Abstract
Background
Interleukin-1 receptor antagonists can reduce mortality in septic shock patients with hepatobiliary dysfunction and disseminated intravascular coagulation (HBD + DIC), an organ failure pattern with inflammatory features consistent with macrophage activation. Identification of clinical phenotypes in sepsis may allow for improved care. We aim to describe the occurrence of HBD + DIC in a contemporary cohort of patients with sepsis and determine the association of this phenotype with known macrophage activation syndrome (MAS) biomarkers and mortality. We performed a retrospective nested case–control study in adult septic shock patients with concurrent HBD + DIC and an equal number of age-matched controls, with comparative analyses of all-cause mortality and circulating biomarkers between the groups. Multiple logistic regression explored the effect of HBD + DIC on mortality and the discriminatory power of the measured biomarkers for HBD + DIC and mortality.
Results
Six percent of septic shock patients (n = 82/1341) had HBD + DIC, which was an independent risk factor for 90-day mortality (OR = 3.1, 95% CI 1.4–7.5, p = 0.008). Relative to sepsis controls, the HBD + DIC cohort had increased levels of 21 of the 26 biomarkers related to macrophage activation (p < 0.05). This panel was predictive of both HBD + DIC (sensitivity = 82%, specificity = 84%) and mortality (sensitivity = 92%, specificity = 90%).
Conclusion
The HBD + DIC phenotype identified patients with high mortality and a molecular signature resembling that of MAS. These observations suggest trials of MAS-directed therapies are warranted.
Similar content being viewed by others
Background
Driven by excessive cellular activation and cytokine overproduction, macrophage activation syndrome (MAS) is an acute hyperinflammatory state characterized by hyperferritinemia, coagulopathy, and hepatobiliary dysfunction [1,2,3,4]. The associated fulminant cytokine storm results in rapidly progressing organ dysfunctions and early death without appropriate therapies. MAS is a form of secondary hemophagocytic lymphohistiocytosis (sHLH) often observed in patients with underlying rheumatic diseases [5], but this syndrome can also arise in a subset of patients with sepsis [6].
Although earlier recognition and increasing compliance to best practices have reduced in-hospital mortality from sepsis over the past decade, high profile clinical trials targeting specific inflammatory pathways have yet to improve survival, as most trial designs work on a presumption that sepsis behaves homogeneously despite presenting as a heterogenous syndrome [7,8,9,10,11]. Consequently, the identification of subgroups of patients who may benefit from specific biological response modifiers represents a strategic opportunity to overcome current limitations and failures [12].
Illustrating this point, in a post-hoc analysis of a randomized clinical trial investigating the effect of anakinra (a recombinant IL-1 receptor antagonist) on mortality in sepsis [9], Shakoory et al. demonstrated that anakinra, compared with placebo, resulted in a 50% relative risk reduction in mortality only in the subset of sepsis patients also presenting with features of MAS [13].
The need for prompt, specific treatment with anakinra requires early recognition, but guidelines developed to facilitate the diagnosis of MAS are impractical, because sepsis patients often present without the classic MAS features. Therefore, the clinical phenotype of concomitant hepatobiliary dysfunction and disseminated intravascular coagulation (HBD + DIC) proposed by Shakoory et al. has been used as a more practical strategy for identifying sepsis patients who may respond to anakinra [13,14,15,16]. Despite its simplicity, it remains unclear whether patients with HBD + DIC truly represent a subgroup of patients with an inflammatory pathophysiology similar to that of MAS.
We sought to describe the frequency of HBD + DIC in a contemporary cohort of patients with septic shock. We also hypothesized that HBD + DIC would be (i) an independent risk factor for mortality and (ii) distinguished by a biomarker signature associated with macrophage activation.
Methods
Study design, population, and setting
We conducted a nested case–control study using de-identified plasma samples and data sets from patients enrolled in the Protocol-Based Care for Early Septic Shock (ProCESS) cohort, approved by the University of Pittsburgh’s Institutional Review Board (PRO16070600). ProCESS enrolled 1341 adult patients from 31 hospitals in the United States between 2008 and 2013. Patients in the emergency department with suspected septic shock were deemed eligible if they met at least two systemic inflammatory response syndrome criteria and had either refractory hypotension or evidence of poor perfusion. Patients randomly received one of three resuscitation strategies: (i) protocol-based early goal directed therapy, (ii) protocol-based standard therapy that did not require placement of a central venous catheter, or (iii) usual care [17].
Definitions, study cohort, and data collection
We assessed the presence of HBD + DIC based on criteria used in prior studies [13,14,15,16]. HBD was defined as a liver Sequential Organ Failure Assessment (SOFA) score ≥ 1 (i.e., total bilirubin ≥ 1.2 mg/dL), and DIC as a platelet count ≤ 100 × 109/L and an international normalized ratio (INR) ≥ 1.5 IU. Cases and controls were both drawn from the ProCESS cohort, allowing for the matching of all patients based on the inclusion and exclusion criteria of the clinical trial, with an equal number of controls selected at random using age as the sole matching criterion. This was due to age being the primary confounder for survival analysis.
Sample collection and human plasma biomarker assays
The ProCESS trial team collected whole blood using EDTA or lithium heparin as an anticoagulant within 30 min of randomization (0 h) [17]. Stored plasma samples were used to quantify the 26 biomarkers investigated in this study, selected based on their role in macrophage activation and/or their association with MAS subsets [1,2,3,4, 13, 18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] (Additional file 1: Table S1).
Personnel performing the biomarker measurements were blinded to patient cohort and outcome. EDTA plasma samples were diluted 25-fold for the measurement of IL-18, IL-18BP, and CXCL9 as described previously [34]. The remaining analytes, with the exception of ferritin, were quantified from EDTA plasma samples diluted fourfold as part of the Human Bio-Plex Pro™ Human Inflammation Panel 1 or Cytokine Group I/II assays (Bio-Rad; Hercules, CA) per the manufacturer’s instructions. Concentrations obtained using the Bio-Plex® MAGPIX™ Multiplex Reader were normalized to the same standards, and an offset (equal to the average difference in controls between plates) was added to each value to minimize batch effects and values close to the lower limit of detection. Human ferritin levels were analyzed from heparinized plasma collected at 0 h using the Beckman Coulter UniCel DxI 800. The tests were performed by the University of Pittsburgh Medical Center (UPMC) Presbyterian Automated Testing Laboratory (Pittsburgh, PA).
Outcomes
The primary outcome was 90-day all-cause mortality. Secondary outcomes included in-hospital mortality, individual differences in the selected biomarkers between cases and controls, and the ability of the biomarker panel to identify HBD + DIC in sepsis patients and to predict 90-day mortality in those with the HBD + DIC phenotype.
Statistical analysis
Data were analyzed using Prism version 9.1.2 (GraphPad; San Diego, CA). Statistical significance was determined for categorical measures by Fisher’s Exact test, with the Freeman–Halton extension where applicable, and for continuous, non-normally distributed measures by the Mann–Whitney U test, with correction for multiple comparisons using the Holm–Šídák method. Linear relationships were determined by Spearman correlation analysis. Receiver operating characteristic (ROC) curves created for each analyte were evaluated for their individual ability to predict HBD + DIC. Multiple logistic regression analysis assessed the discriminatory power of the measured biomarkers for HBD + DIC and 90-day mortality, and we used the log-rank (Mantel–Cox) test for survival comparisons. To generate the heatmap (Partek® Genomics Suite® 6.6), we standardized values at time 0 h for each of the analytes by shifting to a median of zero and scaling to a standard deviation of one. Analyte hierarchical clusters (rows) used Euclidean distance as the measure of dissimilarity and the columns were subjected to forced clustering by group (i.e., sepsis controls and HBD + DIC). In the case of missing data, individuals were excluded from analysis for that particular variable. Missing data are enumerated in their respective tables and figures, as well as in Additional file 1: Table S2.
Sensitivity analysis
A potential limitation of using HBD as a criterion for the identification of cases is that liver SOFA scores conflate chronic and acute liver disease. Therefore, we performed a sensitivity analysis to determine whether (i) the biomarker signature, (ii) 90-day mortality, and (iii) the risk of death at 90 days were different between patients with and without chronic liver disease in the HBD + DIC subset. In addition, as the matching of patients with HBD + DIC to controls occurred only based on age and septic shock, we performed multiple logistic regression analysis to determine whether HBD + DIC was an independent factor associated with mortality in the presence of other potentially confounding variables, which included age, race, gender, ProCESS resuscitation strategy, Charlson Comorbidity Index, Acute Physiology and Chronic Health Evaluation (APACHE) III score, creatinine level, and white blood cell (WBC) count. These confounders were specifically chosen for their inherent association with disease severity and/or mortality in sepsis [14, 37,38,39,40,41,42,43].
Results
Patient characteristics and distribution of the HBD + DIC phenotype in a contemporary septic shock cohort
Of the 1341 individuals enrolled in the ProCESS trial [17], 35% (n = 465) had HBD and 16% (n = 211) had DIC. Only 6.1% (n = 82) of septic shock patients presented with concomitant HBD + DIC, thereby constituting our cases. Notably, the prevalence of HBD + DIC was similar in each of the treatment arms of the ProCESS trial, with cases distributed comparably across the three resuscitation strategies (Additional file 1: Fig. S1A). In addition to having lower total bilirubin levels, higher platelet counts, and lower liver and coagulation SOFA scores, controls differed from the remaining ProCESS patients without HBD + DIC (n = 1177) in gender and in the proportion self-identifying as ‘other race’. However, in-hospital and 90-day mortality were similar (Table 1). Compared with sepsis controls, HBD + DIC patients had lower WBC counts but higher creatinine levels and APACHE III scores. The HBD + DIC cohort was also characterized by a higher Charlson Comorbidity Index, with an increased prevalence of chronic liver disease, renal impairment, and cancer (Table 1).
HBD + DIC is an independent risk factor for death during sepsis
In comparison with controls, HBD + DIC patients experienced higher in-hospital (43% vs 10%, p < 0.001) and 90-day mortality (56% vs 23%, p < 0.001), as would be expected given their higher APACHE III score and Charlson Comorbidity Index (Table 1; Fig. 1; Additional file 1: Fig. S1B). However, HBD + DIC was an independent risk factor for 90-day mortality in the nested case–control cohort after adjusting for these and other clinically relevant confounding variables related to disease severity and mortality in sepsis (n = 163) (OR = 3.1, 95% CI 1.4–7.5, p = 0.008). We further confirmed HBD + DIC as an independent risk factor for 90-day mortality in the entire ProCESS cohort (n = 1315) (OR = 2.5, 95% CI 1.5–4.3, p = 0.001). In the ProCESS trial, no significant differences in primary or secondary outcomes were reported between resuscitation strategy groups [17]; likewise, 90-day mortality was not affected by treatment protocol within our cohort of matched cases and controls (Additional file 1: Fig. S1C).
The HBD + DIC phenotype has a biomarker profile resembling that of MAS
We next investigated the impact of HBD + DIC on biomarker expression in sepsis and whether this biomarker profile was consistent with MAS-like inflammation. Hierarchical clustering of the panel demonstrated marked differences in expression signatures between septic shock patients with and without HBD + DIC (Additional file 1: Fig. S2). Quantitatively, 21 of the 26 biomarkers were significantly different between the two groups (Fig. 2; Additional file 1: Table S3). Relative to sepsis controls, patients with HBD + DIC were characterized by increased expression of ferritin, IL-18, sCD163, sCD25, IL-6, and CXCL10 (Fig. 2; Additional file 1: Table S3), consistent with a biomarker signature of macrophage activation (Additional file 1: Table S1). We also found increased circulating concentrations of IL-18BP and IL-10 in the HBD + DIC cohort (Fig. 2; Additional file 1: Table S3), suggesting the involvement of both pro- and anti-inflammatory soluble factors. Moreover, IL-10 levels were positively correlated with ferritin (ρ = 0.22, p = 0.047) and IL-18 (ρ = 0.33, p = 0.002) in the HBD + DIC subset but not in sepsis controls (ferritin: ρ = 0.06, p = 0.57; IL-18: ρ = 0.16, p = 0.167). Overall, the biomarker pattern associated with the HBD + DIC phenotype closely resembled that reported in association with other MAS subsets (Additional file 1: Table S1), and the most notable differences in patients with HBD + DIC relative to sepsis controls were among biomarkers that have been associated mechanistically with the hyperinflammatory pathophysiology observed in MAS, including ferritin, IL-18, IL-6, and CXCL10 [5] (Fig. 3).
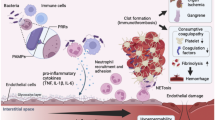
Cell and inflammatory mediator interplay potentially contributes to the development of HBD + DIC in septic patients. Sepsis-induced NK cell deficiency triggers latent viral reactivation, with viral DNA initiating a TLR9-MyD88-mediated signaling cascade [6, 52]. Subsequent inflammasome activation leads to the secretion of IL-18 and IL-1β [6, 56]. In addition to mediating liver injury [59], IL-1β increases transcription and translation of ferritin [60], and production of IL-6 [5]. The release of damage associated molecular patterns (DAMPs), such as mitochondrial DNA or hemoglobin after tissue injury or hemolysis, triggers macrophage activation independent of IFN-γ. Release of free hemoglobin increases hemoglobin-haptoglobin complexes, activating macrophages to produce extracellular ferritin through the CD163 receptor [6, 56]. Ferritin promotes expression of IL-1β and TLR9 [57, 58], resulting in a positive feedback loop with amplification of inflammatory signals [56]. IL-18, in combination with a secondary signal, such as IL-12 or TLR ligands, activates NK cells to produce IFN-γ [53, 64]. We hypothesize, though, that in the context of sepsis with HBD + DIC, NK cells are limited in their responsiveness to IL-18 due to IL-10-mediated downregulation of the IL-18R [55]. As a result, circulating IFN-γ levels are reduced. However, IL-6 enhances signaling through TLRs, increasing the secretion of proinflammatory mediators, including CXCL10 [5]. Persistent NK cell cytolytic dysfunction, stemming from decreased cell number [52] and high levels of IL-6 [54], translates into an impaired ability to induce apoptosis of activated macrophages [6]. In addition, inflammatory mediators produced by macrophages reinforce macrophage (ferritin, IL-6, IL-1β, IL-12, TNF), pDC (ferritin), and lymphocyte (IL-18, IL-12, CXCL10) activation. Thus, the inflammatory cycle continues unabated (cytokine storm), resulting in organ dysfunction and death in the absence of appropriate therapies. The role of pDC- and NK cell-derived IFN-γ in macrophage activation during sepsis remains unclear. In our study, IFN-γ levels were low, although two of its downstream mediators, CXCL10 and IL-18BP, were elevated in patients with sepsis and HBD + DIC
Chronic liver disease does not alter the biomarker signature or the risk of death in HBD + DIC
A limitation of using liver SOFA scores as the HBD criterion for the identification of sepsis patients with HBD + DIC is the inability to distinguish between acute and chronic liver disease. In the matched cohort, chronic liver disease was more prevalent in patients with HBD + DIC than in controls (Table 1; OR = 11.9, 95% CI 4.1–32.4, p < 0.001). Therefore, we investigated whether the biomarker signature and risk of death at 90 days were influenced by the presence of chronic liver disease in patients with HBD + DIC. Chronic liver disease did not affect biomarker expression, with the exception of higher ferritin and M-CSF in patients with HBD + DIC in the absence of chronic liver disease (data not shown). In addition, HBD + DIC patients with and without chronic liver disease had similar 90-day mortality (58% vs 55%, p = 0.82), and HBD + DIC remained an independent risk factor for death at 90 days after including chronic liver disease as a covariate in the multivariate logistic regression model (n = 163) (OR = 2.9, 95% CI = 1.2–7.2, p = 0.018). These results were not surprising given the shared histopathologic and inflammatory features between new onset and chronic HBD [44,45,46], suggesting that, irrespective of cause or eliciting factors, the biomarker signature and mortality related to the HBD + DIC phenotype are driven by this particular pattern of multiple organ dysfunction.
The macrophage activation biomarker panel is highly predictive of HBD + DIC and mortality
We examined which of the 26 biomarkers best discriminated HBD + DIC patients in the setting of septic shock. The biomarker with the highest area under the ROC curve (AUC) for predicting the occurrence of HBD + DIC was IL-18BP (AUC = 0.81, 95% CI 0.74–0.88), followed by sCD163 (AUC = 0.80, 95% CI 0.73–0.87), IL-18 (AUC = 0.79, 95% CI 0.73–0.86), and sCD25 (AUC = 0.78, 95% CI 0.71–0.85) (Fig. 4A; Additional file 1: Table S4). A model including these four biomarkers predicted HBD + DIC (AUC = 0.86, 95% CI 0.81–0.92, p < 0.001), but the inclusion of all 26 proved highly predictive of HBD + DIC, with an AUC of 0.93 (95% CI 0.90–0.97, p < 0.001) and sensitivity and specificity of 82% and 84% (Fig. 4B, C). Notably, this macrophage activation-associated biomarker panel was powerful at discriminating between survivors and non-survivors among the HBD + DIC subset of patients with sepsis (Fig. 4D, E; AUC = 0.95, 95% CI 0.90–1.00, p < 0.001, sensitivity = 92%, specificity = 90%).
Performance of selected biomarkers to predict the presence of HBD + DIC and 90-day mortality during sepsis. A AUC for each of the 26 biomarkers to predict the presence of HBD + DIC in patients with sepsis, organized from highest to lowest. B ROC curve representing the model using 26 biomarkers for predicting the HBD + DIC phenotype in patients with sepsis. C Violin plots showing the distribution of predicted probabilities for the presence of HBD + DIC in sepsis. The model performed well at classifying both sepsis controls and sepsis with HBD + DIC. The majority of sepsis controls had predicted probabilities of presenting with HBD + DIC less than 0.50 (median: 0.12, IQR: 0.03–0.28). By contrast, the cases had predicted probabilities that were overwhelmingly greater than 0.50 (median: 0.96, IQR: 0.65–0.99). D ROC curve representing the model using 26 biomarkers for predicting 90-day mortality among the HBD + DIC subset of patients with sepsis. E Violin plots showing the distribution of predicted probabilities for mortality in the cases. The model performed well at distinguishing between survivors (median: 0.13, IQR: 0.01–0.32) and non-survivors (median: 0.97, IQR: 0.84–0.99) among those with HBD + DIC
Discussion
In accordance with prior reports [13, 16], HBD + DIC was present in approximately 6% of patients with septic shock. The HBD + DIC phenotype was associated with higher mortality and significantly increased expression of key biomarkers implicated in macrophage activation and MAS. Importantly, the macrophage activation-targeted biomarker panel was highly predictive of the HBD + DIC phenotype and mortality.
Chronic liver disease, renal impairment, and cancer were more frequently present among the HBD + DIC sepsis patients, all of which have potential pathophysiologic links to the development of HBD + DIC. First, patients with sepsis and multiple organ failure (MOF) who develop any of three inflammatory pathobiology phenotypes are characterized by an increased proclivity to develop concomitant HBD + DIC and higher mortality. These inflammatory phenotypes include thrombocytopenia associated MOF, defined by new thrombocytopenia (or DIC) and renal dysfunction; immune paralysis associated MOF, defined by immune suppression (i.e., low WBC count); and sequential MOF [14]. Second, MAS commonly arises as a complication of rheumatic conditions, infections, and, importantly, malignancies [5, 47, 48]. Third, numerous features of chronic liver disease predispose to sepsis with HBD + DIC, including a high incidence of bacterial infections (e.g., spontaneous bacterial peritonitis), chronic systemic inflammation [46], and the profound immune dysregulation inherent to chronic liver injury [49, 50]. Fourth, the histopathology of liver tissue from patients with HLH/MAS is consistent with chronic hepatitis [44, 45], suggesting shared features of acute and chronic liver injury that promote organ failure and mortality in the absence of appropriate therapies. Indeed, our data support this pathophysiologic association, as we demonstrated that the HBD + DIC phenotype was more likely to be observed in septic shock patients with chronic liver disease.
Although IFN-γ is a critical driver of the hyperinflammatory state in the spectrum of HLH syndromes [22], it is unclear how IFN-γ contributes to the hyperinflammatory phenotype in sepsis patients with HBD + DIC. HBD + DIC cases exhibited an increase in IFN-γ compared with sepsis controls, but 84% of the HBD + DIC cohort had levels that fell within the normal reference range [51]. The median was also up to 50-fold lower than values reported in association with MAS or sHLH [22, 51]. Despite this, the IFN-γ-stimulated mediators CXCL10 and IL-18BP were increased in patients with HBD + DIC, indicating that IFN-γ signaling may still play a role in the development of this phenotype. Moreover, lower levels of IFN-γ in sepsis-induced HBD + DIC compared to other forms of MAS are not surprising given the effect of sepsis on NK cells. In addition to causing lymphopenia [6, 52], sepsis impairs NK cell cytotoxicity and IFN-γ responses to IL-18 [53, 54]. This mechanism appears to be mediated by IL-10, as serum IL-10 levels negatively correlate with IL-18 receptor (IL-18R) expression on liver NK cells, with neutralization of IL-10 restoring IL-18R expression and IFN-γ responses [55]. In line with these preclinical data, we noted a significant positive correlation between IL-10 and IL-18 in patients with HBD + DIC. Therefore, the counterintuitive pattern of increased IL-18 with only modest elevations in IL-18-induced IFN-γ in the HBD + DIC cohort may be explained by IL-10-mediated downregulation of the IL-18R on NK cells.
Two IFN-γ-independent pathways are also capable of triggering macrophage activation in sepsis. Hemolysis causes an increase in hemoglobin–haptoglobin complexes, which activate macrophages through the CD163 receptor to produce extracellular ferritin [6, 56]. Ferritin increases TLR9 expression and promotes NF-κB-dependent production of IL-1β [57, 58], a proinflammatory cytokine mediating liver injury [59]. Second, sepsis-induced lymphopenia not only dampens host apoptosis of activated macrophages but also contributes to the reactivation of latent viruses [6], initiating a signaling cascade through TLR9 that leads to inflammasome activation and production of IL-18 and IL-1β [6, 56], as well as extracellular ferritin [60], with repeated TLR9 stimulation leading to the clinical appearance of a cytokine storm [19]. Importantly, chronic IL-18 elevation strongly correlates with MAS risk, and excess free IL-18 promotes severe experimental MAS [34]. These events promote a positive feedback loop with amplification of inflammation and liver injury. Thus, we propose that NK cells do not mediate the inflammatory HBD + DIC phenotype in sepsis through IFN-γ but rather that persistent NK cell cytolytic dysfunction, stemming from a quantitative reduction in cell number and high levels of IL-6 [52, 54], translates into an impaired ability to induce apoptosis of activated macrophages [6].
The role of inflammasome activity in macrophage activation indicates HBD + DIC patients may benefit from therapeutic modalities targeting IL-18 and/or IL-1β. Total IL-18 and IL-18BP are typically elevated in sepsis-induced HBD + DIC, albeit to a lesser extent than in MAS [1, 6], but the increase in both suggests IL-18 has bioactive effects. Although increased circulating IL-1β levels in the HBD + DIC cohort appear biologically irrelevant, IL-1β contributes to a feedforward proinflammatory amplification loop even at low concentrations [61]. Furthermore, a causative role for IL-1β has been shown in clinical trials using recombinant IL-1 receptor antagonists to inhibit IL-1 signaling, effectively reducing mortality from 65 to 35% in patients with sepsis and HBD + DIC [13]. Only inhibition of both IL-18 and IL-1β completely protects against mortality in a murine model of lethal endotoxemia [62], and combined blockade of IL-18 and IL-1β has been successfully implemented in a genetic form of MAS resulting from NLRC4 inflammasome hyperactivity [63]. These data suggest that simultaneous neutralization of both IL-1β and IL-18 could have additive value over blocking either cytokine alone in the treatment of HBD + DIC.
Limitations
Matching of patients with HBD + DIC to controls occurred only based on age and septic shock, potentially increasing the risk of confounding variables. However, our results were robust to sensitivity analyses, suggesting that HBD + DIC is a distinct phenotype in sepsis. The panel of biomarkers tested was incomplete and biased toward those previously associated with MAS/hyperinflammation, and not all analytes had the best dynamic range. Finally, as this was a retrospective analysis, we were unable to test anti-cytokine therapies, but our findings substantiate the trialing of MAS-targeted therapeutics to improve outcomes in septic shock patients with HBD + DIC.
Conclusion
Concomitant HBD + DIC is a simple clinical strategy to identify septic shock patients who portray biomarker features of macrophage activation and progress with high mortality. This represents a key subgroup of patients to target for future trials testing treatments for macrophage activation and MAS.
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- APACHE:
-
Acute Physiology and Chronic Health Evaluation
- AUC:
-
Area under the ROC Curve
- DAMP:
-
Damage Associated Molecular Pattern
- DIC:
-
Disseminated Intravascular Coagulation
- HBD:
-
Hepatobiliary Dysfunction
- IL:
-
Interleukin
- IL-18R:
-
IL-18 Receptor
- INR:
-
International Normalized Ratio
- IQR:
-
Interquartile Range
- IU:
-
International Units
- MAS:
-
Macrophage Activation Syndrome
- MOF:
-
Multiple Organ Failure
- ROC Curve:
-
Receiver Operating Characteristic Curve
- sHLH:
-
Secondary Hemophagocytic Lymphohistiocytosis
- SOFA:
-
Sequential Organ Failure Assessment
- WBC:
-
White Blood Cell
References
Mazodier K, Marin V, Novick D, Farnarier C, Robitail S, Schleinitz N, Veit V, Paul P, Rubinstein M, Dinarello CA, Harle JR, Kaplanski G (2005) Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood 106:3483–3489
Ravelli A, Magni-Manzoni S, Pistorio A, Besana C, Foti T, Ruperto N, Viola S, Martini A (2005) Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J Pediatr 146:598–604
Fardet L, Galicier L, Lambotte O, Marzac C, Aumont C, Chahwan D, Coppo P, Hejblum G (2014) Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol 66:2613–2620
Ravelli A, Minoia F, Davi S, Horne A, Bovis F, Pistorio A, Arico M, Avcin T, Behrens EM, De Benedetti F, Filipovic L, Grom AA, Henter JI, Ilowite NT, Jordan MB, Khubchandani R, Kitoh T, Lehmberg K, Lovell DJ, Miettunen P, Nichols KE, Ozen S, Schmid PJ, Ramanan AV, Russo R, Schneider R, Sterba G, Uziel Y, Wallace C, Wouters C, Wulffraat N, Demirkaya E, Brunner HI, Martini A, Ruperto N, Cron RQ, Paediatric Rheumatology International Trials O, Childhood A, Rheumatology Research A, Pediatric Rheumatology Collaborative Study G, Histiocyte S (2016) 2016 classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Arthritis Rheumatol. 68:566–576
Schulert GS, Grom AA (2015) Pathogenesis of macrophage activation syndrome and potential for cytokine- directed therapies. Annu Rev Med 66:145–159
Carcillo JA, Shakoory B, Castillo L (2019) Secondary hemophagocytic lymphohistiocytosis, macrophage activation syndrome, and hyperferritinemic sepsis-induced multiple-organ dysfunction syndrome in the pediatric ICU. In: Mastropietro C, Valentine K (eds) Pediatric Critical Care. Springer, Cham
Angus DC (2011) The search for effective therapy for sepsis: back to the drawing board? JAMA 306:2614–2615
Cohen J, Opal S, Calandra T (2012) Sepsis studies need new direction. Lancet Infect Dis 12:503–505
Opal SM, Fisher CJ Jr, Dhainaut JF, Vincent JL, Brase R, Lowry SF, Sadoff JC, Slotman GJ, Levy H, Balk RA, Shelly MP, Pribble JP, LaBrecque JF, Lookabaugh J, Donovan H, Dubin H, Baughman R, Norman J, DeMaria E, Matzel K, Abraham E, Seneff M (1997) Confirmatory interleukin-1 receptor antagonist trial in severe sepsis: a phase III, randomized, double-blind, placebo-controlled, multicenter trial. The Interleukin-1 Receptor Antagonist Sepsis Investigator Group. Crit Care Med 25:1115–1124
Bone RC (1996) Why sepsis trials fail. JAMA 276:565–566
Marshall JC (2014) Why have clinical trials in sepsis failed? Trends Mol Med 20:195–203
Seymour CW, Kennedy JN, Wang S, Chang CH, Elliott CF, Xu Z, Berry S, Clermont G, Cooper G, Gomez H, Huang DT, Kellum JA, Mi Q, Opal SM, Talisa V, van der Poll T, Visweswaran S, Vodovotz Y, Weiss JC, Yealy DM, Yende S, Angus DC (2019) Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA 321:2003–2017
Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, Cron RQ, Opal SM (2016) Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med 44:275–281
Carcillo JA, Halstead ES, Hall MW, Nguyen TC, Reeder R, Aneja R, Shakoory B, Simon D, Eunice Kennedy Shriver National Institute of Child H, Human Development Collaborative Pediatric Critical Care Research Network I (2017) Three hypothetical inflammation pathobiology phenotypes and pediatric sepsis-induced multiple organ failure outcome. Pediatr Crit Care Med 18:513–523
Kernan KF, Ghaloul-Gonzalez L, Shakoory B, Kellum JA, Angus DC, Carcillo JA (2019) Adults with septic shock and extreme hyperferritinemia exhibit pathogenic immune variation. Genes Immun 20:520–526
Kyriazopoulou E, Leventogiannis K, Norrby-Teglund A, Dimopoulos G, Pantazi A, Orfanos SE, Rovina N, Tsangaris I, Gkavogianni T, Botsa E, Chassiou E, Kotanidou A, Kontouli C, Chaloulis P, Velissaris D, Savva A, Cullberg JS, Akinosoglou K, Gogos C, Armaganidis A, Giamarellos-Bourboulis EJ, Hellenic Sepsis Study G (2017) Macrophage activation-like syndrome: an immunological entity associated with rapid progression to death in sepsis. BMC Med 15:172
Investigators P, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC (2014) A randomized trial of protocol-based care for early septic shock. N Engl J Med 370:1683–1693
Akashi K, Hayashi S, Gondo H, Mizuno S, Harada M, Tamura K, Yamasaki K, Shibuya T, Uike N, Okamura T et al (1994) Involvement of interferon-gamma and macrophage colony-stimulating factor in pathogenesis of haemophagocytic lymphohistiocytosis in adults. Br J Haematol 87:243–250
Behrens EM, Canna SW, Slade K, Rao S, Kreiger PA, Paessler M, Kambayashi T, Koretzky GA (2011) Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J Clin Invest 121:2264–2277
Billiau AD, Roskams T, Van Damme-Lombaerts R, Matthys P, Wouters C (2005) Macrophage activation syndrome: characteristic findings on liver biopsy illustrating the key role of activated, IFN-gamma-producing lymphocytes and IL-6- and TNF-alpha-producing macrophages. Blood 105:1648–1651
Bleesing J, Prada A, Siegel DM, Villanueva J, Olson J, Ilowite NT, Brunner HI, Griffin T, Graham TB, Sherry DD, Passo MH, Ramanan AV, Filipovich A, Grom AA (2007) The diagnostic significance of soluble CD163 and soluble interleukin-2 receptor alpha-chain in macrophage activation syndrome and untreated new-onset systemic juvenile idiopathic arthritis. Arthritis Rheum 56:965–971
Bracaglia C, de Graaf K, Pires Marafon D, Guilhot F, Ferlin W, Prencipe G, Caiello I, Davi S, Schulert G, Ravelli A, Grom AA, de Min C, De Benedetti F (2017) Elevated circulating levels of interferon-gamma and interferon-gamma-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann Rheum Dis 76:166–172
Cartland SP, Genner SW, Martinez GJ, Robertson S, Kockx M, Lin RC, O’Sullivan JF, Koay YC, ManuneedhiCholan P, Kebede MA, Murphy AJ, Masters S, Bennett MR, Jessup W, Kritharides L, Geczy C, Patel S, Kavurma MM (2019) TRAIL-expressing monocyte/macrophages are critical for reducing inflammation and atherosclerosis. iScience 12:41–52
Heyworth CM, Whetton AD, Nicholls S, Zsebo K, Dexter TM (1992) Stem cell factor directly stimulates the development of enriched granulocyte-macrophage colony-forming cells and promotes the effects of other colony-stimulating factors. Blood 80:2230–2236
Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP (1998) IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol 160:3513–3521
Mathy NL, Scheuer W, Lanzendorfer M, Honold K, Ambrosius D, Norley S, Kurth R (2000) Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology 100:63–69
Menten P, Proost P, Struyf S, Van Coillie E, Put W, Lenaerts JP, Conings R, Jaspar JM, De Groote D, Billiau A, Opdenakker G, Van Damme J (1999) Differential induction of monocyte chemotactic protein-3 in mononuclear leukocytes and fibroblasts by interferon-alpha/beta and interferon-gamma reveals MCP-3 heterogeneity. Eur J Immunol 29:678–685
Osugi Y, Hara J, Tagawa S, Takai K, Hosoi G, Matsuda Y, Ohta H, Fujisaki H, Kobayashi M, Sakata N, Kawa-Ha K, Okada S, Tawa A (1997) Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood 89:4100–4103
Samah B, Porcheray F, Gras G (2008) Neurotrophins modulate monocyte chemotaxis without affecting macrophage function. Clin Exp Immunol 151:476–486
Sanchez-Martin L, Estecha A, Samaniego R, Sanchez-Ramon S, Vega MA, Sanchez-Mateos P (2011) The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood 117:88–97
Tamura K, Kanazawa T, Tsukada S, Kobayashi T, Kawamura M, Morikawa A (2008) Increased serum monocyte chemoattractant protein-1, macrophage inflammatory protein-1beta, and interleukin-8 concentrations in hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 51:662–668
Weber GF, Chousterman BG, He S, Fenn AM, Nairz M, Anzai A, Brenner T, Uhle F, Iwamoto Y, Robbins CS, Noiret L, Maier SL, Zonnchen T, Rahbari NN, Scholch S, Klotzsche-von Ameln A, Chavakis T, Weitz J, Hofer S, Weigand MA, Nahrendorf M, Weissleder R, Swirski FK (2015) Interleukin-3 amplifies acute inflammation and is a potential therapeutic target in sepsis. Science 347:1260–1265
Weber GF, Schlautkotter S, Kaiser-Moore S, Altmayr F, Holzmann B, Weighardt H (2007) Inhibition of interleukin-22 attenuates bacterial load and organ failure during acute polymicrobial sepsis. Infect Immun 75:1690–1697
Weiss ES, Girard-Guyonvarc’h C, Holzinger D, de Jesus AA, Tariq Z, Picarsic J, Schiffrin EJ, Foell D, Grom AA, Ammann S, Ehl S, Hoshino T, Goldbach-Mansky R, Gabay C, Canna SW (2018) Interleukin-18 diagnostically distinguishes and pathogenically promotes human and murine macrophage activation syndrome. Blood 131:1442–1455
Xuan W, Qu Q, Zheng B, Xiong S, Fan GH (2015) The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J Leukoc Biol 97:61–69
Canna SW, de Jesus AA, Gouni S, Brooks SR, Marrero B, Liu Y, DiMattia MA, Zaal KJ, Sanchez GA, Kim H, Chapelle D, Plass N, Huang Y, Villarino AV, Biancotto A, Fleisher TA, Duncan JA, O’Shea JJ, Benseler S, Grom A, Deng Z, Laxer RM, Goldbach-Mansky R (2014) An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet 46:1140–1146
Eachempati SR, Hydo L, Barie PS (1999) Gender-based differences in outcome in patients with sepsis. Arch Surg 134:1342–1347
Nachtigall I, Tafelski S, Rothbart A, Kaufner L, Schmidt M, Tamarkin A, Kartachov M, Zebedies D, Trefzer T, Wernecke KD, Spies C (2011) Gender-related outcome difference is related to course of sepsis on mixed ICUs: a prospective, observational clinical study. Crit Care 15:R151
Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC (2008) Racial variation in the incidence, care, and outcomes of severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med 177:279–284
Jones JM, Fingar KR, Miller MA, Coffey R, Barrett M, Flottemesch T, Heslin KC, Gray DT, Moy E (2017) Racial disparities in sepsis-related in-hospital mortality: using a broad case capture method and multivariate controls for clinical and hospital variables, 2004–2013. Crit Care Med 45:e1209–e1217
Yang Y, Yang KS, Hsann YM, Lim V, Ong BC (2010) The effect of comorbidity and age on hospital mortality and length of stay in patients with sepsis. J Crit Care 25:398–405
Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A et al (1991) The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 100:1619–1636
Gomez H, Kellum JA (2016) Sepsis-induced acute kidney injury. Curr Opin Crit Care 22:546–553
Favara BE (1992) Hemophagocytic lymphohistiocytosis: a hemophagocytic syndrome. Semin Diagn Pathol 9:63–74
Ost A, Nilsson-Ardnor S, Henter JI (1998) Autopsy findings in 27 children with haemophagocytic lymphohistiocytosis. Histopathology 32:310–316
Singanayagam A, Triantafyllou E (2021) Macrophages in chronic liver failure: diversity, plasticity and therapeutic targeting. Front Immunol 12:661182
Ravelli A (2002) Macrophage activation syndrome. Curr Opin Rheumatol 14:548–552
Janka G, Imashuku S, Elinder G, Schneider M, Henter JI (1998) Infection- and malignancy-associated hemophagocytic syndromes. Secondary hemophagocytic lymphohistiocytosis. Hematol Oncol Clin North Am 12:435–444
Arroyo V, Moreau R, Jalan R (2020) Acute-on-chronic liver failure. N Engl J Med 382:2137–2145
Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V, Consortium CSIotE-C (2013) Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 144: 1426-1437, 1437 e1421-1429
Yang SL, Xu XJ, Tang YM, Song H, Xu WQ, Zhao FY, Shen DY (2016) Associations between inflammatory cytokines and organ damage in pediatric patients with hemophagocytic lymphohistiocytosis. Cytokine 85:14–17
Halstead ES, Carcillo JA, Schilling B, Greiner RJ, Whiteside TL (2013) Reduced frequency of CD56 dim CD16 pos natural killer cells in pediatric systemic inflammatory response syndrome/sepsis patients. Pediatr Res 74:427–432
Souza-Fonseca-Guimaraes F, Parlato M, Philippart F, Misset B, Cavaillon JM, Adib-Conquy M, Captain study g (2012) Toll-like receptors expression and interferon-gamma production by NK cells in human sepsis. Crit Care 16:R206
Cifaldi L, Prencipe G, Caiello I, Bracaglia C, Locatelli F, De Benedetti F, Strippoli R (2015) Inhibition of natural killer cell cytotoxicity by interleukin-6: implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheumatol 67:3037–3046
Hiraki S, Ono S, Kinoshita M, Tsujimoto H, Takahata R, Miyazaki H, Saitoh D, Seki S, Hase K (2012) Neutralization of IL-10 restores the downregulation of IL-18 receptor on natural killer cells and interferon-gamma production in septic mice, thus leading to an improved survival. Shock 37:177–182
Kernan KF, Carcillo JA (2017) Hyperferritinemia and inflammation. Int Immunol 29:401–409
Elmaagacli A, Steckel N, Koldehoff M, Christoph S, Ottinger H, Trenschel R, Beelen D (2010) Toll-like-receptor expression and cellular immune reconstitution in AML-patients with elevated serum ferritin levels after allogeneic transplant. Blood 116:1049
Ruddell RG, Hoang-Le D, Barwood JM, Rutherford PS, Piva TJ, Watters DJ, Santambrogio P, Arosio P, Ramm GA (2009) Ferritin functions as a proinflammatory cytokine via iron-independent protein kinase C zeta/nuclear factor kappaB-regulated signaling in rat hepatic stellate cells. Hepatology 49:887–900
Szabo G, Petrasek J (2015) Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol 12:387–400
Rogers JT (1996) Ferritin translation by interleukin-1and interleukin-6: the role of sequences upstream of the start codons of the heavy and light subunit genes. Blood 87:2525–2537
Palmblad K, Schierbeck H, Sundberg E, Horne AC, Harris HE, Henter JI, Antoine DJ, Andersson U (2015) High systemic levels of the cytokine-inducing HMGB1 isoform secreted in severe macrophage activation syndrome. Mol Med 20:538–547
Vanden Berghe T, Demon D, Bogaert P, Vandendriessche B, Goethals A, Depuydt B, Vuylsteke M, Roelandt R, Van Wonterghem E, Vandenbroecke J, Choi SM, Meyer E, Krautwald S, Declercq W, Takahashi N, Cauwels A, Vandenabeele P (2014) Simultaneous targeting of IL-1 and IL-18 is required for protection against inflammatory and septic shock. Am J Respir Crit Care Med 189:282–291
Canna SW, Girard C, Malle L, de Jesus A, Romberg N, Kelsen J, Surrey LF, Russo P, Sleight A, Schiffrin E, Gabay C, Goldbach-Mansky R, Behrens EM (2017) Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J Allergy Clin Immunol 139:1698–1701
Anderko RR, Rinaldo CR, Mailliard RB (2020) IL-18 responsiveness defines limitations in immune help for specialized FcRgamma(-) NK cells. J Immunol 205:3429–3442
Acknowledgements
A complete list of investigators in the Protocolized Care for Early Septic Shock (ProCESS) study is provided in the Additional file 1: Appendix. We would like to thank Ali Smith, Octavia Peck Palmer and Vanessa Jackson for their contributions to this study, and Karen Nieri for reviewing and editing the final version of the manuscript.
Funding
This work was supported by National Institutes of Health grants R01GM108618 (JAC, JAK), P50GM076659 (DCA, JAK), R01DK083961 (JAK), R01HD098428 (SWC), and 1K08GM117310-01A1 (HG); The RK Mellon Institute for Pediatric Research (SWC); and The American Association of Immunologists Careers in Immunology Fellowship Program (RRA). The study sponsor had no role in the study design, analysis or writing of this manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
RRA, HG, JAC and JAK had full access to all data and take responsibility for the integrity of the data and accuracy of the analysis. Concept and design: RRA, HG, JAC, JAK, SWC, BS, DCA. Acquisition, analysis, and interpretation of data: RRA, HG, SWC, BS, DCA, DMY, DTH, JAK, JAC. Drafting of manuscript: RRA, HG, SWC, BS, JAK, JAC. Critical revision of the manuscript for important intellectual content: RRA, HG, SWC, BS, DCA, DMY, DTH, JAK, JAC. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the University of Pittsburgh’s Institutional Review Board (PRO16070600).
Consent for publication
Not applicable.
Competing interests
RRA reports stipend support from The American Association of Immunologists; HG, DCA, DTH, and JAK report funding from the National Institutes of Health; SWC reports funding from the National Institutes of Health, the RK Mellon Institute for Pediatric Research, research support unrelated to the current work from AB2Bio and IMMvention therapeutix; DMY reports funding from the National Institutes of Health, research support unrelated to the current work from the National Institutes of Health, royalties from Wolters/Kluwer, Lippincott/Williams and Wilkins, UpToDate Inc, and McGraw Hill Inc., editorial stipend from the American College of Emergency Physicians, payment for expert consultation from multiple legal firms, honorarium for participation on an advisory board with University of Pennsylvania, payment from National Quality Forum committees, and payment from the PA Board of Medicine; JAC reports funding from the National Institutes of Health for research support both related and unrelated to the current work. None of the remaining authors report any disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1. A.
Relative distribution of sepsis controls and HBD + DIC sepsis cases within each treatment arm of the ProCESS trial. Arm 1 refers to protocol-based early goal directed therapy, Arm 2 to protocol-based standard therapy not requiring placement of a central venous catheter, and Arm 3 to usual care [17]. B. Unadjusted in-hospital and 90-day mortality in sepsis controls vs sepsis with HBD + DIC. C. Unadjusted 90-day mortality in sepsis controls and sepsis with HBD + DIC, stratified according to treatment arm, with comparisons in mortality drawn between resuscitation strategies within controls and cases. Statistical significance was determined by Fisher’s Exact test. ***p < 0.001, ns = not significant. Figure S2. Heatmap demonstrating the hierarchical clustering of 26 macrophage activation-associated biomarkers after forced clustering of columns by group. Biomarkers were measured in samples collected within 30 min of admission to the emergency department (time 0 h).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Anderko, R.R., Gómez, H., Canna, S.W. et al. Sepsis with liver dysfunction and coagulopathy predicts an inflammatory pattern of macrophage activation. ICMx 10, 6 (2022). https://doi.org/10.1186/s40635-022-00433-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-022-00433-y