Abstract
Background
Neurofilament light chain (NfL), glial fibrillary acidic protein (GFAP), and total-tau protein (tau) are novel blood biomarkers of neurological injury, and may be used to predict outcomes in critical COVID-19.
Methods
A prospective multicentre cohort study of 117 consecutive and critically ill COVID-19 patients in six intensive care units (ICUs) in southern Sweden between May and November 2020. Serial NfL, GFAP and tau were analysed in relation to mortality, the Glasgow Outcome Scale Extended (GOSE) and the physical (PCS) and mental (MCS) components of health-related quality of life at one year.
Results
NfL, GFAP and tau on ICU admission predicted one-year mortality with an area under the curve (AUC) of 0.82 (95% confidence interval [CI] 0.74\(-\)0.90), 0.72 (95% CI 0.62\(-\)0.82) and 0.66 (95% CI 0.54\(-\)0.77). NfL on admission was an independent predictor of one-year mortality (p = 0.039). Low NfL and GFAP values were associated with good PCS (\(\ge\)45) at one year but not with good MCS (\(\ge\)45) or GOSE (\(\ge\)5).
Conclusions
NfL on ICU admission was an independent predictor of mortality. High levels of NfL, GFAP and tau were associated with mortality but not with poor GOSE in survivors at one year. Low levels of NfL and GFAP were associated with improved physical health-related quality of life.
Graphical Abstract

Similar content being viewed by others
Background
Critical COVID-19
Critical COVID-19 requiring intensive care and ventilatory support is characterised by acute respiratory distress syndrome (ARDS) [1] and multiorgan dysfunction, posing a significant risk of severe morbidity and mortality [2, 3]. Identifying patients likely to develop critical COVID-19 can aid resource allocation and facilitate adequate therapeutic interventions. The search for predictive biomarkers and clinical features that can forecast the disease course may be particularly important in COVID-19 research.
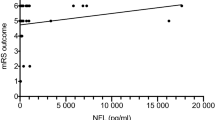
Mortality as a function of neurofilament light chain (NfL) on ICU admission in critical COVID-19. The grey line (with a grey 95% confidence band) is a local polynomial regression of mortality. For clearer visualisation, patients who survived beyond one year (mortality of 0) are indicated as having a mortality range of \(-\)0.15 to 0.15. In contrast, patients who did not survive one year (mortality of 1) are shown as having mortality in the range of 0.85 to 1.15
Correlation network for NfL, GFAP, and tau. The correlation network visualises pairwise correlations between the variables by colouring the edges with the Spearman rank correlation. The deeper the colour, the stronger the correlation. Only variables connected (directly or indirectly) with NfL, GFAP, or tau through absolute correlations > 0.3 are visualised. The spatial arrangement of the variables (nodes) groups them into strongly correlated clusters of variables. Biomarker blood samples were collected on admission to the intensive care unit (ICU). Alb serum albumin, CCI Charlson comorbidity index, CFS clinical frailty scale Consc altered consciousness pre-ICU, Crea serum creatinine, GFAP serum glial fibrillary acidic protein, GOSE Glasgow Outcome Scale Extended, Mort one-year mortality, IL6 serum interleukin-6, MCS short Form-36 Item Questionnaire Health Survey Version 2 mental component summary, NfL serum neurofilament light chain, PCT serum procalcitonin, PCS short Form-36 Item Questionnaire Health Survey Version 2 physical component summary, WBC white blood cell count
Neurological complications of COVID-19
Sepsis-associated encephalopathy (SAE) is a frequent early finding in sepsis, and long-term effects can be severe [4]. Sepsis may induce disruption of the blood–brain barrier (BBB) and may also cause cerebral hypoperfusion [5]. Similar mechanisms have been proposed in COVID-19 [6, 7]. SARS-CoV-2 infection has been associated with neurological complications [8], and the severity of COVID-19 seems to be an important determinant [9].
Post-mortem studies have found evidence of SARS-CoV-2 in the brain tissue of patients who eventually succumbed to COVID-19, suggesting a direct viral invasion targeting neurons via angiotensin-converting enzyme 2 receptors [10, 11]. Moreover, the extensive systemic inflammatory response in critical COVID-19, sometimes resulting in a “cytokine storm”, can also lead to neurological injury [12].
NfL as a function of age and mortality in critical COVID-19. The dash-dotted horizontal line is a local polynomial regression line. The dotted vertical line represents the age of 60. The local polynomial regression lines and vertical lines divide data into sectors with one-year mortality displayed for each sector. Altered consciousness before ICU admission was defined as GCS verbal response <4 after the onset of COVID-19 symptoms and before admission to the intensive care unit. GCS Glasgow Coma Scale, ICU intensive care unit, NfL serum neurofilament light chain
Receiver operator characteristic (ROC) curves and their corresponding areas under the curve (AUC) for one-year mortality prediction in critical COVID-19. All the models were based on logistic regression. The difference between the model using age, serum creatinine on ICU admission, altered consciousness before ICU admission and Charlson comorbidity index and the model using age, serum creatinine on ICU-admission, altered consciousness, Charlson comorbidity index and NfL on ICU-admission was significant (p = 0.029). ICU intensive care unit, NfL serum neurofilament light chain
Biomarkers of CNS injury
Neurofilament light chain (NfL) is a 68-kDa structural protein found in neurons, and elevated levels in cerebrospinal fluid and blood have been associated with various neurological conditions [13, 14]. The normal levels of NfL in the blood increase with age and have been reported to vary from lows around 3 pg/ml in younger adults to highs exceeding 50 pg/ml for those above 65 years of age [15, 16].
Glial fibrillary acidic protein (GFAP) is a type III intermediate filament protein in astrocytes [17]. Increased levels of GFAP have been associated with reactive gliosis, a typical response to central nervous system (CNS) injury, and have been detected in various neurological diseases [18, 19]. Normal blood levels of GFAP are also age-dependent, with values well below 100 pg/ml in young adults, rising fourfold for >65 year-olds [20].
Total-tau protein, commonly referred to as tau, is another neuronal protein that has received considerable attention in the context of Alzheimer’s disease [21]. Further, elevated blood levels of tau have been associated with the appearance of encephalopathy in septic patients [22]. Normal tau levels in the blood are in the 1.8\(-\)2.7 pg/ml range in functionally intact older adults [23].
Elevated NfL and GFAP blood levels have been reported in COVID-19 [24], and higher levels are associated with severe disease. NfL is most extensively studied and high NFL levels are associated with worse clinical outcomes in critical COVID-19 [25]. Health-related quality of life (HrQoL) has been insufficiently investigated in critical COVID-19. The physical and mental component scores of SF-36v2®have shown long-lasting deterioration in hospitalised COVID-19, particularly in women and in those aged 41–60 years [25, 26].
This study aimed to describe associations between three biomarkers of CNS injury, NfL, GFAP, and tau, and one-year mortality as well as recovery at one year using the GOSE and the physical (PCS) and mental (MCS) components of SF-36v2®. We hypothesised that increased levels of all three biomarkers, particularly NfL, were associated with worse clinical outcomes in critical COVID-19.
Methods
Study design
This prospective multicentre cohort study is a part of the SweCrit COVID-19 study [2]. ClinicalTrials.gov identifier: NCT04974775. Surviving participants were invited to a follow-up one year after ICU admission, performed primarily face-to-face but could be replaced by a telephone interview. Certified interpreters were used when participants were deemed non-fluent in the Swedish language.
An age-matched control group was created by collecting blood samples and basic demographic data from healthy volunteers.
The manuscript was prepared per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [27].
Participants
Critically ill patients, 18 years or older, with laboratory-confirmed SARS-CoV-2 infection were included at six intensive care units (ICU) in the Skåne Region, Sweden, between May 11, 2020, and November 30, 2020. The SweCrit COVID-19 study was predetermined to stop inclusion after one year (May 10 2021), and this cohort thus represents an initial pilot study. Patients were excluded if COVID-19 was not the primary cause of ICU admission.
Variables
In survivors, functional outcome was assessed one year after admission using the clinician-reported Glasgow Outcome Scale Extended (GOSE), an ordinal scale ranging from 1 to 8, where 1 represents death and 8 a full recovery [28]. A GOSE \(\ge 5\) was considered a good functional outcome.
To investigate HrQoL, the patient-reported SF-36v2® was used [29]. The SF-36v2® was calculated and presented as two summary scores of physical (Physical Component Summary, PCS) and mental (Mental Component Summary, MCS) HrQoL. Both PCS and MCS are presented as T scores with a range of 0–100, with lower scores indicating a worse HrQoL and 50 representing the mean value of a US normative population. PCS and MCS scores \(\ge 45\) are considered normal or good HrQoL.
Altered consciousness before ICU admission was defined as no verbal response, incomprehensible sounds, or inappropriate words (Glasgow Coma Scale [GCS] verbal response <4) at any point after the onset of COVID-19 symptoms and prior to admission to the intensive care unit.
Data sources
Background and survival data were extracted from the patient administrative system for intensive care units (PASIVA) and the regional quality register COVID-IR. PASIVA is synchronised with the Swedish population register, containing survival data. Data regarding altered consciousness before ICU admission were gathered retrospectively through electronic medical records.
Measurement of NfL, GFAP, and tau
Serial blood samples used to analyse NfL, GFAP, and tau were collected on ICU admission, day 2, and day 7 after ICU admission. Samples were centrifuged, aliquoted, frozen, and stored in the SWECRIT biobank at Region Skåne (BD-47, SC-1922). Samples collected later than 6 h after ICU admission were excluded. If the sampling time was missing, samples were included in the analysis if the freezing time was within 6 h. The frozen plasma samples were transported to the Clinical Neurochemistry Laboratory in Mölndal. Batch analyses of NfL, GFAP, and tau were performed on thawed samples using a commercially available single-molecule array (Simoa) method on an HD-X analyser according to the instructions from the manufacturer (Quanterix, Billerica, United States of America). Frozen plasma samples from the control group were analysed simultaneously. All analyses were performed by board-certified laboratory technicians at the Clinical Neurochemistry Laboratory, University of Gothenburg, Sweden, blinded to clinical data. Intra-assay coefficients of variation were around 5%.
Statistics
Generals
For all hypotheses tests, we considered p-values <0.05 significant. To assess a difference in the location of two independent variables, we used the Wilcoxon rank-sum test (Mann–Whitney U test). Differences in proportions were assessed using Pearson’s \(\chi ^2\) test.
Correlation network analysis
The pairwise Spearman rank correlation was calculated and visualised for absolute correlations >0.3 using the network_plot function of the corrr package in R [30, 31].
Local polynomial regression
Local polynomial regression [32] was used to visualise mortality as a function of the biomarkers using the default settings (loess) of geom_smooth of the ggplot2 package in R [30].
Regression models
Multivariable binary logistic regression was used to analyse one-year mortality, GOSE, and SF-36v2®. Areas under the curve (AUC) were derived from the receiver operating characteristic (ROC) curves [33]. Differences in AUCs were tested with the method of DeLong et al. [34]. The biomarkers were analysed using the base-10 logarithm.
Results
Participants
A total of 117 COVID-19 patients requiring intensive care were included. Healthy controls (n = 119) were used for comparison. Age was similarly distributed with a median age (25th and 75th percentiles) of 66 (56–72) years for the COVID-19 group and 65 (61–71) years for the healthy and age-matched controls.
Biomarker levels and outcomes
The median levels (25th and 75th percentiles) of NfL, GFAP and tau on ICU admission were 20.4 (11.9\(-\)34.3) pg/mL, 176.0 (102.5\(-\)243.3) pg/mL and 7.64 (3.87\(-\)14.23) pg/mL, and were significantly higher in one-year non-survivors compared to survivors for all three biomarkers; see Table 1.
In healthy controls, the corresponding levels for NfL, GFAP and tau were 12.0 (9.0\(-\)15.9) pg/mL, 110.0 (79.8\(-\)154.5) pg/mL and 6.57 (5.27\(-\)7.80) pg/mL. Levels differed significantly between critical COVID-19 and healthy controls for NfL (p \(\le 0.0001\)) and GFAP (p \(\le 0.0001\)) but not for tau.
Hospital mortality was 26% and one-year mortality was 30%. Most included patients had severe ARDS with a median P/F ratio of 12 kPa on day 1. More than half, 52%, required invasive mechanical ventilation. Age, Charlson comorbidity index, clinical frailty scale, SAPS-3 score, creatinine and platelets on admission differed significantly between survivors and non-survivors. There was no significant difference in gender distribution between survivors and non-survivors, while body mass index was significantly lower in non-survivors (Table 1).
Univariate predictions of mortality, GOSE, and SF-36v2®
One-year mortality versus NfL on day 0 (admission), day 2 and day 7 is presented in Fig. 1. Higher NfL levels, particularly on day 0, were associated with increased one-year mortality. Higher GFAP and tau levels were also associated with increased one-year mortality; see Appendix, Fig. 5.
NfL levels on days 0, 2, and 7 for one-year survivors versus non-survivors, good or poor GOSE, PCS, and MCS, are presented in the Appendix, Fig. 6. NfL levels for days 0 (p \(\le 0.0001\)), 2 (p \(\le 0.0001\)), and 7 (p \(\le 0.05\)) were all significantly higher in non-survivors. NfL levels for day 0 (p \(\le 0.05\)) were significantly higher in patients with poor PCS. Corresponding analyses for GFAP and tau are presented in the Appendix, Figs. 7 and 8. GFAP levels for days 0 (p \(\le 0.001\)), 2 (p \(\le 0.01\)), and 7 (p \(\le 0.01\)) were all significantly higher in non-survivors. GFAP levels for days 0 (p \(\le 0.05\)) and 7 (p \(\le 0.01\)) were significantly higher in patients with poor PCS. Tau levels for days 0 (p \(\le 0.01\)) and 7 (p \(\le 0.01\)) were significantly higher in non-survivors. No significant differences in tau levels were observed in patients with poor PCS. Except for day 7 GOSE GFAP levels (p \(\le 0.05\)), no significant differences in biomarkers were observed for good versus poor outcomes in GOSE or MCS.
NfL on admission predicted one-year mortality with an AUC of 0.82 (95% confidence interval [CI] 0.74\(-\)0.90); see Fig. 4. GFAP and tau on admission predicted one-year mortality with an area under the curve (AUC) of 0.72 (95% CI 0.62\(-\)0.82) and 0.66 (95% CI 0.54\(-\)0.77), respectively.
The correlations of NfL, GFAP and tau
To find significant clinical correlations, the correlations (Spearman rank) of NfL, GFAP and tau and the variables presented in Table 1 are visualised in Fig. 2 for absolute correlations > 0.3. Variables with no correlation to NfL, GFAP and tau were removed. NfL correlated with altered consciousness admission before ICU admission, as presented in Fig. 9.
Multivariate predictions of mortality
Figure 3 shows age-adjusted levels for critical COVID-19. For patients younger than 60 years of age and NfL less than the age-adjusted levels, mortality was 0%, whereas, for NfL above the age-adjusted levels, mortality was 17%. Similarly, for patients older than 60 years and NfL lower than for the age-adjusted levels, the mortality was 35%, whereas, for NfL above the age-adjusted levels, the mortality was 53%.
Age, creatinine, CCI, and altered mental status before ICU admission generated an AUC for one-year mortality of 0.84 (95%CI 76–92%). Adding NfL on ICU admission to the model increased the AUC to 0.90 (95%CI 84–96%) (p = 0.028), showing that NfL on admission was an independent predictor of one-year mortality; see Fig. 4. GFAP and tau on admission were not predictors of one-year mortality (p = 0.11 and p = 0.17). No biomarkers were predictors of the functional outcomes or HrQoL (GOSE, MCS or PCS).
Discussion
The main finding of this study is that NfL on ICU admission is a strong independent predictor of mortality in critical COVID-19.
For a better understanding of the role of NfL, GFAP and tau on ICU admission in critical COVID-19, a correlation network was created, revealing that NfL correlates primarily with age, renal function, CCI, CFS, altered consciousness before ICU admission, serum procalcitonin (PCT), and indirectly with interleukin-6 (IL-6) and albumin, apart from being correlated with GFAP and tau. GFAP and tau, consequently, had similar correlation patterns.
It is well-established that NfL increases with age and that age is a strong predictor of intensive care outcomes in COVID-19—in fact, better than the ICU gold standard Simplified Acute Physiology Score 3 (SAPS-3) [2]. Due to the correlation analyses and previously described associations of NfL, the multivariable logistic regression analyses of mortality included age, creatinine, CCI, and altered consciousness before ICU admission. GFAP and tau were also predictors of one-year mortality in critical COVID-19, albeit not independent predictors.
In univariate analyses, HrQoL in survivors showed that levels of NfL and GFAP on ICU admission differed for good versus poor PCS at one year, while no differences were seen for good versus poor MCS. Levels of tau did not differ for PCS or MCS at one year. There is no clear explanation for these differences, but our results are in line with those of Needham et al. [25].
The findings that NfL, GFAP and tau on ICU admission, i.e. before lengthy intensive care, showed predictive capabilities of one-year mortality in critical COVID-19 may suggest (1) a direct effect of SARS-CoV-2 on the CNS in critical COVID-19, (2) that they measure (possibly sub-clinical) pre-existing degenerative CNS disease, or (3) that they reflect a CNS effect of the secondary organ failure (e.g. hypoxia or hypoperfusion). These three possible explanations may also be viewed in light of a proposed increased BBB permeability in critical COVID-19 [6, 7].
Since levels of NfL in the cerebrospinal fluid (CSF) are much higher than in blood, initial studies primarily focused on analysing the CSF in neurological disease. The degree to which the BBB and blood–CSF barrier permeability influence the blood NfL levels is unclear. Explorative work used the CSF and serum albumin ratio to estimate BBB permeability. However, this ratio is rather a marker of the blood–CSF barrier [35, 36]. Patients with the highest CSF-to-serum albumin ratio also had the highest CSF and blood NfL levels, suggesting that an altered permeability of the blood–CSF barrier contributes to serum NfL [37, 38]. This relationship, however, was not observed in all studies [37,38,39,40]. Of note, there is an increased disruption of the BBB with ageing which may contribute to the increased levels of blood NfL in age-related diseases [41]. The increase in blood NfL levels with age may be driven by an increasing burden of comorbidities rather than the ageing process, such as the disruption of the BBB. Blood NfL levels have also been reported to depend on renal function and sex [42, 43].
The NfL and GFAP levels in critical COVID-19 in this study are within previously suggested reference intervals in healthy individuals [15, 16, 20]. Compared to previous COVID-19 studies, however, biomarker levels in the present study, especially NfL, are similar [24, 25]. NfL and GFAP levels in our age-matched controls were also significantly lower than in critical COVID-19. The previously reported slow release of NfL compared to GFAP is clearly shown in Figs. 6 and 7 [24], which also impacts absolute levels. Blood samples were collected within a relatively short time frame in the ICU and NfL levels in critical COVID-19 have been reported to normalise over time, up to 6 months later [24]. Our samples were collected at the defined start of ICU care, corresponding to an average 11 days after the onset of symptoms [2]. The differing levels of NfL and GFAP thus seem to adequately reflect health versus critical disease.
The precise cause of elevated NfL values in non-survivors cannot be determined in the present study. We have, however, corrected for comorbidities using the Charlson comorbidity index, making a pre-existing degenerative disease a less likely cause.
We speculate that the most likely cause for NfL elevation on admission in critical COVID-19, particularly in non-survivors, reflects a secondary CNS effect due to organ failure (hypoxia or hypoperfusion and consequent CNS injury) and recommend further studies to investigate this hypothesis in critical disease. If our hypothesis is correct, NfL could be used to evaluate intensive care in general, e.g. different sedation or drug strategies, blood pressure or ventilatory targets, using a biomarker reflecting perhaps the most important endpoint of intensive care—preserving cognitive abilities and emotional well-being.
Limitations
The most important limitation of our study is the small sample size, as it limits our search for a more precise cause for the higher biomarker levels of CNS injury in non-survivors.
Strengths
This study presents a prospectively and carefully studied population of critically ill COVID-19 patients. Serial biomarkers of CNS injury were collected over time and batch analysed together with healthy and age-matched controls, allowing for correct comparisons. We also allowed for corrections for renal function, comorbidities, and altered consciousness prior to admission. The detailed and mainly face-to face follow-up, including GOSE and HrQoL as measured by SF-36v2®one year after critical disease, is also a strength.
Conclusion
NfL, GFAP, and tau on ICU admission for critical COVID-19 are predictors of one-year mortality. NfL is an independent (age, creatinine, comorbidities, altered consciousness before ICU admission) predictor of one-year mortality and may be a potential outcome predictor in critical disease in general. In addition, NfL and GFAP are associated with good physical HrQoL at 1 year.
Availability of data and materials
The datasets generated and analysed during the current study are not publicly available due to limitations in the ethical approval of the study and data management policies of Region Skåne. However, they are available from the corresponding author upon reasonable request.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- AUC:
-
Area under the curve
- BBB:
-
Blood–brain barrier
- CNS:
-
Central nervous system
- CI:
-
Confidence interval
- CSF:
-
Cerebrospinal fluid
- GCS:
-
Glasgow Coma Scale
- GFAP:
-
Glial fibrillary acidic protein
- GOSE:
-
Glasgow Outcome Scale Extended
- HrQoL:
-
Health-related quality of life
- ICU:
-
Intensive care unit
- IL-6:
-
Interleukin-6
- NfL:
-
Neurofilament light chain
- MCS:
-
SF-36v2® mental component summary
- PASIVA:
-
Patient administrative system for intensive care units
- PCT:
-
Procalcitonin
- PCS:
-
SF-36v2® physical component summary
- ROC:
-
Receiver operating characteristic
- SAPS 3:
-
Simplified Acute Physiology Score III
- SAE:
-
Sepsis-associated encephalopathy
- STROBE:
-
Strengthening the Reporting of Observational Studies in Epidemiology
References
Ranieri VM et al (2012) Acute respiratory distress syndrome: the Berlin Definition. JAMA 307:2526–2533
Didriksson I et al (2023) Intensive care unit burden is associated with increased mortality in critically ill COVID-19 patients. Acta Anaesthesiol Scand 67:329–338
NIH (2023) Clinical Spectrum of SARS-CoV-2 Infection
Chaudhry N, Duggal AK (2014) Sepsis associated encephalopathy. Adv Med 2014:762320
Ehler J et al (2017) Translational evidence for two distinct patterns of neuroaxonal injury in sepsis: a longitudinal, prospective translational study. Crit Care 21:262
Bonetto V et al (2022) Markers of blood-brain barrier disruption increase early and persistently in COVID-19 patients with neurological manifestations. Front Immunol 13:1070379
Hernández-Parra H et al (2023) Alteration of the blood-brain barrier by COVID-19 and its implication in the permeation of drugs into the brain. Front Cell Neurosci 17:1125109
Granholm AC (2023) Long-term effects of SARS-CoV-2 in the brain: clinical consequences and molecular mechanisms. J Clin Med 12(9):3190
Mao L et al (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 77:683–690
Bocci M et al (2021) Infection of brain pericytes underlying neuropathology of COVID-19 patients. Int J Mol Sci 22(21):11622
Matschke J et al (2020) Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol 19:919–929
Poyiadji N et al (2020) COVID-19-associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology 296:E119–E120
Forgrave LM, Ma M, Best JR, DeMarco ML (2019) The diagnostic performance of neurofilament light chain in CSF and blood for Alzheimer’s disease, frontotemporal dementia, and amyotrophic lateral sclerosis: A systematic review and meta-analysis. Alzheimers Dement (Amst) 11:730–743
Karantali E et al (2022) Neurofilament light chain in patients with a concussion or head impacts: a systematic review and meta-analysis. Eur J Trauma Emerg Surg 48:1555–1567
Simrén J et al (2022) Establishment of reference values for plasma neurofilament light based on healthy individuals aged 5–90 years. Brain Commun 4:fcac174
Hviid CVB, Knudsen CS, Parkner T (2020) Reference interval and preanalytical properties of serum neurofilament light chain in Scandinavian adults. Scand J Clin Lab Invest 80:291–295
Middeldorp J, Hol EM (2011) GFAP in health and disease. Prog Neurobiol 93:421–443
Eng LF, Ghirnikar RS (1994) GFAP and astrogliosis. Brain Pathol 4:229–237
Petzold A (2015) Glial fibrillary acidic protein is a body fluid biomarker for glial pathology in human disease. Brain Res 1600:17–31
Tybirk L, Hviid CVB, Knudsen CS, Parkner T (2022) Serum GFAP—reference interval and preanalytical properties in Danish adults. Clin Chem Lab Med 60:1830–1838
Olsson B et al (2016) CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol 15:673–684
Zhao T, Xia Y, Wang D, Pang L (2019) Association between elevated serum tau protein level and sepsis-associated encephalopathy in patients with severe sepsis. Can J Infect Dis Med Microbiol 2019:1876174
Gala I et al (2021) Plasma tau and neurofilament light in frontotemporal lobar degeneration and Alzheimer disease. Neurology 96:e671–e683
Sutter R et al (2021) Serum neurofilament light chain levels in the intensive care unit: comparison between severely ill patients with and without Coronavirus disease 2019. Ann Neurol 89:610–616
Needham EJ et al (2022) Brain injury in COVID-19 is associated with dysregulated innate and adaptive immune responses. Brain 145:4097–4107
Koullias E et al (2022) Long-term effect on health-related quality of life in patients with COVID-19 requiring hospitalization compared to non-hospitalized COVID-19 patients and healthy controls. Cureus 14:e31342
Von Elm E et al (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 147:573–577
McMillan T et al (2016) The Glasgow Outcome Scale—40 years of application and refinement. Nat Rev Neurol 12:477–485
Ware JE, Gandek B (1998) Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol 51:903–912
Wickham H (2016) ggplot2: Elegant graphics for data analysis, 2 edn. Use R! Springer International Publishing, Basel, Switzerland
Kuhn M, Jackson S, Cimentada J (2022) corrr: Correlations in R. Https://github.com/tidymodels/corrr, https://corrr.tidymodels.org
Cleveland WS, Grosse E, Shyu WM (1992) Local regression models. Wadsworth & Brooks/Cole Advanced Books & Software, Monterey, USA
Fawcett T (2006) An introduction to ROC analysis. Pattern Recogn Lett 27:861–874
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44(3):837–845
Reiber H (1994) Flow rate of cerebrospinal fluid (CSF)—a concept common to normal blood–CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci 122:189–203
Freedman MS et al (2005) Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Arch Neurol 62:865–870
Kalm M et al (2017) Serum concentrations of the axonal injury marker neurofilament light protein are not influenced by blood–brain barrier permeability. Brain Res 1668:12–19
Uher T et al (2021) Neurofilament levels are associated with blood–brain barrier integrity, lymphocyte extravasation, and risk factors following the first demyelinating event in multiple sclerosis. Mult Scler 27:220–231
Novakova L et al (2017) Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 89:2230–2237
Wurster CD et al (2020) Neurofilament light chain in serum of adolescent and adult SMA patients under treatment with nusinersen. J Neurol 267:36–44
Sweeney MD, Sagare AP, Zlokovic BV (2018) Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol 14:133–150
Rebelos E et al (2022) Circulating neurofilament is linked with morbid obesity, renal function, and brain density. Sci Rep 12:7841
Mielke MM et al (2019) Comparison of variables associated with cerebrospinal fluid neurofilament, total-tau, and neurogranin. Alzheimers Dement 15:1437–1447
Acknowledgements
We thank Dr Märta Läffler, who organised the COVID-IR registry, all study participants and their families, as well as all staff at the ICUs of Skåne University Hospital in Malmö and Lund, Helsingborg Hospital and Kristianstad Hospital for contributing to this study.
Funding
Open access funding provided by Lund University. AF: Regional research support, Region Skåne #2022-1284; Governmental funding of clinical research within the Swedish National Health Service (ALF) #2022:YF0009 and #2022-0075; Crafoord Foundation grant number #2021-0833; Lions Skåne research grants; Skåne University Hospital grants; Swedish Heart and Lund Foundation (HLF) #2022-0352 and #2022-0458.
HF: Regional research support, Region Skåne; Government funding of clinical research within the Swedish National Health Service (ALF) #2022-0226; Swedish Heart and Lund Foundation (HLF) #2021-10233; Hans-Gabriel and Alice Trolle-Wachtmeister Foundation for Medical Research
KB: Swedish Research Council (#2017-00915 and #2022-00732), the Swedish Alzheimer Foundation (#AF-930351, #AF-939721 and #AF-968270), Hjärnfonden, Sweden (#FO2017-0243 and #ALZ2022-0006), Governmental funding of clinical research within the Swedish National Health Service (ALF) (#ALFGBG-715986 and #ALFGBG-965240), the European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236), the Alzheimer’s Association 2021 Zenith Award (ZEN-21-848495), and the Alzheimer’s Association 2022-2025 Grant (SG-23-1038904 QC).
HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2022-01018 and #2019-02397), the European Union’s Horizon Europe research and innovation programme under grant agreement No 101053962, Governmental funding of clinical research within the Swedish National Health Service (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C, and #ADSF-21-831377-C), the Bluefield Project, the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022-0270), the European Union’s Horizon 2020 research and innovation programme under the Marie Sk?odowska-Curie grant agreement No 860197 (MIRIADE), the European Union Joint Programme—Neurodegenerative Disease Research (JPND2021-00694), the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre, and the UK Dementia Research Institute at UCL (UKDRI-1003).
The funding organisations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
AF and HF designed and funded the study. KB and HZ analysed NfL, GFAP, and tau in the blood samples. GL conducted one-year follow-ups, and TS collected data. TS and AF interpreted the data and performed statistical analyses. TS and AF wrote the first draft of the manuscript. All authors read, provided critical revisions, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
TS, ID, MS, GL, KB, AF, and HF declare no competing interests. HZ has served on scientific advisory boards or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave. In addition, HZ has given lectures at symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work).
Ethics approval and consent to participate
Ethical approval and consent to participate: Ethical approval was acquired from the Swedish Ethical Review Authority (2020/01955, 2020/03483 and 2020/05233). Written informed consent was obtained from all surviving participants on admission, before discharge or at follow-up. For deceased patients, consent was presumed according to the approval from the Ethical Review Authority. Trial registration: NCT04974775. Registered 23rd July 2021—retrospectively registered, link.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Mortality as a function of glial fibrillary acidic protein (GFAP) and tau on ICU admission in critical COVID-19. The grey line (with a grey 95% confidence band) is a local polynomial regression of mortality. For clearer visualisation, patients who survived beyond one year (mortality = 0) are indicated as having mortality in the range of \(-\)0.15 to 0.15. In contrast, patients who did not survive one year (mortality = 1) are shown as having mortality in the range of 0.85 to 1.15. GFAP serum glial fibrillary acidic protein
NfL levels for one-year outcomes mortality, GOSE, PCS and MCS in critical COVID-19. The Wilcoxon rank-sum test was used to assess the difference in NfL levels between dead and alive. GOSE Glasgow Outcome Scale Extended, ICU intensive care unit, NfL serum neurofilament light chain, MCS defined as short Form-36 item Questionnaire Health Survey version 2 mental component summary, PCS defined as short Form-36 item Questionnaire Health Survey version 2 physical component summary, ns: p \(> 0.05\) * p \(\le 0.05\) p \(\le 0.01\) *** p \(\le 0.001\) **** p \(\le 0.0001\)
GFAP levels for one-year outcomes mortality, GOSE, PCS and MCS in critical COVID-19. The Wilcoxon rank-sum test was used to assess the difference in GFAP levels between dead and alive. GFAP serum glial fibrillary acidic protein, GOSE Glasgow Outcome Scale Extended, ICU intensive care unit, MCS defined as short Form-36 item Questionnaire Health Survey version 2 mental component summary, PCS defined as short Form-36 item Questionnaire Health Survey version 2 physical component summary, ns: p \(> 0.05\) *p \(\le 0.05\) p \(\le 0.01\) ***p \(\le 0.001\) ****p \(\le 0.0001\)
Tau levels for one-year outcomes mortality, GOSE, PCS and MCS in critical COVID-19. The Wilcoxon rank-sum test was used to assess the difference in tau levels between dead and alive. GOSE Glasgow Outcome Scale Extended, ICU intensive care unit, MCS defined as short Form-36 item Questionnaire Health Survey version 2 mental component summary, PCS defined as short Form-36 item Questionnaire Health Survey version 2 physical component summary, ns: p \(> 0.05\) *p \(\le 0.05\) p \(\le 0.01\) ***p \(\le 0.001\) ****p \(\le 0.0001\)
NfL levels on ICU-admission for altered consciousness before ICU-admission. Altered consciousness before ICU admission was defined as a GCS verbal response <4 after the onset of COVID-19 symptoms and before admission to the intensive care unit. The Wilcoxon rank-sum test was used to assess the difference in NfL levels between dead and alive. GCS Glasgow Coma Scale, ICU intensive care unit, NfL serum neurofilament light chain, ns: p \(> 0.05\) *p \(\le 0.05\) p \(\le 0.01\) ***p \(\le 0.001\) ****p \(\le 0.0001\)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sievert, T., Didriksson, I., Spångfors, M. et al. Neurofilament light chain on intensive care admission is an independent predictor of mortality in COVID-19: a prospective multicenter study. ICMx 11, 66 (2023). https://doi.org/10.1186/s40635-023-00547-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-023-00547-x