tissue samples were digested in 5 × Trypsin/DPBS (Sigma-Aldrich) for 1 h at 4 °C followed by 1 U/mL Dispase (Stemcell) in DMEM/F-12 incubation for 2 min. Cells were passed through a 100 μm cell strainer and flow through was resuspended in 3 ml PCPM (DMEM/F12, 5% FCS, 200 mM L-glutamine, Penicillin-Streptamycin, 1 μg/ml Charybdotoxin, 200 μM Hydrocortisone, 20 mg/ml Adenine (all Sigma-Aldrich), 10 μM Y-27632 (Miltenyi Biotec, Bergisch Gladbach, Germany), 12.5 mg/ml insulin (PAN Biotech, Aidenbach, Germany), 1 mg/ml hEGF (Sigma-Aldrich)) and seeded into 12-well plates.
Cloning of immortalization factors into pSBbi vectors
Plasmids (pCMV(CAT)T7-SB100 (#34879), pSBbi-GN (#60517), pSBbi-RP (#60513), pBABE-neo-hTERT (#1774), pBABE-neo largeTcDNA (#1780) were purchased from Addgene. cDNAs were PCR amplified and cloned into the pSBbI vectors via either directional SfiI cloning for hTERT [12] or by cloning using the In-Fusion® HD Cloning Kit (Clontech, Mountain View, CA, USA) for SV40 large T antigen (SV40LT). Primer sequences are listed in Additional file 2: Table S2. Immortalization vectors contained either hTERT or SV40LT under the control of the EF1α promotor as well as a GFP/RFP-2A-puromycin/neomycin selection cassette under the control of the synthetic RPBSA promoter (Additional file 1: Figure S1).
Electroporation of immortalization factors and antibiotic selection
Electroporation followed the Amaxa™ Basic Epithelial Cells Nucleofector™ Kit (Lonza, Cologne, Germany) protocol, using program T0-13. 6 × 105 cells were utilized for electroporation of 2 µg total DNA (0.1 µg pCMV(CAT)T7-SB100 plus either 1.9 µg SV40LT or hTERT plasmids in single experiments or 850 ng of both immortalization plasmids in combinatorial approaches). Upon electroporation, cells were reseeded in 6-well plates. Antibiotic selection was administered 5–7 days post-electroporation using 1 mg/ml G418 (PAN Biotech) and/or 1 µg/ml puromycin (PAN Biotech). G418 was administered for 15–20 days, whereas puromycin treatment lasted for 5–7 days.
Cell culture
The human metastases derived prostate cancer cell lines 22Rv1, LNCaP and PC-3 were purchased from the Leibniz-Institute DSMZ GmbH (Braunschweig, Germany). The human benign prostate epithelial cell line RWPE-1 was purchased from ATCC (Manassas, VA, USA). All cell lines were cultured under matching protocols at 37 °C and 5% CO2. Media was purchased from Sigma-Aldrich. Trypsin–EDTA, phosphate-buffered saline and FCS were purchased from Thermo Fisher Scientific. Hormonal treatment was performed using 10 nM R1881 (Sigma-Aldrich) or DMSO (Applichem, Darmstadt, Germany).
Immunofluorescence and flow cytometry
For immunofluorescence analysis, cells were fixated with 4% formaldehyde (Sigma-Aldrich) in DPBS and permeabilized using 1% Triton-X (Sigma-Aldrich) in PBS. Blocking was performed using 1% BSA (Sigma-Aldrich) in PBS. Primary antibodies were pan-cytokeratin (KRT), clone MNF116, 1:1.00, epithelial cell adhesion molecule (EpCAM), clone EPR20532-225, 1:500 (both Abcam, Cambridge, UK); AR, clone D6F11, 1:600 (Cell Signaling, Danvers, MA, USA) along with respective secondary, conjugated antibodies (1:500, Thermo Fisher Scientific). DAPI (Sigma-Aldrich) was used as DNA staining solution.
For flow cytometry analysis 1 × 105—1 × 106 cells were stained in 0.5% BSA/PBS antibody dilution buffer and labelled with the respective antibodies (CD49f-Pacific Blue, clone GoH3, 1:200; CD26-APC, clone BA5b, both Biolegend, Amsterdam, The Netherlands) and analyzed on a FACS Aria II device. Unstained cells were used as a control. Analysis was performed using FlowJo™ 10.
Analysis of integration and expression of immortalization factors
Genomic DNA (gDNA) was isolated using the NEB Monarch® Genomic DNA Purification Kit (New England Biolabs (NEB), Ipswich, MA, USA). PCR for analysis of integration was performed using primers located within the respective integration cassette. Primers targeting endogenous GAPDH were used as control. For analysis of immortalization factor expression, total RNA was isolated using the RNeasy® Mini Kit (Qiagen, Hilden, Germany) following the manufactures guide. 500 ng of total RNA were reverse transcribed using the Primescript® Reverse Transcription Kit (Takara, Tokyo, Japan). qPCR runs for SV40LT and hTERT expression analysis were performed along with primers for housekeeping genes RPL37A and ACTB. qPCR reactions were run using the PowerUp™ SYBR™ Green Master Mix (Thermo Fisher Scientific) on a QuantStudio 3 cycler (Thermo Fisher Scientific). Primer sequences are listed in Additional file 2: Table S2.
RNA sequencing
Library preparation of total RNA was performed with the NEB Next Ultra II RNA directional Kit and single read sequencing was performed using a NextSeqR® 2000 System with a read length of 72 bp. Using a molecular barcode, the samples were demultiplexed (bcl2fastq2) to fastq data and quality controlled (FastQC). Trimmomatic was used for adapter trimming and read filtering [15]. The resulting reads were aligned to the Ensembl GRCh38 reference genome using Hisat2 [16]. The aligned reads were sorted using samtools [17]. The sorted and aligned reads were counted into genes using htsec-counts [18]. The test for differential expression were performed using the r-package deseq2 [19]. Principal component analysis was executed using ClustVis [20].
Luciferase assay
For determination of AR activity using a luciferase assay, cells were co-transfected with an androgen receptor responsive elements (ARE) Firefly luciferase reporter plasmid (pGL3-4xARE-E4-luc, a gift from Dr. M Carey, Department of Biological Chemistry, UCLA, USA) and Renilla luciferase transfection control plasmid (pRL-TK, Promega, Madison, WI, USA). Subsequently, cells were cultured in presence of either 10 nM R1881 or DMSO.
Dual luciferase reporter assays were performed according to the manufacturer’s protocol using the Dual-Glo® Luciferase Assay System (Promega) on a Varioskan Lux microplate reader (Thermo Fisher). Firefly luciferase activity was normalized to Renilla luciferase activity. Transcriptional activation of Firefly luciferase reporter in R1881 treated cells was presented as luciferase activity relative to the DMSO control.
In vivo experiments
In vivo assays were performed at the Max-Planck Institute for Molecular Biomedicine, Münster, Germany. 5 × 106 cells of MS-pPC-191ST, MS-pPC-192S, MS-pPC-193S, MS-pPC-193ST and 22Rv1 were injected subcutaneously into the nuchal fold of SCID (severe combined immunodeficient) mice along with a 1:1 mixture of PCPM and Matrigel® (Corning, NY, USA). 22Rv1 mice were sacrificed after 2 weeks when the tumor reached a growth of about 2 cm3. MS-pPC-193ST mice were sacrificed after 14 weeks, at this time point three out of four mice displaying a palpable tumor tissue at the site of injection. All other mice were sacrificed after 16 weeks.
Pathological analysis
After tumor resection, the specimens were macroscopically examined by a pathologist, fixated in 4% buffered paraformaldehyde and sectioned. Preparation for histological examination, including dehydration, paraffin embedding and hematoxylin and eosin (HE) staining were carried out due to standard protocols at the Gerhard-Domagk-Institute, University Hospital Münster. Histological examination was performed by two pathologists (A.K and S.H.).
Immunohistochemistry was done at the Gerhard-Domagk-Institute using the automated Ventana BenchMark ULTRA IHC staining system (Roche Diagnostics, Basel, Switzerland) according to the manufacturer’s instructions. Concisely, 3 µm sections from paraffin-embedded tissue were deparaffinized and pre-treated with Cell Conditioning 1 solution (CC1, Ventana/Roche, Basel, Switzerland) for 24–64 min at 95–100 °C. Subsequently, incubation with primary antibodies (AR, clone SP107, CellMarque, Rocklin, CA, USA; SV40LT, clone PAb101-412, Invitrogen, Waltham, MA, USA) was implemented for 16–32 min at 36 °C. Visualization of immunoreaction was done via Optiview DAB IHC Detection Kit. Tissue sections were counterstained with hematoxylin and blueing solution (all Ventana/Roche).
STR genotyping and authentication
The STR profiling technique took place at the DSMZ under ISO-9001 certified conditions, according to guidelines of the global standard ANSI/ATCC ASN-0002.1-2021 (2021). PCR reaction was performed using 1–2 ng of gDNA with a fluorescent primer set according to ASN-0002.1 and analyzed using capillary separator Genome Lab GenExp Genetic Analysis System (SciEx, Darmstadt, Germany). Allele calling of fragments was carried out by subjecting the reaction products to Genetic Fragment Analyzer software (BeckmannCoulter) and obtained STR profiles were compared for uniqueness in the international STR database using the online tool of the DSMZ (https://celldive.dsmz.de) [21]. A defined search algorithm was also used to check whether cell lines with related profiles or a mix of STR profiles exist. The genetic identity of the established cell lines was authenticated by matching the STR profiles of respective cell lines with generated STR profiles of tumor tissue from FFPE.
Results
Patient characteristics
Patient characteristics are given in Additional file 2: Table S1. All patients had pathologically confirmed prostate adenocarcinomas displayed by HE and nuclear AR immunohistochemical analysis (Fig. 1A).
Patients radical prostatectomy tissue samples and early cell cultures. A Upper panel: Hematoxylin and eosin staining shows acinar and glandular adenocarcinoma of the prostate with predominantly poorly formed and fused glands as well as microglandular pattern, preponderantly according to Gleason ‘s Pattern 4 (magnification: 10x; bars represent 200 µm). Lower panel: Immunohistochemical staining of AR showing a nuclear positivity in atypical glands in all samples (magnification: 10x; bars represent 200 µm). B Initial cell cultures of RPE specimen, inlays show senescence of cell cultures without genetic treatment (Bars represent 100 µm)
Initial cell culture of prostate specimen
Cell cultures obtained by mechanical and enzymatical dissociation were cultivated until reaching confluency and passaged routinely. All four cell cultures (MS-pPC-184, MS-pPC-191, MS-pPC-192 and MS-pPC-193) reached a state of cellular senescence within passages 11–15 without any further cellular proliferation (Fig. 1B). Thus, our approach to establish primary prostate cancer cell lines from human primary prostatic cancer tissue, failed.
Immortalization of primary prostate cells
In parallel, primary epithelial cell cultures were electroporated at about passage 4–7 using different immortalization factor combinations, i.e., SV40LT only (MS-pPC-184S, MS-pPC-191S, MS-pPC-192S; MS-pPC-193S), hTERT only (MS-pPC-184 T) and SV40LT and hTERT (MS-pPC-184ST, MS-pPC-191ST, MS-pPC-193ST).
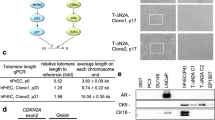
MS-pPC-184S, -T and -ST cells displayed senescence within passages 15, 15 and 20, respectively, demonstrating no successful immortalization of MS-pPC-184 specimen. MS-pPC-191S showed senescence at passage 45, whereas -191ST could be passaged further (> 100 passages). Furthermore, MS-pPC-192S, MS-pPC-193S and -193ST showed no signs of senescence. These four cell cultures (MS-pPC-191ST, MS-pPC-192S, MS-pPC-193S and MS-pPC-193ST) acquired common characteristics of cell lines, i.e., unique cell morphology and growth characteristics (Fig. 2A). Stable integration was determined by PCR on gDNA of electroporated cells. Amplicons span the immortalization cassette including integration factors hTERT or SV40LT, EF1α promotor, RPBSA promotor and RFP or GFP, respectively (Fig. 2B, Additional file 1: Figure S1). Additionally, immortalized cell lines showed both SV40LT and hTERT expression, whereas parental cell cultures were negative for both factors (Fig. 2C, D). These results indicate a successful immortalization of primary prostatic epithelial cells into four different cell lines.
Immortalization of cell lines by stable integration. A Established immortalized cell lines at high passage numbers (Bars represent 100 µm). B Genomic integration of immortalization displayed by PCR on gDNA of immortalized cell lines. C Expression of immortalization factors on mRNA level. D Expression of immortalization factors on protein level (Bars represent 50 µm)
Characterization of primary epithelial cell cultures
For authentication, primary cell cultures and immortalized cell lines were analyzed for consistency with patient tumor tissue. Therefore, short tandem repeat (STR) analyses on both, cell lines and matching tumor tissues was performed (Additional file 2: Table S3). All cell lines showed identical STR profiles compared to patients’ tumor tissues. Furthermore, none of the primary cell lines showed contamination with established cell lines used in the laboratory. Thus, we demonstrate successful establishment of cell lines derived from matched patient samples.
Next, we investigated expression of epithelial prostate (cancer) markers KRT, AR and EpCAM (Fig. 3A). KRT and EpCAM expression was detected in MS-pPC-192S, 193S and 193ST, whereas 191ST were negative for both markers. Additionally, none of these cell lines displayed expression of AR. Benign prostate epithelial cell line RWPE-1 showed similar expression pattern, with KRT and EpCAM, but no AR expression (Fig. 3B). In contrast, LNCaP—a metastasis-derived prostate cancer cell line—showed expression of all markers. These results suggest an epithelial, yet not prostate cancer related origin of cell lines MS-pPC-192S, 193S and 193ST. When analyzing AR activity in response to hormonal induction using luciferase assays, we noticed a drastic increase in AR activity in LNCaP cells, whereas no changes in activity were observed in newly derived cell lines (Fig. 3C).
Characterization of newly established cell lines. A, B Immunofluorescence analysis of prostate and epithelial markers KRT, AR and EpCAM in immortalized cell lines (A) and in control prostate cancer cell line LNCaP and benign prostate epithelial cell line RWPE-1 (B) (Bars represent 100 µm). C AR activity upon hormonal treatment in LNCaP and newly derived cell lines using Luciferase assay. D Flow cytometry analysis of luminal (CD26) and basal (CD49f) prostate markers. E RNAseq based principal component analysis of cell lines compared to prostate cancer cell lines LNCaP and PC-3 as well as benign prostate epithelial cell line RWPE-1
Thereafter, we performed flow cytometry analysis for expression of basal (CD49f) and luminal (CD26) markers (Fig. 3D). No cell line displayed a sole luminal expression pattern but either a mixture of both markers or basal marker only. Subsequently, we performed RNAseq analysis of four cell lines along with prostate (cancer) cell lines RWPE-1, LNCaP and PC-3. Principal component analysis (PCA) demonstrated closest proximity of MS-pPC-193S, -ST and -192S to RWPE-1 cell line (Fig. 3E). MS-pPC-191ST showed less proximity, although closer to RWPE-1 than any other reference cell line. Prostate cancer metastasis derived cell lines PC-3 and LNCaP cell lines showed high distance to immortalized cell lines. These results suggest that immortalized cell lines reflect healthy prostate cells rather than metastasis derived prostate cancer cells.
In vivo analysis
Given that the resected tissue originating from prostatectomy specimens presumably containing both, normal epithelial and prostate cells, we aimed to analyze the oncogenic capability of cell lines by subcutaneous injection into male SCID mice. Highly tumorigenic 22Rv1 prostate cancer cells served as control. 22Rv1 cells showed tumor development within 2 weeks in all 5 mice injected. Three cell lines (MS-pPC-191ST, MS-pPC-192S and MS-pPC-193S) did not show any signs of tumor development within 16 weeks, while the fourth cell line, MS-pPC-193ST showed tumor formation (approx. 1 cm3) 14 weeks after injection in three out of four mice (Fig. 4A).
In vivo tumor formation ability of immortalized cell lines. A Tumors derived via injection of prostate cancer cell line 22Rv1 and newly derived cell line MS-pPC-193ST in SCID mice. 22Rv1 tumors and MS-pPC-193ST tumors were resected 2 weeks and 14 weeks after injection, respectively (Bars represent 1 cm). B Hematoxylin and eosin staining (upper panel) revealed prostatic adenocarcinoma in 22Rv1 tumors, whereas MS-pPC-193ST tumors displayed squamous differentiation. Middle panel: 22Rv1 showed strong nuclear AR expression, whereas no AR expression was observed in MS-pPC-193ST tumors. Lower panel: MS-pPC-193ST tumors displayed nuclear expression of SV40LT, which was absent in 22Rv1 tumors (magnification: 10X; bars represent 200 µm)
Macroscopically, 22Rv1 tumors appeared elastic with visible blood vessels and focal bleedings. Contrary, MS-pPC-193ST derived tumors displayed higher stiffness without observable vascularity.
Histological examination of 22Rv1 tumors revealed an adenocarcinoma in a mainly solid growth pattern containing few cribriform glands (Fig. 4B). Immunohistochemically, 22Rv1 tumors showed a strong nuclear positivity for AR, resembling a prostate adenocarcinoma. HE staining of MS-pPC-193ST derived resections displayed a tumor with squamous differentiation with multilayered intertwined cords and keratinized areas. Nuclei showed marked atypia, e.g. hyperchromasia. No specific staining was observed for AR, while tumor cells exhibited a strong nuclear positivity for SV40LT, demonstrating MS-pPC-193ST cells as origin of tumors. Additionally, STR profiling of tumor DNA revealed origin of tumor from matched patient sample derived cells (MS-pPC-193ST; Additional file 2: Table S3). These results indicate immortalization, yet not oncogenic transformation in MS-pPC-191ST, 192S and 193S cell lines. In MS-pPC-193ST, cells underwent both immortalization and oncogenic transformation. However, oncogenic transformation does not mimic prostate cancer due to absence of immunohistochemical and molecular prostate cancer characteristics.
Discussion
Although prostate cancer occurs with high incidence worldwide, causal research is hampered by the lack of early-stage prostate cancer models. In this study, we aimed to establish primary cancer cell lines derived from prostate cancer specimen. Given the complexity of primary prostate cancer cell line derivation, we also intended to apply a non-viral based immortalization of prostate epithelial cells.
Whereas we were not able to establish primary prostate cancer cell lines from human primary prostate cancer, we demonstrated for the first time successful immortalization of four cell lines derived from human prostatic tissue using the non-viral Sleeping Beauty transposase system. Thereby, we circumvent safety regulatory issues as well as viral-based risk of alteration of regulatory elements or actively transcribed gene expression using viral integration. The newly derived cell lines might be used as a preclinical model for stages of either prostate cancer initiation or early localized prostate cancer progression.
Research on prostate cancer is mostly performed using cell lines derived from metastatic tissue samples rather than primary prostate cancer. A recent review describes four primary prostate cancer cell lines, i.e., 1013 L, UM-SCP-1, PSK-1 and PPC-1 [22]. However, these lines represent either non-adenocarcinomas of the prostate (1013 L (primary prostate urothelial carcinoma), UM-SCP-1 (prostatic squamous cell carcinoma), a Klinefelter syndrome patient derived cell line representing a rare prostate small cell carcinoma (PSK-1)) or have been described being a derivative of established metastasis derived PC-3 cell line (PPC-1) [23, 24]. The most widely used cell lines, e.g., LNCaP, VCaP, PC-3 or 22Rv1, are derived either from distant metastases (LNCaP, supraclavicular lymph node metastasis; VCaP, vertebral metastatic lesion; PC-3, bone metastasis) or from xenografts, passaged in mice after castration-induced regression and relapse of the parental, androgen-dependent CWR22 xenograft (22Rv1). Thus, none of these cell lines mimics the early stage of prostate cancer.
Regarding our initial aim, we were not able to establish primary prostate cancer cell lines demonstrated by senescence of cell cultures at early passages. Additionally, none of the established cell lines did show prostate cancer specific characteristics., e.g., AR or solely luminal gene expression. Moreover, none of the cell lines possessed the capability to form prostate adenocarcinoma in vivo. Our results are in line with reports demonstrating derivation of primary prostate patient derived organoids from healthy epithelial, yet not prostate cancer cells [25, 26]. It is well established, that tumor microenvironment plays an important role for tumorigenesis and cancer progression, a fact that is also known for prostate adenocarcinoma [27, 28]. The problem not yet overcome in establishment of primary prostate cancer cell lines is the assumed dependency of primary prostate cancer cells on the tumor environment including adjacent prostate glands, prostate stroma and circulatory supply of hormones and growth factors, limiting their growth ability in isolated cell culture [29, 30].
Primary epithelial cells are not capable of innumerable cell divisions but rather reach a state of cellular senescence, thereby impeding long term research. Immortalization of these cells has been used as a remedy of early proliferation termination. Viral integration is the most widely used approach for stable expression of immortalization factors. We now present for the first time the non-viral, SB based immortalization of human prostatic tissue derived epithelial cell lines. Integration of both SV40LT and a combination of SV40LT and hTERT led to immortalization of cell lines demonstrated by high proliferative capacity without any signs of senescence for more than 100 passages. Using this system, we present an alternative to viral integration—even in primary epithelial cells—circumventing the risks and issues of usage of viral approaches, e.g., alteration of the cellular transcriptional machinery, posttranscriptional deregulation of gene expression and risk factor environments, e.g., safety levels of laboratories.
Comprehensive characterization of the newly established cell lines by RNA sequencing revealed highest similarity to prostate epithelial cell line RWPE-1 rather than prostate cancer cell lines. Supposing both cancer and normal prostate cells within the initial tissue sample, we surmise that cancer cells have been overgrown by non-cancer, epithelial cells due to the lack of cancer cell supporting factors as well as higher capacity of non-cancer, epithelial cells to adapt to cell culture conditions. However, prostate epithelial cells might have lost some of their original characteristics. Although lack of AR expression points to a more basal rather than luminal phenotype, flow cytometry analysis of both basal and luminal marker genes, i.e. CD26 and CD49f, respectively, did neither demonstrate specific basal nor luminal subpopulations. Thus, we hypothesize acquisition of both basal and luminal characteristics during adaption to cell culture conditions. One cell line, MS-pPC-191ST, showed characteristics of neither epithelial cells, e.g., EpCAM or KRT expression nor prostate cancer cells. Morphologically, this cell line resembled a non-epithelial, presumably mesenchymal phenotype.
Interestingly, one cell line (MS-pPC-193ST) showed tumor formation capacity in SCID mice, a main characteristic of oncogenic cell lines [31]. Whereas mice tumor derived tissue displayed matching STR profile with patient tissue, it did not reflect neither patient tumor characteristics nor any other characteristics of prostate adenocarcinomas. Hence, we hypothesize that integration and strong expression levels of both SV40LT and hTERT, led to immortalization and highly proliferative capacity, which finally caused acquisition of mutations causing oncogenic transformation resulting in a tumor-bearing cell line. This cell line, however, has been merely transformed into a tumorigenic epithelial cell line displaying squamous differentiation and showed no histological resemblance to prostatic adenocarcinoma tissue. Oncogenic transformation of primary epithelial cells by SV40LT, hTERT and the H-ras oncogene, resulting in altered cellular differentiation not fully resembling the tissue of origin, has also been described in mammary cells [31, 32]. In these cells, stable expression of the H-ras oncoprotein led to tumor formation in mice. However, tumor formation resulted in poorly differentiated carcinomas, suggesting an oncogenic transformation due to stable expression of the H-ras oncogene. Decisively, MS-pPC-193ST and the aforementioned MS-pPC-191ST cell lines should not be regarded as primary prostate cancer cell lines, whereas two cell lines MS-pPC-192S and MS-pPC-193S are definitive prostate epithelial cell lines, providing a tool for early prostate cancer research.
A limitation of our study is the usage of SV40LT as immortalization factor. This oncoprotein is involved in p53 and pRB inactivation to avoid cell cycle arrest [33]. The tumor suppressor gene TP53—the most altered gene in human cancer—is involved in cell cycle arrest, DNA repair and regulation of apoptotic genes [34]. Additionally, inactivation of the Rb protein has been described being a driver event in early prostate cancer progression [35]. Thus, inactivation of p53 and Rb by SV40LT might be of risk of introducing artificial tumorigenic alterations into normal cells. In future approaches, we intend to apply CRISPR-Cas9 technology to excise the integrated immortalization cassette. Given that this study was aimed to proof the concept of primary epithelial cell line establishment by using the non-viral integrating Sleeping Beauty transposase system, we did not perform excision of integrated immortalization factors.
For initiation and early progression of primary prostate cancer, several driver mutations or alterations have been described e.g., inactivating SPOP mutations, activating FOXA1 mutations, TMPRRS-ERG fusion, loss of PTEN or AR alterations. The newly derived cell lines offer the possibility of research on prostate cancer initiation and early progression as well as analyses of early drug treatment approaches. Thus, we intend to introduce targeted mutations in combination with reprogramming and/or differentiation strategies into organoids to provide insights into the development and evolution of prostate cancer.
Conclusion
In this study, we aimed to establish primary prostate cancer and prostate epithelial cells lines as basic research models for early prostate cancer. We were not able to derive primary prostate cancer cell lines, a problem not yet overcome, demonstrating challenge to specifically cultivate prostate cancer rather than prostate epithelial cells in vitro. Though, for the first time, we provide evidence for non-viral based immortalization of primary prostate cells using the SB transposase system. Thereby, both regulatory and molecular issues based on viral immortalization approaches might be circumvented. Although, none of these newly derived cell lines displayed prostate cancer characteristics, future approaches, e.g., targeted mutation of SPOP, FOXA1 or PTEN, which have been discussed as prostate cancer driver alterations, offer a tool for research on prostate cancer initiation and early cancer progression.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AR:
-
Androgen receptor
- ARE:
-
Androgen receptor responsive element
- cDNA:
-
Complementary DNA
- EpCAM:
-
Epithelial cell adhesion molecule
- gDNA:
-
Genomic DNA
- GFP:
-
Green fluorescent protein
- hTERT:
-
Human telomerase reverse transcriptase
- KRT:
-
Cytokeratin
- PCA:
-
Principal component analysis
- PCa:
-
Prostate cancer
- PCR:
-
Polymerase chain reaction
- RFP:
-
Red fluorescent protein
- SB:
-
Sleeping beauty
- SCID:
-
Severe combined immunodeficient
- STR:
-
Short tandem repeats
- SV40LT:
-
Simian virus 40 large T antigen
References
Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Pineros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021. https://doi.org/10.1002/ijc.33588.
Tomlins SA, Laxman B, Dhanasekaran SM, Helgeson BE, Cao X, Morris DS, et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 2007;448(7153):595–9.
Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012;44(6):685–9.
Baca SC, Prandi D, Lawrence MS, Mosquera JM, Romanel A, Drier Y, et al. Punctuated evolution of prostate cancer genomes. Cell. 2013;153(3):666–77.
Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470(7333):214–20.
Ahmed D, Eide PW, Eilertsen IA, Danielsen SA, Eknaes M, Hektoen M, et al. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis. 2013;2(9):e71.
Ku JL, Shin YK, Kim DW, Kim KH, Choi JS, Hong SH, et al. Establishment and characterization of 13 human colorectal carcinoma cell lines: mutations of genes and expressions of drug-sensitivity genes and cancer stem cell markers. Carcinogenesis. 2010;31(6):1003–9.
Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10(6):515–27.
Zhao Z, Fowle H, Valentine H, Liu Z, Tan Y, Pei J, et al. Immortalization of human primary prostate epithelial cells via CRISPR inactivation of the CDKN2A locus and expression of telomerase. Prostate Cancer Prostatic Dis. 2021;24(1):233–43.
Lundberg AS, Randell SH, Stewart SA, Elenbaas B, Hartwell KA, Brooks MW, et al. Immortalization and transformation of primary human airway epithelial cells by gene transfer. Oncogene. 2002;21(29):4577–86.
Moiani A, Paleari Y, Sartori D, Mezzadra R, Miccio A, Cattoglio C, et al. Lentiviral vector integration in the human genome induces alternative splicing and generates aberrant transcripts. J Clin Invest. 2012;122(5):1653–66.
Kowarz E, Loscher D, Marschalek R. Optimized sleeping beauty transposons rapidly generate stable transgenic cell lines. Biotechnol J. 2015;10(4):647–53.
Yant SR, Wu X, Huang Y, Garrison B, Burgess SM, Kay MA. High-resolution genome-wide mapping of transposon integration in mammals. Mol Cell Biol. 2005;25(6):2085–94.
He S, Li Y, Chen Y, Zhu Y, Zhang X, Xia X, et al. Immortalization of pig fibroblast cells by transposon-mediated ectopic expression of porcine telomerase reverse transcriptase. Cytotechnology. 2016;68(4):1435–45.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–20.
Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–60.
Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27(21):2987–93.
Anders S, Pyl PT, Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31(2):166–9.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.
Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 2015;43(W1):W566–70.
Koblitz J, Dirks WG, Eberth S, Nagel S, Steenpass L, Pommerenke C. DSMZCellDive: diving into high-throughput cell line data. F1000Res. 2022;11:420.
Sailer V, von Amsberg G, Duensing S, Kirfel J, Lieb V, Metzger E, et al. Experimental in vitro, ex vivo and in vivo models in prostate cancer research. Nat Rev Urol. 2023;20(3):158–78.
Capes-Davis A, Theodosopoulos G, Atkin I, Drexler HG, Kohara A, MacLeod RA, et al. Check your cultures! A list of cross-contaminated or misidentified cell lines. Int J Cancer. 2010;127(1):1–8.
van Bokhoven A, Varella-Garcia M, Korch C, Hessels D, Miller GJ. Widely used prostate carcinoma cell lines share common origins. Prostate. 2001;47(1):36–51.
Karthaus WR, Iaquinta PJ, Drost J, Gracanin A, van Boxtel R, Wongvipat J, et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell. 2014;159(1):163–75.
Drost J, Karthaus WR, Gao D, Driehuis E, Sawyers CL, Chen Y, et al. Organoid culture systems for prostate epithelial and cancer tissue. Nat Protoc. 2016;11(2):347–58.
Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–22.
Ge R, Wang Z, Cheng L. Tumor microenvironment heterogeneity an important mediator of prostate cancer progression and therapeutic resistance. NPJ Precis Oncol. 2022;6(1):31.
Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO classification of tumours of the urinary system and male genital organs-part b: prostate and bladder tumours. Eur Urol. 2016;70(1):106–19.
Andreoiu M, Cheng L. Multifocal prostate cancer: biologic, prognostic, and therapeutic implications. Hum Pathol. 2010;41(6):781–93.
Dimri G, Band H, Band V. Mammary epithelial cell transformation: insights from cell culture and mouse models. Breast Cancer Res. 2005;7(4):171–9.
Elenbaas B, Spirio L, Koerner F, Fleming MD, Zimonjic DB, Donaher JL, et al. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 2001;15(1):50–65.
Ahuja D, Saenz-Robles MT, Pipas JM. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005;24(52):7729–45.
Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351(6326):453–6.
Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18(1):11–22.
Acknowledgements
The authors thank all patients and their families for supporting this study. We also appreciate support of Dr. Michele Boiani from the Max-Planck-Institute for Molecular Biomedicine, Münster for in vivo studies. We also thank Thorsten König from the Institute of Molecular Tumor Biology, University Hospital Muenster for flow cytometry analysis. This work was supported by Janssen-Cilag GmbH, IIS-N-ARN-20-DEU-0198-V01. This study was further supported by the Deutsche Forschungsgemeinschaft ((DFG), BE5347/4-1).
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by Janssen-Cilag GmbH, IIS-N-ARN-20-DEU-0198-V01. This study was further supported by the Deutsche Forschungsgemeinschaft ((DFG), BE5347/4-1).
Author information
Authors and Affiliations
Contributions
Conception and design: NW, AK, SL, CB. Acquisition of data: NW, AK, SL, TR, VH, WGD, SH, JV, CB. Analysis and interpretation of data: NW, AK, SL, VH, WGD, SH, JV, AJS, MB, KS, CB. Drafting of the manuscript: NW, AK, SL, CB. Critical revision of the manuscript for important intellectual content: All authors. Obtaining funding: CB. Supervision: AJS, CB.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The local ethical committee approved the study (2007-467-f-S), and all patients gave informed consent.
Consent for publication
Not applicable.
Competing interests
CB, AJS, MB and KS received travel expenses and consulting fees from Janssen Cilag GmbH. CB received research funding by Janssen Cilag GmbH. MB also declares employment by Jannsen Cilag GmbH. All other authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Integration cassette of immortalization vectors. Immortalization vectors containing either SV40LT or hTERT under the control of the EF1α promotor as well as a GFP/RFP-2A-neomycin/puromycin selection cassette under the control of the synthetic RPBSA promoter. ISPA: Integration specific PCR amplicons.
Additional file 2: Table S1.
Patient characteristics. Table S2. Primer sequences. Table S3. STR profiling.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lange, S., Kuntze, A., Wüstmann, N. et al. Establishment of primary prostate epithelial and tumorigenic cell lines using a non-viral immortalization approach. Biol Res 57, 21 (2024). https://doi.org/10.1186/s40659-024-00507-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40659-024-00507-z