Abstract
Background
Veno-arterial extracorporeal membrane oxygenation (ECMO-CPR) is a life-saving rescue for selected patients when standard cardiopulmonary resuscitation fails. The use is increasing although the treatment modality is not fully established. Resuscitated patients typically develop a detrimental early post-arrest cardiac dysfunction that also deserves main emphasis. The present study investigates an ECMO-CPR strategy in pigs and assesses early post-arrest left ventricular function in detail. We hypothesised that a significant dysfunction could be demonstrated with this model using magnetic resonance imaging (MRI), not previously used early post-arrest.
Methods
In eight anaesthetised pigs, a 15-min ventricular fibrillation was resuscitated by an ECMO-CPR strategy of 150-min veno-arterial ECMO aiming at high blood flow rate and pharmacologically sustained aortic blood pressure and pulse pressure of 50 and 15 mmHg, respectively. Pre-arrest cardiac MRI and haemodynamic measurements of left ventricular function were compared to measurements performed 300-min post-arrest.
Results
All animals were successfully resuscitated, weaned from the ECMO circuit, and haemodynamically stabilised post-arrest. Cardiac output was maintained by an increased heart rate post-arrest, but left ventricular ejection fraction and stroke volume were decreased by approximately 50 %. Systolic circumferential strain and mitral annular plane systolic excursion as well as the left ventricular wall thickening were reduced by approximately 50–70 % post-arrest. The diastolic function variables measured were unchanged.
Conclusions
The present animal study demonstrates a successful ECMO-CPR strategy resuscitating long-lasting cardiac arrest with adequate post-arrest haemodynamic stability. The associated severe systolic left ventricular dysfunction could be charted in detail by MRI, a valuable tool for future cardiac outcome assessments in resuscitation research.
Trial registration
Institutional protocol number: FOTS 4611/13.
Similar content being viewed by others
Background
Patients with cardiac arrest not responding to standard cardiopulmonary resuscitation (CPR) can be resuscitated by veno-arterial extracorporeal membrane oxygenation (ECMO-CPR). Advances in technology and equipment have made ECMO-CPR much more attractive during recent years, and its use is increasing. The enthusiastic quest for novel resuscitation techniques may be due to the fact that standard CPR frequently fails. Although ECMO-CPR is claimed to represent “a frontier of resuscitation” [1], it is not an established treatment modality. No controlled clinical ECMO-CPR studies exist, but observational studies have been published [2–7]. Many centres have reported positively on their clinical experiences as reviewed recently by Fagnoul et al. [8]. Studies on ECMO-CPR usually focus on survival until hospital discharge and on post-arrest neurological sequelae. End-points in experimental studies are frequently the regain of spontaneous circulation (ROSC) and post-arrest brain tissue damage. A clinical problem after ROSC, however, is the early post-arrest cardiac dysfunction with circulatory failure-increasing the risk of adverse neurological function and poor outcome in resuscitated patients [9]. Detailed knowledge of cardiac function post-arrest is therefore needed. Few data exist on cardiac dysfunction after ECMO-CPR. The purpose of the present study was twofold; (1) to investigate ECMO-CPR in an experimental pig model and (2) to assess the early post-arrest left ventricular (LV) function by cardiac magnetic resonance imaging (MRI). We hypothesised that a significant early post-arrest LV dysfunction could be demonstrated with this model using MRI. Cardiac MRI allows accurate measurements of motions, volumes and flow in the beating heart and is not previously used to describe early post-arrest cardiac dysfunction.
Methods
Overall design and details
The study was a controlled experimental animal study. A listing of experiment details is presented in Tables 1 and 2.
Animal welfare
The experimental protocol was approved by the Norwegian National Animal Research Authority. The animal experiments were performed in accordance with the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (European Council, ETS No. 170). Personnel who handled the animals were certified with Federation of Laboratory Animal Science Associations category C. Detailed information according to the ARRIVE guidelines (Animals in Research: Reporting In Vivo Experiments) [10] is presented in Additional file 1: Table S1. A pig model was chosen because of the close similarities to human cardiac anatomy and physiology, also adequate for the use of standard equipment and clinical MRI sequences.
Animals and anaesthesia
Eight pigs were included in the study; each pig was kept fasting overnight-with free access to water, pre-medicated in the pig box housing and weighted before a short transport to the MRI research operating theatre. A mixture of intravenous anaesthesia standardised by weight and suspended in Ringer’s acetate solution was infused at 10 ml/kg/h (Table 1). The anaesthesia left no reaction to intermittent sharp hoof pinching or surgery and was chosen also to provide haemodynamic stability and the safe use of neuromuscular blockade [11, 12].
Preparation
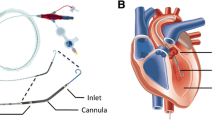
Each pig received a tracheostomy and intravascular catheters by a midline neck incision. A micro-manometer pressure transducer was positioned in the LV via an 8 French vascular introducer in the right carotid artery (Table 2). Both internal jugular veins were cannulated with 9 French vascular introducers and used to place a pulmonary artery (PA) catheter and a temporary pacing lead to the right ventricle. The femoral artery and the right external jugular vein were cannulated with 14 French and 21 French ECMO cannulae, respectively, using a guiding obturator placed over a guide-wire during fluoroscopy. To prevent ECMO clotting, a bolus of intravenous heparin 2 mg/kg followed by infusion 0.5 mg/kg/h was given to obtain an activated clotting time ≥300 s. Mechanical ventilation was set to keep PaO2 within 20 ± 5 kPa and PaCO2 within 5.0 ± 0.5 kPa. Each pig had standard monitoring during the experiment-including electrocardiography (ECG), pulse oximetry, end-tidal expiratory CO2, hourly urine output and oesophageal temperature.
Experimental protocol
Baseline assessments
After preparation and a following 30-min stabilisation period, baseline cardiac MRI and haemodynamic measurements of LV function were obtained. An arterial and a mixed venous blood sample were also analysed.
Establishment of ECMO
After baseline assessments, the pig was connected to a custom-made femoro-jugular veno-arterial ECMO circuit (Table 2). The ECMO consisted of an 86 ml centrifugal blood pump and a 270 ml micro-porous polypropylene hollow fibre oxygenator with a 2.5 m2 surface area. An oxygen/air mixer enabled an adjusted sweep gas oxygen fraction and sweep gas flow rate. The circuit was without surface coating and primed with Ringer’s acetate solution. The machinery was initially set to standby with the vascular lines clamped.
Cardiac arrest and resuscitation
Ventricular fibrillation (VF) was induced by an electrical stimulator connected to the right ventricular pacing lead. VF was confirmed by ECG shape and aortic blood pressure drop, and the ventilator was set to standby. After 15 min of untreated VF, ECMO-CPR was initiated with initial blood pump speed at the maximum 4540 rounds per minute. The sweep gas was set at the same flow rate as the ECMO blood flow rate, the fraction of oxygen to 100 % and the circuit temperature to 38.0 °C (normothermia in the pig). After 5 min of ECMO-CPR, fibrillation was terminated by external damped sinusoidal waveform 360 Joule monofasic shocks through standard anterior/anterior pads until sinus rhythm was obtained. The extracorporeal support continued at the same blood flow rate for a total of 150 min. Repeated blood gas analyses were performed, and the sweep gas flow rate and the oxygen/air mixture were adjusted according to the protocol. The lowest acceptable mean aortic pressure (MAP) and pulse pressure after defibrillation and regain of spontaneous cardiac beating (ROSB) was set by protocol consensus to 50 and 15 mmHg, respectively. Intravenous adrenaline (epinephrine) 10–25 μg boluses were given to ensure these criteria were met initially. If in need of continued inotropic support, infusion of the beta-adrenergic agonist dobutamine 5 μg/kg/min was started [13], and the infusion rate was adjusted if necessary. Each pig was weaned from ECMO during a 60 min period with successive 0.7 l/min reductions in blood flow rate every 10 min, to zero support. A 60-min stabilisation period followed before post-arrest assessments.
Post-arrest assessments
At 285 min post-arrest, cardiac function was re-assessed by the PA catheter and the LV pressure transducer. Arterial and mixed venous blood samples were analysed, and a second cardiac MRI was made. Finally, the pig was euthanized.
Cardiac MRI measurements
Image acquisition, interpretation and post-processing were done according to the recommendations from the Society for Cardiovascular Magnetic Resonance [14]. Cardiac MRI images were analysed by the software Segment version 1.9 R2585 (http://segment.heiberg.se) [15] and by Diagnosoft HARP 3.0.
The LV was assessed by adjacent parallel short-axis slices covering the full volume of the LV and by long axis slices. Series of cinematographic images of the cardiac cycle were recorded by retrospective ECG-gating without breath-holds. The images were analysed to estimate end-diastolic volume (EDV), end-systolic volume (ESV), stroke volume (SV = EDV-ESV), ejection fraction (EF = SV/EDV) and cardiac output (CO = SV · heart rate (HR)). In addition, mid LV radial wall thickening and mitral annular plane systolic excursion (MAPSE) were measured. Wall thickening was averaged from measurements in the septum and the lateral wall, and MAPSE averaged from antero-septal-and postero-lateral measurements.
Slices in short axis were also magnetically gridded throughout the cardiac cycle (tagging) to assess peak global systolic LV circumferential (Lagrangian) strain by harmonic phase analysis [16]. Strain assessment provides information on the process of myocardial deformation; it can be extended to include fine details [17], and it also known to quantify myocardial function during echocardiography [18, 19].
Phase-contrast imaging with blood velocity encoded through-plane images of the mid-ascending aorta was also included to assess LV stroke volume (SVphase) and cardiac output (COphase). It has been stated that this technique “should provide the most accurate measurement available of cardiac output” [20].
For further descriptions of cardiac MRI measurements the reader is kindly referred to Additional files 2 and 3 with the accompanying illustrations in Additional file 4, 5, 6, 7 and 8.
Haemodynamic measurements
The PA thermodilution CO measurements (COPA) were performed with injections of 10-ml ice-cold isotonic glucose, and the COPA was calculated as the average value of three consecutive measurements. Central venous pressure (CVP) was measured, and the SV (SVPA = COPA/HR) and the systemic vascular resistance (SVRPA = (MAP-CVP)/COPA∙80) were calculated.
The LV pressure transducer measurements were recorded on a separate PC monitoring system and analysed offline with custom-built software based on a visual programming language (Table 2) [21]. The maximum systolic LV pressure (LVPmax) and the maximum positive and negative first time derivate of LV pressure (dP/dtmax and dP/dtmin) were measured, in addition to the end-diastolic LV pressure (EDP) and the end-systolic LV pressure (ESP). Systolic duration was measured from ECG R-peak to dP/dtmin and diastolic duration from dP/dtmin to onset of systole. Isovolumetric contraction time (IVC) was measured from ECG R-peak to dP/dtmax. The isovolumetric relaxation constant tau was calculated using Weiss method [22] in a 50-millisec time-frame starting at dP/dtmin. LV afterload was estimated from arterial elastance (Ea = ESP/SV).
Measurements of oxygen consumption and oxygen delivery
The arterial and venous oxygen content (CaO2 and CvO2) were calculated from the arterial and mixed venous blood samples, respectively. Oxygen delivery (DO2 = CO∙CaO2) and oxygen consumption (VO2 = CO∙arterio-venous oxygen difference (CaO2 -CvO2)) were calculated.
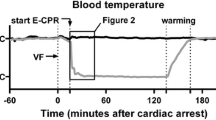
Outside the MRI scanner continuous measurements of HR, MAP and mean pulmonary artery pressure (MPAP) were recorded throughout the experiments (Fig. 1).
Continuous haemodynamic measurements (outside the MRI scanner). Continuous HR (heart rate), MAP (mean aortic pressure) and MPAP (mean pulmonary artery pressure) during the experiment, outside MRI scanner, shown as mean values (black line) ± standard deviation (grey). Cardiac arrest (VF) was induced at time 0 (bottom timeline, black triangle at dotted line). ECMO-CPR was started at time 15 (separate dotted line) and defibrillation was done at time 20 (open triangle). ECMO continued for 150 min before a 60-min stepwise weaning. Cardiac MRI of the beating heart was made ~60 min pre-arrest, and ~300 min post-arrest. The pig was euthanized after the post-arrest cardiac MRI (bullet point)
Statistical analysis
Statistical analyses of normality (D’Agostino-Pearson omnibus and Shapiro-Wilk) and of equality of variances (Levenes) supported the use of parametric tests. Data are reported as mean ± standard deviation if not otherwise stated. The statistical significance level α was set to 0.05. A paired two-tailed t test was used to compare baseline- and post-arrest measurements. The degree to which volumetrically and phase-contrast estimates of SV change correlated was quantified by a Pearson’s correlation analysis.
Results
The pigs weighed 49.7 ± 2.3 kg, preparation took 119 ± 29 min and cardiac MRI scan time was 63 ± 15 min. Prior to induction of cardiac arrest activated clotting time was 349 ± 83 s, and ECMO circuit priming volume was 538 ± 17 ml. The experiments lasted 747 ± 71 min.
Success of defibrillation
All animals were successfully defibrillated by a median of 1 shock (range 1–4). MAP at the time of defibrillation was 52 ± 8 mmHg with an ECMO blood flow rate of 4.60 ± 0.15 l/min. VF spontaneously reemerged after ROSB in two pigs demanding one additional defibrillation in both.
Continuous haemodynamic measurements
Continuous HR, MAP and MPAP outside the MRI scanner are presented in Fig. 1. A considerable leap in HR was observed immediately following the defibrillation. The HR slowed gradually during the period of ECMO-CPR, but severe tachycardia with an approximate frequency of 150 beats/min remained throughout the experiment. MAP increased to a maximum of 77 ± 15 mmHg 2 min after defibrillation. MAP then dropped over the next 13 min to a low point of 49 ± 9 mmHg 20 min after initiation of ECMO-CPR. A further decrease in MAP was prevented by intravenous boluses of adrenaline and infusion of dobutamine. The median total dose of dobutamine and adrenaline was 10.3 mg (range 0–19.5 mg) and 80 μg (range 0–340 μg), respectively. MPAP changed in parallel with MAP to a maximum of 20 ± 5 mmHg 2 min after defibrillation. Both pressures stabilised as adrenaline and dobutamine were added. During the period of stepwise reductions in ECMO blood flow rate, MPAP increased in steps, reaching 22 ± 4 mmHg when weaning was completed. All animals could be successfully weaned from ECMO as dictated by the protocol. During the 60-min stabilisation period following ECMO separation, the haemodynamics remained unchanged (Fig. 1).
Baseline and post-arrest haemodynamic measurements and blood gas values
Haemodynamic measurements and blood gas values at baseline and post-arrest are presented in Table 3. The substantial increase in HR post-arrest limited the reductions in CO. Systolic duration and isovolumetric contraction time were shortened by 33 ± 8 % and 17 ± 12 %, respectively, whereas diastolic duration decreased by 57 ± 17 %. LV afterload estimated by Ea increased post-arrest, and there was also a noticeable rise in MPAP. There was a minor increase in dP/dtmax whereas LVPmax did not significantly change; neither did EDP, dP/dtmin nor tau.
Arterial blood gas values remained virtually unaltered post-arrest, and the haemoglobin concentration was stable (Table 3). The DO2 was maintained due to the increased HR, but mixed venous oxygen saturation dropped from 69.4 ± 13.1 % to 44.0 ± 7.9 % (p < 0.001) post-arrest, corresponding to a 66 ± 38 % increase in VO2 at almost unchanged DO2 (mean difference –74 ml/min (p = 0.29)).
Baseline and post-arrest cardiac MRI
Cardiac MRI measurements at baseline and at 308 min ± 8 min post-arrest are presented in Table 4 and Fig. 2. LV function was severely reduced post-arrest, as EF decreased from 61 ± 5 % to 34 ± 8 % (p < 0.001) and SV decreased from 62 ± 9 ml to 30 ± 7 ml (p < 0.001). The lowered EF was related to a 58 ± 39 % increase in ESV as EDV was maintained (mean difference −8.9 ml (p = 0.22)). The reduction in SV was consistent with the change in SVphase (Pearson r = 0.7). The minor reduction in CO (0.5–0.7 l/min) was not statistically significant. Consistent with the reduced SV the absolute value of global systolic circumferential strain decreased from −17.4 ± 4.2 % to −5.1 ± 3.3 % (p < 0.001) and MAPSE was reduced from 12 ± 1 mm to 6 ± 1 mm (p <0.001). Similarly, mid LV radial wall thickening was severely affected by a reduction from 58 ± 18 % to 20 ± 9 % (p < 0.001). The reductions in MAPSE and wall thickening were consistent between the different measuring locations in the LV.
Baseline and post-arrest cardiac MRI. EF (ejection fraction), SV (stroke volume), CO (cardiac output), MAPSE (mitral annular plane systolic excursion), strain (peak global systolic left ventricular circumferential strain) and wall thickening before (baseline) and at ~300 min post-arrest. A paired two-tailed t test was used to compare baseline- and post-arrest measurements; asterisk: p value < 0.001
Discussion
In this study, ECMO-CPR emerged as an effective tool resuscitating the heart from long-lasting cardiac arrest. Fifteen minutes of VF induced a severe early post-arrest LV dysfunction that was demonstrated in detail by cardiac MRI. This is the first study to describe early post-arrest LV dysfunction by cardiac MRI.
There are no ECMO-CPR guidelines for patients with refractory cardiac arrest. As a consequence, several strategies are implemented targeting various circuit flows, arterial pressures, oxygen saturations and core temperatures. Indications may also differ between centres, although a refractory normothermic arrest with a no-flow period of less than 5 min will generally be accepted for ECMO-CPR. A 60-min time-frame is often stated as the clinical accepted limit of low-flow (the period of standard CPR) before ECMO is established. In animals, without extracorporeal support the probability of successful ROSC decreases drastically when VF is untreated for more than 10–12 min, and it is almost impossible to achieve sustainable ROSC in pigs after 15 min of untreated VF [23–26]. In the present study, the 15 min duration of cardiac arrest followed by ECMO-CPR was chosen to allow early survival [23, 27–30], but with a significant post-arrest cardiac dysfunction that could be examined by cardiac MRI in a stable state.
ECMO-CPR strategy
All pigs were successfully resuscitated by our protocol using a short (150 min) ECMO-CPR run with maximal flow, initially 100 % oxygen, normothermia, MAP above 50 mmHg and pulse pressure above 15 mmHg. The diameter, length and position of the venous cannula usually limit the ECMO blood flow rate. In our study, the venous cannula was placed as deep as the diaphragm, with its multiple side holes covering the full length of the caval veins and the right atrium. With this placement, the ECMO blood flow rate during ECMO-CPR could be kept as high as baseline CO pre-arrest without incidents of “suck down”, and increased CVP could be avoided. The low MPAP during ECMO-CPR indicated a relatively low blood flow through the pulmonary circulation, and thus a limited amount of blood entering the LV. This venous unloading, combined with adequate myocardial perfusion, is known to be particularly important to the post-arrest myocardium [31].
In peripheral veno-arterial ECMO, blood from the arterial cannula flows upwards in the descending aorta, increasing LV afterload. LV distension may then develop with compromised myocardial perfusion and hydrostatic pulmonary oedema. Although coronary perfusion was not measured, serious hypo-perfusion was most likely prevented by our protocol with the attention to adequate MAP and pulse pressure, the latter to assure LV emptying. The pressures were maintained above the lower limits by the customised dosage of positive inotropic medication. Dobutamine enhance LV function post-arrest [32]. All pigs could be weaned from extracorporeal support in accordance with the study protocol—this success would not have been possible after ECMO-CPR with severely compromised myocardial perfusion.
LV function after ECMO-CPR
Cardiac MRI is often considered as the reference standard for the assessments of LV volumes and function. In addition, we assessed post-arrest LV haemodynamics by a PA catheter and a LV pressure transducer. Cardiac MRI demonstrated a severe impairment of LV function after ECMO-CPR. The systolic dysfunction was evident both by the global volume and flow measurements and by the LV motion measurements, mid LV radial wall thickening, MAPSE, and global systolic circumferential strain. Interestingly, post-arrest dysfunction was characterised by almost similar reductions (approximately 50–70 %) of systolic LV motion in all three deformation directions, indicating a global LV impairment.
When compared to cardiac MRI the LVP and dP/dt measurements did not reflect the severity of systolic LV dysfunction. LVP was maintained because MAP was pharmacologically sustained as prescribed by the protocol. An increased inotropic state post-arrest-a result of the dobutamine infusion and an endogenous catecholamine response [33, 34] probably contributed to the observed increase in dP/dtmax and HR [35, 36]. In addition, an increased afterload (Ea) post-arrest and a shorter systolic duration and IVC may also have influenced the dP/dt variable [37, 38]. The COPA and SVPA measurements, however, corresponded well with the MRI measurements, but PA assessments gave less information.
In addition to the success of ECMO-CPR, our study demonstrates that a detailed assessment of early post-arrest LV dysfunction is feasible by cardiac MRI. The model may be relevant for future research on ECMO-CPR strategies and cardiac outcome.
Diastolic LV dysfunction was not demonstrated by the LV function variables included in this study. Post-arrest ESV increased without changes in EDV or EDP, indicating reduced systolic emptying of a ventricle adequately filled in diastole. A preserved diastolic function was supported by an unaltered tau and dP/dtmin post-arrest, indicating normal LV relaxation. Taken together, this can imply that LV dysfunction after ECMO-CPR is a failure of myocardial contractile function, not predominantly related to LV relaxation ability. Increased HR and dP/dtmax with reduced SV, and preserved diastolic function with maintained dP/dtmin, tau, and end-diastolic pressure-volume relationship have also been demonstrated after hypoxic cardiac arrest in pigs [27]. Early post-arrest LV dysfunction in pre-arrest healthy hearts thus seems similar whether cardiac arrest is induced electrically or by hypoxia.
The reduced SV post-arrest was compensated for by an increased HR, maintaining CO and DO2. However, the substantial drop in mixed venous oxygen saturation indicates that DO2 was insufficient for the increased post-arrest metabolic needs. The increased VO2 may be due to recovery from oxygen debt and activation of other oxygen consuming processes such as systemic inflammation, induced by ischemia and reperfusion [39].
Limitations
Our experimental model was different from a clinical ECMO-CPR scenario. In our study, healthy pigs were included, and cardiac arrest was introduced electrically. The duration of no-flow-exceeding usual clinical limits was intended to achieve a combination of survival and a certainly measurable post-arrest cardiac dysfunction. Cardiovascular function was in focus, and no attention was paid to neurological or other organ outcome. Pre-arrest heparinisation is a common experimental approach that also differs from the later on (or omitted) clinical heparin administration. Sham-operated animals were decided not included as the experiments were completed without complications, haemodynamic stability was adequate (Fig. 1), and veno-arterial ECMO only does not seem to affect intrinsic function of the porcine heart [40]. The reversibility of post-arrest myocardial LV dysfunction (stunning) was not addressed as it may take several days for the heart to fully recover. Some myocardial necrosis cannot be excluded, encouraging future investigations of myocardial damage related to ECMO-CPR in this model.
Conclusions
An ECMO-CPR strategy of 150-min veno-arterial ECMO aiming at high blood flow rate and pharmacologically sustained aortic blood pressure and pulse pressure allowed successful resuscitation of long-lasting cardiac arrest with adequate post-arrest haemodynamic stability in pigs. MRI imaging of the in vivo beating heart demonstrated a severe early post-arrest LV dysfunction. Systolic LV function variables were substantially reduced whereas diastolic function was preserved. Cardiac MRI will be a valuable tool for future research on post-arrest cardiac dysfunction including assessment of cardiac outcome after ECMO-CPR.
Abbreviations
- HR:
-
heart rate
- COphase and SVphase :
-
cardiac output and stroke volume by cardiac MRI phase-contrast technique
- EDV:
-
end-diastolic volume
- ESV:
-
end-systolic volume
- Wall thickening septum and Wall thickening lat :
-
mid left ventricular radial wall thickening in septum and lateral wall
- MAPSEantsept and MAPSEpostlat :
-
mitral annular plane systolic excursion measured anteroseptally and posterolaterally
- HRPA, COPA and SVPA :
-
pulmonary artery catheter assessments of heart rate, cardiac output and stroke volume
- MAP:
-
mean aortic pressure
- CVP:
-
central venous pressure
- Wedge:
-
pulmonary arterial wedge pressure
- SVR:
-
systemic vascular resistance
- LVPmax :
-
systolic left ventricular pressure maximum
- EDP:
-
end diastolic pressure
- dP/dtmax and dP/dtmin :
-
maximum and minimum LVP time derivates
- tau:
-
tsovolumetric relaxation constant
- Ea:
-
arterial elastance
- DD:
-
diastolic duration
- SD:
-
systolic duration
- IVC:
-
isovolumetric contraction time
- SVO2 :
-
mixed venous oxygen saturation
- DO2 :
-
oxygen delivery
- VO2 :
-
oxygen consumption
References
Fiser RT, Morris MC (2008) Extracorporeal cardiopulmonary resuscitation in refractory pediatric cardiac arrest. Pediatr Clin N Am 55:929–941
Chen YS, Lin JW, Yu HY, Ko WJ, Jerng JS, Chang WT, Chen WJ, Huang SC, Chi NH, Wang CH, Chen LC, Tsai PR, Wang SS, Hwang JJ, Lin FY (2008) Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. Lancet 372:554–561
Shin TG, Choi JH, Jo IJ, Sim MS, Song HG, Jeong YK, Song YB, Hahn JY, Choi SH, Gwon HC, Jeon ES, Sung K, Kim WS, Lee YT (2011) Extracorporeal cardiopulmonary resuscitation in patients with inhospital cardiac arrest: a comparison with conventional cardiopulmonary resuscitation. Crit Care Med 39:1–7
Fagnoul D, Taccone FS, Belhaj A, Rondelet B, Argacha JF, Vincent JL, Backer DD (2013) Extracorporeal life support associated with hypothermia and normoxemia in refractory cardiac arrest. Resuscitation 84:1519–1524
Haneya A, Philipp A, Diez C, Schopka S, Bein T, Zimmermann M, Lubnow M, Luchner A, Agha A, Hilker M, Hirt S, Schmid C, Müller T (2012) A 5-year experience with cardiopulmonary resuscitation using extracorporeal life support in non-postcardiotomy patients with cardiac arrest. Resuscitation 83:1331–1337
Stub D, Bernard S, Pellegrino V, Smith K, Walker T, Sheldrake J, Hockings L, Shaw J, Duffy SJ, Burrell A, Cameron P, Smit DV, Kaye DM (2015) Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation 86:88–94
Huang SC, Wu ET, Wang CC, Chen YS, Chang CI, Chiu IS, Ko WJ, Wang SS (2012) Eleven years of experience with extracorporeal cardiopulmonary resuscitation for paediatric patients with in-hospital cardiac arrest. Resuscitation 83:710–714
Fagnoul D, Combes A, De Backer D (2014) Extracorporeal cardiopulmonary resuscitation. Curr Opin Crit Care 20:259–265
Laurent I, Monchi M, Chiche JD, Joly LM, Spaulding C, Bourgeois B, Cariou A, Rozenberg A, Carli P, Weber S, Dhainaut JF (2002) Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol 40:2110–2116
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8, e1000412
Grund F, Tjomsland O, Sjaastad I, Ilebekk A, Kirkeboen KA (2004) Pentobarbital versus medetomidine-ketamine-fentanyl anaesthesia: effects on haemodynamics and the incidence of ischaemia-induced ventricular fibrillation in swine. Lab Anim 38:70–78
Fannelop T, Dahle GO, Matre K, Segadal L, Grong K (2004) An anaesthetic protocol in the young domestic pig allowing neuromuscular blockade for studies of cardiac function following cardioplegic arrest and cardiopulmonary bypass. Acta Anaesthesiol Scand 48:1144–1154
Vasquez A, Kern KB, Hilwig RW, Heidenreich J, Berg RA, Ewy GA (2004) Optimal dosing of dobutamine for treating post-resuscitation left ventricular dysfunction. Resuscitation 61:199–207
Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E (2013) Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson: Off J Soc Cardiovascular Magnetic Resonance 15:35
Heiberg E, Sjogren J, Ugander M, Carlsson M, Engblom H, Arheden H (2010) Design and validation of segment-freely available software for cardiovascular image analysis. BMC Med Imaging 10:1
Osman NF, McVeigh ER, Prince JL (2000) Imaging heart motion using harmonic phase MRI. IEEE Trans Med Imaging 19:186–202
Rosen BD, Gerber BL, Edvardsen T, Castillo E, Amado LC, Nasir K, Kraitchman DL, Osman NF, Bluemke DA, Lima JA (2004) Late systolic onset of regional LV relaxation demonstrated in three-dimensional space by MRI tissue tagging. Am J Physiol Heart Circ Physiol 287:H1740–H1746
Skulstad H, Urheim S, Edvardsen T, Andersen K, Lyseggen E, Vartdal T, Ihlen H, Smiseth OA (2006) Grading of myocardial dysfunction by tissue Doppler echocardiography: a comparison between velocity, displacement, and strain imaging in acute ischemia. J Am Coll Cardiol 47:1672–1682
Gjesdal O, Helle-Valle T, Hopp E, Lunde K, Vartdal T, Aakhus S, Smith HJ, Ihlen H, Edvardsen T (2008) Noninvasive separation of large, medium, and small myocardial infarcts in survivors of reperfused ST-elevation myocardial infarction: a comprehensive tissue Doppler and speckle-tracking echocardiography study. Circ Cardiovasc Imaging 1:189–196, 182 p following 196
Kilner PJ, Gatehouse PD, Firmin DN (2007) Flow measurement by magnetic resonance: a unique asset worth optimising. J Cardiovasc Magn Reson: Off J Soc Cardiovascular Magnetic Resonance 9:723–728
Halvorsen PS, Fleischer LA, Espinoza A, Elle OJ, Hoff L, Skulstad H, Edvardsen T, Fosse E (2009) Detection of myocardial ischaemia by epicardial accelerometers in the pig. Br J Anaesth 102:29–37
Weiss JL, Frederiksen JW, Weisfeldt ML (1976) Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. Eur J Clin Investig 58:751–760
Trummer G, Foerster K, Buckberg GD, Benk C, Heilmann C, Mader I, Feuerhake F, Liakopoulos O, Brehm K, Beyersdorf F (2010) Successful resuscitation after prolonged periods of cardiac arrest: a new field in cardiac surgery. J Thorac Cardiovasc Surg 139:1325–1332, 1332 e1321–1322
Staffey KS, Dendi R, Brooks LA, Pretorius AM, Ackermann LW, Zamba KD, Dickson E, Kerber RE (2008) Liquid ventilation with perfluorocarbons facilitates resumption of spontaneous circulation in a swine cardiac arrest model. Resuscitation 78:77–84
Pytte M, Bendz B, Kramer-Johansen J, Eriksen M, Stromme TA, Eilevstjonn J, Brosstad F, Sunde K (2008) Prearrest administration of low-molecular-weight heparin in porcine cardiac arrest: hemodynamic effects and resuscitability. Crit Care Med 36:881–886
Derwall M, Brucken A, Bleilevens C, Ebeling A, Fohr P, Rossaint R, Kern KB, Nix C, Fries M (2015) Doubling survival and improving clinical outcomes using a left ventricular assist device instead of chest compressions for resuscitation after prolonged cardiac arrest: a large animal study. Crit Care 19:123
Ali AA, White P, Xiang B, Lin HY, Tsui SS, Ashley E, Lee TW, Klein JR, Kumar K, Arora RC, Large SR, Tian G, Freed DH (2011) Hearts from DCD donors display acceptable biventricular function after heart transplantation in pigs. Am J Transplant: Off J Amer Soc Transplantation Amer Soc Transplant Surg 11:1621–1632
Ao H, Tanimoto H, Yoshitake A, Moon JK, Terasaki H (2001) Long-term mild hypothermia with extracorporeal lung and heart assist improves survival from prolonged cardiac arrest in dogs. Resuscitation 48:163–174
Leonov Y, Sterz F, Safar P, Radovsky A (1990) Moderate hypothermia after cardiac arrest of 17 minutes in dogs. Effect on cerebral and cardiac outcome. Stroke; J Cerebral Circ 21:1600–1606
Ostadal P, Mlcek M, Kruger A, Horakova S, Skabradova M, Holy F, Svoboda T, Belohlavek J, Hrachovina V, Taborsky L, Dudkova V, Psotova H, Kittnar O, Neuzil P (2013) Mild therapeutic hypothermia is superior to controlled normothermia for the maintenance of blood pressure and cerebral oxygenation, prevention of organ damage and suppression of oxidative stress after cardiac arrest in a porcine model. J Transl Med 11:124
Safar P, Abramson NS, Angelos M, Cantadore R, Leonov Y, Levine R, Pretto E, Reich H, Sterz F, Stezoski SW, Tisherman S (1990) Emergency cardiopulmonary bypass for resuscitation from prolonged cardiac arrest. Am J Emerg Med 8:55–67
Kern KB, Hilwig RW, Berg RA, Rhee KH, Sanders AB, Otto CW, Ewy GA (1997) Postresuscitation left ventricular systolic and diastolic dysfunction. Treatment with dobutamine. Circulation 95:2610–2613
Wortsman J, Frank S, Cryer PE (1984) Adrenomedullary response to maximal stress in humans. Am J Med 77:779–784
Niemann JT, Garner D (2005) Post-resuscitation plasma catecholamines after prolonged arrest in a swine model. Resuscitation 65:97–101
Weidemann F, Jamal F, Sutherland GR, Claus P, Kowalski M, Hatle L, De Scheerder I, Bijnens B, Rademakers FE (2002) Myocardial function defined by strain rate and strain during alterations in inotropic states and heart rate. Am J Physiol Heart Circ Physiol 283:H792–H799
Eising GP, Hammond HK, Helmer GA, Gilpin E, Ross J Jr (1994) Force-frequency relations during heart failure in pigs. Am J Physiol 267:H2516–H2522
Wallace AG, Skinner NS Jr, Mitchell JH (1963) Hemodynamic determinants of the maximal rate of rise of left ventricular pressure. Am J Physiol 205:30–36
Mason DT (1969) Usefulness and limitations of the rate of rise of intraventricular pressure (dp-dt) in the evaluation of myocardial contractility in man. Am J Cardiol 23:516–527
Adrie C, Laurent I, Monchi M, Cariou A, Dhainaou JF, Spaulding C (2004) Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care 10:208–212
Shen I, Levy FH, Vocelka CR, O’Rourke PP, Duncan BW, Thomas R, Verrier ED (2001) Effect of extracorporeal membrane oxygenation on left ventricular function of swine. Ann Thorac Surg 71:862–867
Acknowledgements
The work was performed at the Intervention Centre at Rikshospitalet, Oslo University Hospital. Departmental funds from the Intervention Centre and the Department of Research and Development, Oslo University Hospital, supported the costs of the experiments. The authors wish to acknowledge the animal research veterinary and the staff at the Department of Comparative Medicine for care of the animals. We would also like to thank the staff at the Intervention Centre for their excellent assistance during the experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HAB contributed to the study design, data collection, data analyses and manuscript preparation. PSH contributed to the study design, data collection, data analyses and revision of manuscript. HS contributed to the study design, revision of manuscript. TE contributed to the cardiac MRI design and analysis, revision of manuscript. EF contributed to the study design and revision of manuscript. JFB contributed to the study design, data collection, data analyses and manuscript revision. All authors read and approved the final manuscript.
Additional files
Additional file 1:
Table S1. (DOCX 14 kb)
Additional file 2:
Cardiac MRI. (DOCX 13 kb)
Additional file 3:
Table S2. (DOCX 29 kb)
Additional file 4:
Figure S1. (TIFF 17954 kb)
Additional file 5:
Figure S2. (JPEG 3675 kb)
Additional file 6:
Figure S3. (JPEG 6822 kb)
Additional file 7:
Figure S4. (TIFF 14029 kb)
Additional file 8:
Figure S5. (TIFF 5090 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bergan, H.A., Halvorsen, P.S., Skulstad, H. et al. Successful ECMO-cardiopulmonary resuscitation with the associated post-arrest cardiac dysfunction as demonstrated by MRI. ICMx 3, 25 (2015). https://doi.org/10.1186/s40635-015-0061-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-015-0061-2