Abstract
Background
To evaluate the objective response rate (ORR) at 2 months of treatment with regorafenib according to RECIST 1.1, Choi, and modified Choi (mChoi) criteria in patients with metastatic colorectal cancer (mCRC).
Methods
Baseline and 2-month contrast-enhanced computed-tomography (CECT) scans of 55 patients with mCRC, prospectively enrolled in phase II TEXCAN trial, were centrally assessed. The primary endpoint was 2-month ORR by RECIST 1.1, Choi, and mChoi criteria. Final outcome was overall survival (OS).
Results
Of 55 patients included in this study (Intention-to-treat [ITT1] population), 35 had CECT at 2 months (ITT2 population). According to RECIST 1.1 criteria, 20 (57%) patients were SD and 15 were PD (43%) in the ITT2 population. According to Choi criteria, 18 (51%) patients were responders and 17 (48%) were non-responders. Median OS was 5.3 months (95% CI 3.7–8.6) in the ITT1 population and 8.9 months (95% CI 5.1–12.6) in the ITT2 population. In the ITT2 population, median OS was 16 months (95% CI 6.6–17.5) in SD patients (n = 20) and 4.6 months (95% CI 3.3–5.8) in PD patients (n = 15), according to RECIST 1.1 criteria (HR = 6.48). Median OS was 7.9 months (95% CI 4.2–17.5) in responders (n = 18) and 9.9 months (95% CI 3.7-NA) in non-responders (n = 17) according to Choi criteria (HR = 1.06). All patients except one were classified as non-responders with mChoi criteria.
Conclusion
At 2 months, unlike RECIST 1.1, Choi and mChoi criteria could not identify mCRC patients with regorafenib survival benefit.
Trial registration
ClinicalTrials.gov Identifier: NCT02699073.Registered March 4, 2016, Retrospectively registered.
Similar content being viewed by others
Key points
-
At 2 months, Choi and mChoi criteria were not able to identify mCRC patients with a regorafenib OS benefit.
-
RECIST 1.1 criteria identify survival benefit at 2 months despite the absence of partial responders with these criteria.
Background
Until now, the Response Evaluation Criteria in Solid Tumors (RECIST) based on anatomic measurement of the tumor size have been the most widely used for tumor imaging in drug trials or in clinical practice. These criteria provide standardized assessment of the tumor response in terms of progression-free survival (PFS) or time to progression, considered acceptable surrogate endpoints for overall survival (OS) [1]. However, traditional RECIST usually evaluate the responses late and are of limited use as primary endpoint in clinical trials evaluating targeted therapies, especially those with an antiangiogenic and/or an anti-proliferative effect. The ability to differentiate between response or stable disease (SD) and progressive disease (PD) early during therapy is crucial for allowing adjustment of therapy. This is the reason why Choi et al. proposed to take into account not only the size of the tumor, but also drug-induced necrosis [2]. These new response criteria combined a change in tumor size (10% decrease of the largest diameter regardless the attenuation change) or tumor attenuation (15% decrease in Hounsfield units [HU] regardless the size change) on contrast-enhanced computed-tomography (CECT) scans. Choi criteria correlated better than RECIST with disease-specific survival of imatinib-treated gastrointestinal stromal tumors (GIST) patients [3]. Given that tyrosine kinase inhibitor (TKI) can induce necrosis, in metastatic renal cell carcinomas (mRCCs) but little change in size, some authors proposed to extend Choi criteria to sunitinib-treated mRCCs [3, 4]. They found that predictive PFS and OS values of Choi criteria measured during the portal phase at 2 months were significantly better than RECIST 1.0 criteria. However, this finding was not confirmed in two other studies [5, 6] whose authors concluded that neither RECIST nor standard Choi criteria successfully discriminate between TKI-treated mRCC patients with short versus long-term clinical benefit. Nathan et al. [6] proposed using modified Choi (mChoi) criteria, which require a 10% size decrease and a 15% attenuation decrease to define a partial response (PR), while others [7, 8] proposed using only a 10% decrease of the longest diameter as a threshold for classifying PR. Those two last criteria seemed to correspond better with time to progression than RECIST and standard Choi criteria in mRCC patients treated with TKI [6]. Choi criteria appeared more appropriate than RECIST 1.1 to identify responders with long survival among advanced hepatocellular carcinoma patients benefiting from sorafenib [9]. The study of mCRC evaluating an optimal morphologic response (based on qualitative assessment of necrosis) to preoperative chemotherapy with bevacizumab, also supported the potential use of Choi or mChoi criteria [10].
Among new targeted therapies, regorafenib, an oral agent that blocks multiple protein kinases, demonstrated significant clinical efficacy in mCRC patients. In an international pivotal phase III CORRECT study of refractory or advanced mCRC, patients with progression during or within 3 months of the last standard therapy showed both PFS and OS benefit compared to placebo [11]. Median OS was 6.4 months in the regorafenib arm versus 5 months in the placebo arm (HR 0.77; 95% CI 0·64–0·94; one-sided p = 0.0052). It is possible that a potential subpopulation of patients with mCRC can benefit more from regorafenib therapy. Hence, a more appropriate selection of patients and identification of predictors of early clinical benefit would be useful.
the objective of this study was to evaluate RECIST1.1, CHOI, mCHOI, or RECIST modified with a 10% decrease of the longest diameter as a threshold for classifying PR (RECIST10%) criteria to assess which provides the best early response assessment to regorafenib in patients with mCRC.
Materials and methods
Patients
A total of 55 patient with mCRC who had been treated with regorafenib after a fluoropyrimide-based, an anti-vascular endothelial growth factor (VEGF) and an anti-epidermal growth factor receptor (EGFR) therapy in prospective, open-label, single-arm phase II TEXCAN trial in seven French centers from February 2016 to June 2017, were analyzed. Patients were treated until progression or unacceptable adverse events. Main inclusion criteria were i) disease progression in patients with histologically proven mCRC, who had been previously treated with, or were not considered candidates for, available therapies including fluoropyrimidine-based chemotherapy, ii) an anti-VEGF and an anti-EGFR therapy (if patients were KRAS wild-type), iii) Eastern Cooperative Oncology Group Performance Status (ECOG PS) 0 or 1, iv) at least one target lesion on CT scan, and v) adequate renal, hematological and liver functions. The initial and maximal dose of regorafenib was 160 mg (4 tablets of 40 mg) taken once daily for 3 weeks followed by 1 week off therapy. Dose interruptions and/or reductions were required based on individual safety. Patients continued regorafenib until PD according to RECIST, unacceptable toxic effects, withdrawal of patient’s consent, decision of discontinuation taken by the investigator in the patient’s best interest, or death. If PD according to RECIST criteria, but tumor response by CHOI criteria, the patient continued regorafenib at the investigator’s discretion in case of evident clinical benefit.
Imaging acquisition
Thoracic–abdominal–pelvic CECT scans after non-standardized intravenous (IV) injection of a low-osmolar, non-ionic contrast agent with an iodine concentration of 300–350 mg/mL at 2 mL/sec were collected. Two acquisition protocols were used according to each center’s usual procedures. For each patient, the same acquisition protocol was used at baseline and follow-up CECT. Thoracic–abdominal–pelvic CECT images for 32 patients were obtained in one acquisition during the portal phase (70–90 s post-injection). Thorax CECT images were obtained during the arterial phase of enhancement (25 s post-injection), while abdomen and pelvis (from the dome of the diaphragm to the pubis) CECT images were acquired during the portal phase (70–90 s post-injection) for the other 23 patients. Multidetector computed tomography (MDCT) parameters were: tube current 150 mAs, tube voltage 120 Kv, detector collimation of 2–3 mm.
Images were taken by the picture archiving and communication systems (PACS) system in each center. An anonymized copy of the exam was collected by the center in charge of the central reading (Radiology Department of La Pitié Salpêtrière Hospital, Paris).
Imaging analysis
A central radiological review for imaging interpretation was composed by two radiologists with 25 and 4 years, respectively, of experience in the evaluation of abdominal imaging. Radiologists performed a consensus reading of all CECT scans and were blinded to the patients’ clinical data and outcome.
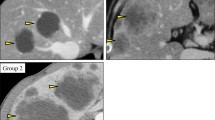
First, only the baseline CECT was displayed on a workstation (Myrian® 1.13.1, Intrasense, Paris, France) to perform the target lesions selection. A maximum of five (two per organ) target lesions >10 mm for the largest diameter were selected for being representative of all involved organs as recommended by RECIST 1.1 [12]. The largest axial diameter of each target lesion was measured manually with calipers, except for the lymph nodes whose small diameters were measured. The attenuation values in Hounsfield units (HU) were obtained in free-hand region of interest (ROI) drawn about 1 mm inside the lesion boundaries on the CECT section whose largest diameter had been measured. For lung lesions, a narrow window was used to avoid inclusion of voxels containing air in the measured area. Then, follow-up CECT images were also displayed on the workstation and were compared to baseline scans to achieve rigorous concordance in the identification of all target lesions during follow-up. The largest axial diameter and the attenuation measurements were recorded as described above.
Data analysis
For each patient, the sum of all the largest axial diameters was computed and the averages of mean and median attenuations in ROI images of all target lesions were calculated. Absolute and percent change between baseline and the 2-month evaluation was calculated for each patient. Tumor response was assessed according to RECIST 1.1, Choi, mChoi, and RECIST10% (Table 1). For evaluation by RECIST 1.1, responders had complete response (CR) and partial response (PR). CR, PR, and SD were considered disease controlled responses. For evaluation by Choi criteria, responders were defined as having ≥10% decrease of the sum of the largest tumor diameter or ≥ 15% decrease of the mean attenuation computed for the ROIs of all target lesions. For evaluation by mChoi criteria, responders were defined as having ≥10% decrease of sum of the largest tumor diameter and ≥ 15% decrease of mean attenuation computed for the ROIs of all target lesions. For evaluation by RECIST10%, responders had CR and PR defined by a − 10% of the longest diameter threshold. Patients who did not meet the RECIST 1.1, Choi, mChoi, and RECIST 10 response criteria were considered non-responders.
The primary endpoint was the tumor response rate (ORR) at 2 months according to RECIST 1.1, Choi, mChoi, and RECIST10% criteria. Final outcome was OS.
Statistical analyses
The primary objective of this study was to evaluate which of criteria, RECIST1.1, Choi, provide the best early (at 2 months) response assessment to regorafenib treatment in patients receiving regorafenib for mCRC after standard therapy. Secondary objective were also to evaluate mChoi and RECIST10% at 2 months.
The primary population for efficacy analyses was the intention-to-treat population (ITT1), which was defined as all patients having received at least one tablet of study drug. For the primary endpoint evaluation it was the population defined as patients who underwent CT scans at baseline and at 2 months (ITT2). Primary endpoint was described using percent with 95% confidence intervals (CI). OS was measured from the administration date of regorafenib to the date of death, regardless of the cause, or censored at the time of the last follow-up visit. Survival curves were prepared using the Kaplan-Meier method and were compared using the log-rank Mantel-Cox test in accordance with the final response outcomes. Cox proportional hazards regression model was used to compare survival according to radiological responses using Choi, mChoi, RECIST 1.1, and RECIST10%. All statistical tests were two-tailed. A p value of .05 was considered significant and 95% CI were calculated. A Fisher’s exact test was used for comparison of frequency. The results of safety analysis will be published in a separate paper.
Results
The study flow chart is presented in Fig. 1. Fitty-five patients were included in the study and all received at least one tablet of regorafenib (ITT1 population). Twenty patients were not reevaluated at 2 months because of RECIST or clinical, biological progression at 1 month (n = 7), absence of CECT at 2 months (n = 5), death (n = 4), adverse event (n = 3), or consent withdrawal (n = 1). Therefore, the primary endpoint was assessed in 35 patients (ITT2 population). Patient and tumor characteristics are given in Table 2.
Seventy five target lesions were identified and studied in the ITT2 population. Overall, four (11%) patients had only one target lesion, 25 (71%) had two, three (8%) had three, three (8%) had four, and none (3%) had five for a mean of 2.1 target lesions per patient.
The variations of the imaging parameters (variation of the longest diameter according to RECIST1.1 and attenuation according to Choi criteria) of all the target lesions between baseline and 2 months are summarized in Fig. 2. There were no newly lesions identified. At 2 months, according to RECIST 1.1, there were no responders in the ITT2 population; 20 (57%) were SD (disease control rate) and 15 were PD (43%). At 2 months by CHOI criteria, 18 (51%) patients (95% CI 34–68.6%) were responders and 17 (48%) were non-responders (Table 3). At 2 months by mCHOI criteria, only one (3%) patient was responder and 34 (97%) were non responders. At 2 months by RECIST10%, one (3%) patient was responder, 19 (54%) were SD, and 16 (43%) were PD.
Median OS was 5.3 months (95% CI 3.7–8.6) In the ITT1 population and it was 8.9 months (95% CI 5.1–12.6) in the ITT2 population. In the ITT2 population, median OS was 16 months (95% CI 6.6–17.5) in SD patients at 2 months (n = 20) and 4.6 months (95% CI 3.3–5.8) in PD patients (n = 15), according to RECIST 1.1 (HR = 6.48, 95% CI 2.23–18.79; Fig. 3a). Median OS was 7.9 months (95% CI 4.2–17.5) in responders (n = 18) and was 9.9 months (95% CI 3.7-NA) in non-responders (n = 17) according to Choi (HR = 1.06, 95% CI 0.48–2.35 p = 0.89; Fig. 3b). Overall survival as a function of mChoi at 2 months was not calculated because all patients except one were classified as non-responders. Similarly, RECIST10% did not differ significantly from RECIST 1.1 with only one patient having PR.
Discussion
Multikinase inhibitors may reduce tumor attenuation due to decreased perfusion or metabolic activity with subsequent necrosis. The use of Choi criteria [2] allows a simple assessment of this phenomenon, even if an increase of size of the tumor is observed during treatment. Those criteria were successfully used to select GIST patients who will benefit from treatment with imatinib, mainly because the necrosis-induction effect of this biotherapy, which overcomes the intrinsic limitations of Choi criteria. Indeed, according to the Choi criteria, fast-growing untreated tumor with spontaneous central necrosis induced by locally insufficient angiogenesis, which leads to decreased enhancement of the central part of the lesion, would be expected to respond to treatment. This limitation might explain the contradictory results reported for different types of treatment for GIST or other tumor types, in which the drug-induced necrosis is less obvious than in case of imatinib therapy in patients with GIST [3]. Indeed, RECIST 1.1 and Choi criteria used to assess regorafenib activity in patients with advanced GIST after failure of imatinib and sunitinib showed similar clinical benefit rates [13]. Although the detection of response occurred sooner with Choi criteria, Choi criteria showed less concordance with OS than RECIST 1.1. These results had however retrospective nature and were based on the small number of patients. In our study, Choi criteria were less sensitive than those of RECIST1.1 criteria in predicting OS in mCRC patients treated by regorafenib.
An alternative Choi criteria (mChoi), in which a decrease of ≥15% in attenuation and of ≥10% in tumor size are require to define a responder have been proposed by Nathan et al. [6] in order to avoid the bias in assessing a fast-growing tumor with spontaneous necrosis. With mChoi criteria, the number of responders is expected to be lower compared to Choi criteria. Indeed, unlike Nathan et al. [6], who studied tyrosine kinase inhibitor (TKI), we found that mChoi criteria assigned all patients but one in the non-responder group and consequently was not discriminant at all. The difference could be probably explained by the fact that regorafenib induces few attenuation changes and almost no change of the size of the lesion. This might also explain why RECIST10% and RECIST 1.1 showed very similar results.
Patients who showed benefit from regorafenib were considered SD according to RECIST1.1 and RECIST10%. All these patients except one exhibited a tumor growth rate between + 4.4% and + 20%. Hence, despite the fact that regorafenib could not stop the tumor burden to increase in size, patients could live longer when the percentage of size change was below 20% at 2 months.
Conclusion
This study supports the postulate that SD at 2 months assessed with RECIST 1.1 represents true clinical benefit for mCRC patients treated with regorafenib. One of the major limitations of our study is the low patient sample size of the ITT2 population. However, this findings suggest that the simple assessment of attenuation change within the tumor using Choi criteria or mChoi criteria does not provide useful information about the efficacy of regorafenib in patients with mCRC.
Availability of data and materials
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- AOI:
-
Area of interest
- CECT:
-
Contrast-enhanced computed tomography
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- HU:
-
Hounsfield units
- LD:
-
Largest diameter
- mChoi:
-
Modified Choi criteria
- mCRC:
-
Metastatic colorectal carcinoma
- ORR:
-
Overall response rate
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- PS:
-
Performance status
- RECIST:
-
Response evaluation criteria in solid tumors
- ROI:
-
Region of interest
- SD:
-
Standard deviation
- TL:
-
Target lesion
- VOI:
-
Volume of interest
References
Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with Imatinib Mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753–9. https://doi.org/10.1200/JCO.2006.07.3049.
Schmidt N, Hess V, Zumbrunn T, et al. Choi response criteria for prediction of survival in patients with metastatic renal cell carcinoma treated with anti-angiogenic therapies. Eur Radiol. 2013;23:632–9. https://doi.org/10.1007/s00330-012-2640-x.
van der Veldt a a M, Meijerink MR, van den Eertwegh a JM, et al (2010) Choi response criteria for early prediction of clinical outcome in patients with metastatic renal cell cancer treated with sunitinib. Br J Cancer 102:803–809. doi: https://doi.org/10.1038/sj.bjc.6605567.
Krajewski KM, Guo M, Van den Abbeele AD, et al. Comparison of four early posttherapy imaging changes (EPTIC; RECIST 1.0, tumor shrinkage, computed tomography tumor density, Choi criteria) in assessing outcome to vascular endothelial growth factor-targeted therapy in patients with advanced renal cell ca. Eur Urol. 2011;59:856–62. https://doi.org/10.1016/j.eururo.2011.01.038.
Nathan PD, Vinayan A, Stott D, et al. CT response assessment combining reduction in both size and arterial phase density correlates with time to progression in metastatic renal cancer patients treated with targeted therapies. Cancer Biol Ther. 2010;9:15–9.
Krajewski KM, Franchetti Y, Nishino M, et al. 10% tumor diameter shrinkage on the first follow-up computed tomography predicts clinical outcome in patients with advanced renal cell carcinoma treated with angiogenesis inhibitors: a follow-up validation study. Oncologist. 2014;19:507–14. https://doi.org/10.1634/theoncologist.2013-0391.
Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. https://doi.org/10.1016/S0140-6736(08)61039-9.
Ronot M, Bouattour M, Wassermann J, et al. Alternative response criteria (Choi, European association for the study of the liver, and modified response evaluation criteria in solid tumors [RECIST]) versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist. 2014;19:394–402. https://doi.org/10.1634/theoncologist.2013-0114.
Shindoh J, Loyer EM, Kopetz S, et al. Optimal morphologic response to preoperative chemotherapy: an alternate outcome end point before resection of hepatic colorectal metastases. J Clin Oncol. 2012;30:4566–72. https://doi.org/10.1200/JCO.2012.45.2854.
Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12. https://doi.org/10.1016/S0140-6736(12)61900-X.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Shinagare AB, Jagannathan JP, Kurra V, et al. Comparison of performance of various tumour response criteria in assessment of regorafenib activity in advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib. Eur J Cancer. 2014;50:981–6. https://doi.org/10.1016/j.ejca.2013.11.037.
Acknowledgements
We thank Magdalena Benetkiewicz for an editorial assistance. Her work was founded by GERCOR.
Funding
This study has received funding by Bayer in compliance with Ethical Standards.
Author information
Authors and Affiliations
Contributions
OL and TA have made substantial contribution to the conception, the design of the work, and the interpretation of data, OL wrote the manuscript, TA was a major contributor in writing the manuscript, PG and MW performed the CHOI, mCHOI and RECIST analyses, JBB, BR, TM, CL, BC, RC, MLG-L, and AG have made substantial contribution to the data acquisition and analysis, they also substantially revised the drafted work. JH performed the statistical analyses. All authors read and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The TEXCAN study (ClinicalTrials.gov Identifier: NCT02699073) was conducted in accordance with the declaration of Helsinki and Good Clinical Practice guidelines. All patients provided a written informed consent. Approval of the protocol was obtained from an independent ethics committee and the French Health Authority.
Consent for publication
“Not applicable”
Competing interests
Some authors of this manuscript declare relationships with the following companies:
- OL: Roche, Bracco, Siemens and Intrasense.
- JBB: Amgen, Bayer, Merck Serono, Pierre Fabre, Roche, Sanofi, Servier.
- BR: Bayer, Servier, Roche, Novartis, Astellas, Gilead, Abbvie.
- TM: ROCHE and AMGEM, Sanofi, Bayer, BMS.
- CL: MSD, Roche, Halozyme, Servier, Astra-Zeneca.
- BC: Roche, Sanofi, Bayer, Servier, Takeda, Merck, Roche, Amgen.
- RC: Servier, Amgen and Sanofi.
- TA: Roche/Genentech and Ventana, Baxter, Bayer, Amgen, MSD Oncology, Sanofi, Servier, Novartis Roche/Genentech/ventana, and Bristol-Myers Squibb.
- MW, PG, SK, ML G-L, AG and JH have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lucidarme, O., Wagner, M., Gillard, P. et al. RECIST and CHOI criteria in the evaluation of tumor response in patients with metastatic colorectal cancer treated with regorafenib, a prospective multicenter study. Cancer Imaging 19, 85 (2019). https://doi.org/10.1186/s40644-019-0271-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40644-019-0271-z