Abstract
Background
Neutrophil extracellular traps (NETs) are a central player in the host response to bacteria: neutrophils release extracellular DNA (nucleosomes) and neutrophil elastase to entrap and kill bacteria. We studied the role of NETs in Burkholderia pseudomallei infection (melioidosis), an important cause of Gram-negative sepsis in Southeast Asia.
Methods
In a prospective observational study, circulating nucleosomes and neutrophil elastase were assayed in 44 patients with Gram-negative sepsis caused by B. pseudomallei (melioidosis) and 82 controls. Functional assays included human neutrophil stimulation and killing assays and a murine model of B. pseudomallei infection in which NET function was compromised using DNase. Specified pathogen-free 8- to 12-week-old C57BL/6 mice were sacrificed post-infection to assess bacterial loads, inflammation, and pathology.
Results
Nucleosome and neutrophil elastase levels were markedly elevated in patients compared to controls. NETs killed B. pseudomallei effectively, and neutrophils stimulated with B. pseudomallei showed increased elastase and DNA release in a time- and dose-dependent matter. In mice, NET disruption with intravenous DNase administration resulted in decreased nucleosome levels. Although DNase treatment of mice resulted in diminished liver inflammation, no differences were observed in bacterial dissemination or systemic inflammation.
Conclusion
B. pseudomallei is a potent inducer of NETosis which was reflected by greatly increased levels of NET-related components in melioidosis patients. Although NETs exhibited antibacterial activity against B. pseudomallei, NET formation did not protect against bacterial dissemination and inflammation during B. pseudomallei-induced sepsis.
Similar content being viewed by others
Background
Melioidosis (Gram-negative infection caused by Burkholderia pseudomallei) is a major cause of severe community-acquired sepsis in Southeast Asia and northern Australia [1],[2]. The clinical manifestations of B. pseudomallei infection range from chronic skin abscess to acute fulminant sepsis. Despite antibiotic treatment, melioidosis patients with bacteremia or pneumonia have a mortality rate of approximately 40% [3],[1],[2]. Melioidosis is therefore a good clinical model in which to study Gram-negative sepsis [4]-[6].
Neutrophils constitute the central line of the innate immune defense against many bacteria [7],[8]. Their arsenal is impressive and ranges from killing and phagocytosis to the production and release of antimicrobials and immunoregulatory cytokines [9],[2]. Neutrophils play a critical role in the host defense against B. pseudomallei. Although excessive neutrophil recruitment may be detrimental to the host [10], neutrophils are essential for early bacterial containment. Activated neutrophils are rapidly recruited to the lungs upon infection, and neutrophil depletion leads to accelerated mortality in mice [11],[12]. Not surprisingly, conditions that predispose individuals to melioidosis, most notably diabetes mellitus, are associated with impaired neutrophil function [3],[13]-[15].
Neutrophil extracellular traps (NETs; another effector mechanism of neutrophils) consist of extracellular DNA and histone-based structures decorated by antimicrobials such as neutrophil elastase and myeloperoxidase. They ensnare bacteria, degrade virulence factors, and ultimately kill their target [16]. Besides their antimicrobial function, NETs also induce a strong procoagulant response via extracellular nucleosomes (extracellular frames consisting of DNA and histones) within the NETs, which stimulates the proteolytic activity of neutrophil elastase and in turn promotes coagulation [17]-[20]. Virtually, all microbes that cause sepsis (including B. pseudomallei) have been shown to induce NET formation in vitro[7],[21],[16]. In a mouse model of intraperitoneal Escherichia coli infection, it was recently shown that NETs released into the vasculature were able to trap bacteria from the bloodstream and prevent bacterial dissemination [22]. Intriguingly, some bacteria (such as certain strains of Streptococcus pneumoniae and Pseudomonas aeruginosa) have developed mechanisms to circumvent NET-mediated killing, for example, via DNase [21],[23],[8],[19]. In theory, overwhelming NETosis or a reduced clearance capacity of NETs could be detrimental to the host during sepsis and contribute to ongoing inflammation, organ damage, and/or exhaustion of the immune system [21]. In the context of sepsis, free circulating DNA is regarded as an endogenous danger signal or ‘danger-associated molecular pattern’ (DAMP). DAMPs are released during inflammatory stress and trigger the host immune response [24]-[26]. The role and consequences of NET formation during human sepsis, however, remain ill-defined.
In this study, we examined the expression of NET-related markers in patients with melioidosis. We examined next the functional role of NETs in an in vitro model of B. pseudomallei infection, then examined the role of NETs in vivo by treating mice infected with B. pseudomallei with DNase to disrupt the DNA backbone of NETs.
Methods
Studies of clinical melioidosis
The clinical study was approved by the Oxford Tropical Research Ethics Committee (OXTREC 018-07) and the Ethics Committee of Mahidol University (MUTM 2008-001-01). This co-host has been described previously [27]: 44 patients with culture-proven melioidosis and sepsis, from whom ethylenediaminetetraacetic acid (EDTA) plasma was obtained on day of recruitment, 7 days after, and at a follow-up clinic ≥28 days after discharge. Patients were recruited within 48 h of admission. All patients had culture-proven melioidosis and had two out of four criteria for systemic inflammatory response syndrome (SIRS) [28]. Eligible patients had received active antimicrobial chemotherapy for less than 48 h (ceftazidime, amoxicillin-clavulanate, meropenem, or imipenem). Thirty-four patients were classed as diabetic if they had an HbA1c ≥7.8% at enrollment or a diagnosis of diabetes made prior to admission. Eighty-two healthy subjects served as controls (52 with diabetes and 30 without; Additional file 1: Table S1).
Peripheral leukocyte gene expression was determined via microarray analysis, as described previously [29]. RNA was assayed using HumanWG-6 v3.0 Expression BeadChips (Illumina®, Illumina Inc., San Diego, CA, USA), and data have been deposited at ArrayExpress, EMBL-EBI (accession-number E-TABM-852-n). We interrogated this database for proteins co-localizing to NETs [17]. To assess human neutrophil elastase, complexes together with α1-antitrypsin were assayed in plasma by ELISA, as elastase in plasma is immediately inactivated by the formation of these covalent complexes and is hardly detectable in its active form [30],[31]. Nucleosome levels were determined in EDTA plasma by enzyme-linked immunosorbent assay (ELISA) [32],[33]. In brief, the capture antibody was CLB-ANA/60 (which recognizes histone-3). The detector antibody was biotinylated CLB-ANA/58 (which recognizes an epitope exposed on complexes of histone-2A, histone-2B, and dsDNA). The reaction was read using poly-HRP and 3,3′,5,5′-tetramethylbenzidine as the chromogenic substrate.
Human neutrophil stimulation
B. pseudomallei strain 1026b [34] was cultured overnight and transferred to fresh Luria broth for 3 h in a 37°C shaker to yield bacteria at mid log-phase. Staphylococcus aureus strain LAC (USA 300; a known NET inducer [35]) was grown to stationary phase for 20 h in tryptic soy and brain-heart infusion broth at 37°C. Bacteria were centrifuged for 2 min at 300×g and the resulting pellet resuspended in Hanks' balanced salt solution (HBSS) with Ca2+ and Mg2+ (HBSS+/+; Gibco, Invitrogen, Carlsbad, CA, USA) before use.
Human neutrophils were isolated from peripheral blood of healthy donors using Polymorphprep™ (Axis-Shield, Oslo, Norway). Following erythrocyte lysis, neutrophils were washed and resuspended with HBSS without Ca2+ and Mg2+ (HBSS−/−) [7]; ≥99% of neutrophils were viable as determined by trypan blue exclusion [36]. Human NETs were induced as described previously [7],[17]. In brief, neutrophils were resuspended in HBSS−/− (final concentration 2 × 105 cells/100 μL) and infected with 100 μL HBSS+/+ containing live B. pseudomallei (final concentration 2 × 105 to 2 × 107 cfu) at 37°C and 5% CO2 to induce NET formation. HBSS+/+ medium alone served as the negative control; 20 nM phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich Corporation, St. Louis, MO, USA) and S. aureus (2 × 105 cfu) served as positive controls. Supernatant was harvested at 0, 1, and 4 h and passed through a 0.2-μm filter (Millipore, Billerica, MA, USA) to remove live bacteria prior to storage at −20°C. For DNA quantification, cells were washed once with medium before adding HBSS−/−, Ca2+ and Mg2+ (5 mM), and 0.1 U/mL DNase (Roche Diagnostics, Indianapolis, IN, USA) and incubated for a further 2 h at 37°C. Sterile 20 mM EDTA (Thermo Scientific, Waltham, MA, USA) was added to stop DNase activity. In control wells, EDTA was added prior to incubation with DNase.
To examine the growth-inhibiting properties of NETs on B. pseudomallei, 3 × 105 human neutrophils per well were resuspended in 50 μL Iscove's modified Dulbecco's medium (IMDM; Gibco) in a 96-well plate, then stimulated with 100 nM PMA for 5 h at 37°C (5% CO2) to induce NET formation [7]. Following NET formation, the cells were preincubated for 30 min at 37°C with 50 U DNase, heat-inactivated DNase (80°C for 10 min), or phosphate-buffered saline (PBS). NETs were stained with Sytox Green 5 mM (Molecular Probes, Eugene, OR, USA) and then visualized using a confocal microscope (Olympus IX81, Olympus Corporation, Tokyo, Japan). The supernatant was discarded, and approximately 7 × 102 cfu/100 μL IMDM of log-phase B. pseudomallei were added to the wells and spun down for 5 min at 300×g. After 10 h, bacterial loads were quantified by culture on horse blood agar.
Free elastase in the supernatant was assayed by ELISA as in the absence of plasma no complexes are formed with α1-antitrypsin, as described previously [37],[30]. Since NET formation is quantified by the measurement of DNA release after DNase treatment, and DNA will degrade the epitope of the anti-nucleosome antibody, measurement of nucleosomes by this ELISA is not suitable [38]. Therefore extracellular DNA was quantified in the supernatant using a Quant-iT™ Picogreen® dsDNA Assay Kit (Invitrogen) according to the manufacturer's instructions and was analyzed with a SpectraFluor Plus absorbance reader (Tecan Group, Männedorf, Switzerland).
Mouse experiments
Mouse experiments were approved by the Academic Medical Center Animal Use and Welfare Committee (DIX16AB). Pathogen-free 8- to 12-week-old C57BL/6 mice (Charles River, Wilmington, MA, USA) were inoculated intranasally with 1,000 (LD100) or 125 (LD50) cfu B. pseudomallei 1026b in a 50-μL saline solution (eight per group) as described previously [39],[40]. Mice were treated intravenously with 2,000 U DNase I (Roche) in 200 μL PBS at 0, 4, 20, 26, 42, and 50 h post-inoculation to disrupt the NET backbone; control mice received 200 μL PBS intravenously as described [22]. Lung, broncheoalveolar lavage fluid (BALF), liver, and EDTA blood were collected for bacterial culture and cytokine assays as described previously [41]. Nucleosomes were assayed in plasma and BALF to quantify NET formation.
Neutrophil counts in BALF were determined by FACS (FACSCalibur; Becton Dickson, San Jose, CA, USA) using directly labeled antibodies against Gr-1 (Ly-6G; Gr-1 FITC; BD Pharmingen, BD Biosciences, San Jose, USA). Nucleosome levels were measured by ELISA [32],[33]. Tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-10, and chemokine (C-X-C motif) ligand 1 (CXCL1 or KC) in lung homogenates were measured by sandwich ELISA (R&D Systems, Minneapolis, MN, USA) [41]. Plasma cytokine concentrations (TNF-α, IL-6, interferon (IFN)-γ, IL-10, monocyte chemotactic protein-1 [MCP-1 or CCL2], and IL-12p70) were measured by cytometric bead array (BD Biosciences). Lactate dehydrogenase (LDH), aspartate aminotransferase (AST), alanine transaminase (ALT), urea, and creatinine were measured in plasma with spectrophotometry (Roche Diagnostics). Lung and liver inflammation were scored histologically as described previously [42],[41],[40]. In brief, lung inflammation and damage of tissue were analyzed with respect to the following parameters: surface with pneumonia, necrosis and/or formation of abscess, interstitial inflammation, endothelialitis, bronchitis, edema, thrombus formation, and pleuritis. Liver pathology was analyzed with respect to the following parameters: area of liver with parenchymal inflammation, necrosis and/or abscess formation, portal inflammation, and thrombus formation.
Statistical analysis
Gene expression data were analyzed using R-Bioconductor, as described previously [29]. We log-transformed neutrophil elastase and nucleosome concentrations to correct for heteroscedasticity. For correlations, Pearson r is reported. In the kinetic assay, P values reported are for the slope of the linear regression model with elastase or DNA as the dependent variable and time point or stimulus as independent variables. Differences between murine groups were analyzed by the Mann-Whitney U or unpaired t test where appropriate. Analyses were done in GraphPad Prism version 6.0 for Mac OS X (GraphPad Software), and data are expressed as mean and SD unless stated otherwise.
Results
Nucleosome and neutrophil elastase levels are markedly elevated in patients with melioidosis and correlate with disease severity
To establish the presence of NETs during clinical melioidosis, we measured nucleosomes (complexes of extracellular DNA and histones and a useful measure of cell-free DNA in plasma) and neutrophil elastase (a measure of neutrophil degranulation) in the plasma of 44 patients with septic melioidosis and 82 healthy controls. Both are key NET-related markers [17],[18],[43], and both nucleosome (P < 0.0001) and elastase levels (P < 0.0001) were abundant in the plasma of patients with melioidosis on day of recruitment compared to healthy controls (Figure 1A,D).
NET-related markers are abundantly present in plasma from patients with melioidosis with or without diabetes. Increased levels of NET-related markers such as nucleosomes and neutrophil elastase are measured in plasma of patients on day of initial presentation with sepsis caused by B. pseudomallei (melioidosis) compared to healthy control subjects. No difference was found between melioidosis patients with or without diabetes (A, D). No difference was seen between melioidosis survivors versus non-survivors (B, E). Straight lines represent the mean, and dashed lines mark the lower limit of detection. Nucleosome levels and neutrophil elastase were measured in all surviving patients on day of enrollment, day 7, and day 28 (follow-up). There is no difference when looking at the normalization of the NET-related makers over time between melioidosis patients with or without diabetes (C, F). Mean and SEM are shown. ***P < 0.001 (unpaired t test).
The mortality rate of 27% (12 of 44) in this cohort enabled us to correlate the presence of these NET-related markers with disease severity. No differences were seen in the admission nucleosome and neutrophil elastase levels when comparing survivors and non-survivors (Figure 1B,E). No differences were seen in concentration levels of both NET markers in non-survivors on day of enrollment and day 7. All non-survivors died before the 28 day time point. However, a marked difference in nucleosome concentrations between survivors and non-survivors at day 7 was seen: at this time point, patients that went on to die had significantly higher nucleosome levels (192.3 ± 5) compared to survivors (33.6 ± 4; P = 0.001). Evidence for an association between nucleosome and neutrophil elastase plasma levels and disease severity was obtained in patients who survived, a second blood sample was drawn during treatment (at day 7) and after successful completion of therapy (≥28 days from discharge). In all surviving patients, plasma nucleosome and elastase concentrations moved toward normal (Figure 1C,F). Moreover, neutrophil activation (evidenced by elastase-α1-antitrypsin complexes) was strongly correlated with nucleosome levels at admission (P < 0.001, Figure 2), supporting the hypothesis that neutrophils are the source of nucleosomes. Of note, gene expression of proteins known to be associated with NET formation [17] was determined in total peripheral leukocytes derived from patients with culture-confirmed septic melioidosis and healthy controls. In line with the protein measurements, a marked up-regulation of genes encoding the central NET-associated proteins nucleosome histone-2B and histone-4 was found (Additional file 2: Table S2).
No influence of diabetes on the release of the NET-related markers
Since diabetes is the main risk factor for melioidosis and diabetes is known to negatively influence neutrophil function [3],[15],[14], we next sought to determine whether the presence of diabetes influenced the release of NET-related markers in our cohort of patients of whom 72% were diabetic. We found no difference in the release of the NET-related markers in melioidosis when comparing patients with or without diabetes on day of recruitment. In addition, NET-related markers were not different in healthy controls with or without diabetes (Figure 1A,D).
Elastase and extracellular DNA are released by neutrophils in a dose- and time-dependent fashion upon stimulation with B. pseudomallei
To examine whether neutrophils were the common source of NET-related marker release in patients with melioidosis, we isolated human neutrophils from healthy individuals and stimulated them with various known NET inducers as well as B. pseudomallei upon which the kinetics of elastase and extracellular DNA in culture supernatants was determined. Consistent with the patient data, elastase levels increased following stimulation with B. pseudomallei, and this occurred in a dose-dependent (P < 0.001, Figure 3A) and time-dependent manner (P = 0.005, Figure 3C). Extracellular DNA release also increased in a time- and dose-dependent manner following B. pseudomallei infection: extracellular DNA concentrations increased as the multiplicity of infection (MOI) increased (P < 0.001, Figure 3C) and over time (P < 0.001, Figure 3D). Of note, B. pseudomallei-infected cells released similar amounts of elastase and DNA as S. aureus (Additional file 3: Figure S1), itself a potent NET inducer [44]. Elastase levels and DNA release were also strongly correlated, reflecting the clinical data (Figure 3E).
NET-related markers are released by neutrophils in a dose- and time-dependent fashion. The kinetics of B. pseudomallei-induced NET formation at various multiplicities of infections (MOI; 10°, 101, 102) and time points (0, 1, and 4 h) were assessed by quantifying elastase (A, C) and extracellular DNA using a Picogreen dsDNA kit (B, D), in the supernatant of stimulated isolated human neutrophils. The negative control (−) was medium (HBSS−/−), and the positive control (+) was PMA, a known inducer of NETs. Both elastase and DNA increase in a time- and dose-dependent manner following B. pseudomallei (Bps) stimulation. The mean and SD are expressed. (E) The two NET markers, elastase and DNA, were highly correlated, suggesting that neutrophils are the source of this extracellular DNA. All experiments used whole blood from three to six different healthy human volunteers. Elastase results were log-transformed to correct for heteroscedasticity. P values reported are for the slope of linear regression models with elastase or DNA as the dependent variables and time point or MOI as independent variables. **P < 0.01; ***P < 0.001.
NETs exhibit antibacterial activity against B. pseudomallei
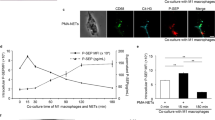
We next sought to examine whether activated NETs were able to kill B. pseudomallei. We pre-stimulated human neutrophils with PMA for 5 h to induce NET formation [7],[17], then preincubated the cells with PBS or DNase prior to infection with B. pseudomallei. Extracellular degradation of DNA by DNase treatment was confirmed by confocal microscopy (Figure 4A,B). Disrupting the backbone of the NETs with active DNase impaired the killing of B. pseudomallei by NETs from human neutrophils (Figure 4C). Taken together, these data confirm the involvement of NETs in the killing of B. pseudomallei by neutrophils.
NETs exhibit antibacterial activity against B. pseudomallei. Representative confocal micrographs of human neutrophils stimulated with PMA (A) to produce NETs and post-stimulation treated with PBS, heat-inactivated (HI) DNase, or active DNase (B), before infection with 7 × 102 cfu B. pseudomallei (C). Data are expressed as mean and SD. P value was determined via one-way ANOVA. Blood from three different healthy human volunteers was used.
NETs do not protect against bacterial dissemination in B. pseudomallei-induced sepsis
Having confirmed that NETs are capable of killing of B. pseudomallei in vitro, we sought to replicate this finding in a murine model of melioidosis. We infected mice with a lethal challenge of B. pseudomallei (103 cfu) and treated half of them with intravenous DNase twice daily in order to disrupt NET formation, as described previously [22]. Animals were sacrificed after 24 or 72 h to determine bacterial loads in BALF and lung (primary sites of the infection), and the liver and blood (to evaluate the extent of bacterial dissemination). In this murine model of Gram-negative sepsis, untreated mice showed an increase of nucleosome levels in plasma (17.6 ± 15) and BALF (202.7 ± 110) 24 h post-infection (Figure 5A,D). In comparison, uninfected untreated mice (t = 0) had low to undetected plasma and BALF nucleosome levels (5.6 ± 3 and 12.9 ± 5, respectively). DNase treatment resulted in successful disruption of intravascular NET formation, as illustrated by significantly decreased nucleosome levels in both plasma (5.3 ± 3, P = 0.03) and BALF (93.9 ± 29, P = 0.02) compared to untreated mice 24 h post-infection (Figure 5A,D). Disruption of intravascular NETs did not, however, result in changes in bacterial loads in BALF, lung, blood, or liver (Figure 5). In line with the bacterial loads, no differences were seen in total cell count or neutrophil influx in BALF between DNase-treated and untreated mice after infection (data not shown). Because cytokines are important regulators of the inflammatory response to bacterial pneumonia [45], we measured pulmonary (TNF-α, IL-6, IL-10, CXCL1) and systemic (TNF-α, MCP-1, IL-6, IFN-γ, IL-10, IL12p70) cytokine and chemokine levels (Table 1). Levels of all cytokines were elevated in both pulmonary and systemic compartments (with the exception of IL-10) upon B. pseudomallei infection. However, in complete correspondence with bacterial loads and cellular influx, no differences were seen between the DNase-treated animals and controls.
Disruption of NETs does not influence bacterial dissemination during experimental melioidosis. C57/Bl6 wild-type (WT) mice were intranasal infected with B. pseudomallei (103 cfu) and either treated with intravenous PBS (control group) or 2,000 U DNase I recombinant (DNase-treated group) at various time points [22]. Compared to control mice, DNase-treated mice show reduced nucleosome levels in plasma (A) and bronchoalveolar lavage fluid (BALF) (D) after 24 h of infection. Data are expressed as box-and-whisker diagrams depicting the smallest observation, lower quartile, median, upper quartile, and largest observation. Equal bacterial titers were seen in BALF (B), lung homogenates (C), blood (E), and liver homogenates (F) during all time points after infection in the DNase I-treated mice compared to control mice. Median values (straight lines) and the lower limit of detection (dashed lines) are shown. Graphs depict eight mice per group and time point. Statistical significance was determined using the unpaired Mann-Whitney U test. *P < 0.05.
We speculated that the infectious challenge might have been too high to reveal an effect of NET blockade in our model and therefore repeated the experiments with a lower inoculum (1.25 × 102 cfu which is the LD50). Again, although significant reductions of nucleosomes of DNase-treated mice were seen in BALF (P = 0.002) compared to untreated mice, no differences were seen in bacterial burdens in BALF, lung, blood, or liver nor local or systemic inflammatory cytokine levels 72 h post-infection (data not shown). These results indicate that disrupting NET release by degradation of the DNA backbone does not impair the host defense against B. pseudomallei infection.
Intravascular NETs cause collateral liver damage during experimental melioidosis
It has been reported previously that NETs are implicated in tissue damage during E. coli sepsis [22]. We therefore examined lung and liver tissue histologically for inflammation. No differences in lung pathology scores were observed between groups (Figure 6A,B,C). In accordance with a previous report, however, we found that significantly less liver inflammation was seen in the DNase-treated group when compared to controls at 72 h post-infection (P = 0.04, Figure 6D,E,F). It should be noted, however, that serum levels of AST and ALT, both markers of hepatocellular injury, as well as urea, creatinine, and LDH, markers of kidney and systemic organ injury, showed a trend toward lower values in the DNase-treated mice, although this did not reach statistical significance (Table 1). We conclude that there is only a limited role for NETs during B. pseudomallei infection in vivo.
Effect of DNase treatment on pulmonary and hepatic inflammation after infection with B. pseudomallei. Representative hematoxylin and eosin stained slides of lung (A, B) and liver (D, E) of control and DNase-treated mice 72 h post-infection with B. pseudomallei (103 cfu). Marked inflammation was seen post-infection, and areas of necrosis (marked with a number sign) and thrombosis formation (marked with an asterisk) were seen in both groups. Significantly less liver inflammation was observed in the DNase-treated group when compared to controls 72 h after infection. Total organ pathology score (lung and liver (C, F)) was determined 24 and 72 h post-infection according to the scoring system as described [41],[39],[40]. Graphs depict eight mice per group and time point. Median values are shown, and statistical significance was determined using the unpaired Mann-Whitney U test. *P < 0.05.
Discussion
NETs have emerged as key players in the innate host response to many bacteria. Neutrophils release extracellular DNA (including nucleosomes) and neutrophil elastase to entrap and capture bacteria [16], and animals depleted of neutrophils are markedly more susceptible to B. pseudomallei infection [12]. Our study extends the description of the role of NETs in the host response to Gram-negative sepsis caused by B. pseudomallei in both clinical melioidosis and a murine model of melioidosis. We found that the key NET components, nucleosomes and neutrophil elastase, were both elevated in clinical melioidosis. In vitro observations supported the notion that neutrophils are the source of these proteins and isolated human neutrophils infected with B. pseudomallei released NET-related markers in a time- and dose-dependent manner. We further showed that NETs killed B. pseudomallei in an in vitro human neutrophil infection model, although in a murine model of experimental melioidosis, in which DNase was used to disrupt NET formation, no protective role for NETs in the host defense against B. pseudomallei infection could be demonstrated.
Our data corroborate a previous report in which it was shown that bactericidal NETs were released from human neutrophils in response to B. pseudomallei in an in vitro model [46]. In addition, we recently showed that inhibition of endogenous activated protein C, which exerts cytoprotective effects on the endothelium by cleavage of histones [47], leads to excessive nucleosome release in BALF during murine experimental melioidosis [48]. We now demonstrate that there is an abundant release of NET-related markers in plasma from melioidosis patients. In vitro experiments demonstrated that NET-related markers are released by neutrophils and exhibit NET-dependent antibacterial activity upon infection with B. pseudomallei.
Surprisingly, we found that NETs played no role in bacterial clearance in our experimental mouse model of sepsis. In DNase-treated mice, circulating nucleosomes were decreased but there was no difference in cellular influx nor bacterial counts and cytokine release. This also argues against a role for nucleosomes (which can become harmful to the host when released in excessive amounts [25],[26]) as significant endogenous danger signals in melioidosis. This correlates with our clinical finding that the concentration of circulating NET markers in melioidosis patients was not different between survivors and non-survivors on day of admission. We did however find a difference between survivors and non-survivors on day 7, which is in line with our previous finding that circulating nucleosomes correlated with severity of the inflammatory response and outcome in a cohort of children suffering from meningococcal sepsis [49].
ELISAs detecting nucleosomes have proven to be a good indicator of NET release in patients [43] but are not specific for nucleosomes released by neutrophils. We therefore cannot exclude that nucleosomes released into the circulation by other cell types, such as endothelial and parenchymal cells, are detected as well. However, the positive correlation with neutrophil elastase together with the marked up-regulation of genes encoding the central NET-associated proteins does suggest that neutrophils are the main source of the detected nucleosomes. Although B. pseudomallei can induce NET release, NETs are predominantly a mechanism for clearing extracellular pathogens, and B. pseudomallei may escape NETosis by hiding intracellularly [50],[21],[51]. Indeed, it was found that both the type 3 secretion system (an important virulence factor of B. pseudomallei) and the bacterial capsule may play a role in evading NETs [46]. Furthermore, one could hypothesize that like S. aureus[52], B. pseudomallei is able to cause leukocyte toxicity by converting NETs into a bioproduct thereby inducing macrophage apoptosis. However, this mechanism would be unfavorable for B. pseudomallei as it is already known that macrophage destruction is one of the host immune defense strategies to restrict intracellular growth of B. pseudomallei[2]. Another possibility could be that plasma from patients with severe sepsis induces platelet-neutrophil interactions in a TLR4-dependent fashion leading to the production of NETs [53],[22], whereas TLR4 does not seem to play an important role in the clearance of B. pseudomallei[2],[41].
At least a third of all patients with melioidosis have diabetes mellitus as a predisposing factor [3],[1],[2]. An explanation for this increased susceptibility was sought in the function of neutrophils from diabetic patients, which displayed impaired phagocytosis of B. pseudomallei, reduced migration, and the inability to produce an oxidative burst to kill intracellular bacteria [54]. Furthermore, it was shown that isolated neutrophils from patients with diabetes mellitus released less extracellular DNA upon in vitro stimulation with B. pseudomallei, which was associated with a reduced bacterial killing capacity when compared to neutrophils derived from healthy controls [46]. However, in our study, we did not observe a difference in the release of NET-related markers between diabetic and non-diabetic melioidosis patients. The combination of clinical data and data from our murine model argues against an important role for the NETs in melioidosis. Our data also suggest that impaired NET release does not provide an explanation for the increased susceptibility to B. pseudomallei infection in patients with diabetes.
NET formation may also injure the host [21]: our finding that DNase-treated mice have diminished hepatocellular injury after infection is consistent with previous reports in which mice deficient in NET components have decreased hepatocellular inflammation compared to controls in an E. coli-induced sepsis model [55],[22].
Conclusion
We have shown that B. pseudomallei is a potent inducer of NETosis, as reflected by increased concentrations of NET-related components in patients with melioidosis. Although NETs exhibit antibacterial activity against B. pseudomallei in vitro, NET formation does not protect against bacterial dissemination and inflammation in a murine model of B. pseudomallei infection. This correlates with our clinical finding that levels of NET components do not correlate with mortality.
Meetings at which work has been presented
This work was presented in part at the International Federation of Shock Societies meeting, June 2012, Miami Beach, Florida, USA; the 15th International Congress of Immunology, August 2013, Milan, Italy; and the 7th World Melioidosis Congress, September 2013, Bangkok, Thailand.
Additional files
Abbreviations
- ALT:
-
alanine transaminase
- AST:
-
aspartate aminotransferase
- B. pseudomallei :
-
Burkholderia pseudomallei
- BALF:
-
bronchoalveolar lavage fluid
- CXCL1/KC:
-
chemokine (C-X-C motif) ligand 1
- DAMPS:
-
danger-associated molecular patterns
- IFN-γ:
-
interferon-gamma
- IL:
-
interleukin
- LDH:
-
lactate dehydrogenase
- MCP-1/CCL2:
-
monocyte chemotactic protein-1
- MOI:
-
multiplicity of infection
- NET:
-
neutrophil extracellular traps
- PMA:
-
phorbol 12-myristate 13-acetate
- S. aureus :
-
Staphylococcus aureus
- TNF-α:
-
tumor necrosis factor-alpha
References
Cheng AC, Currie BJ: Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev 2005, 18(2):383–416. 10.1128/CMR.18.2.383-416.2005
Wiersinga WJ, Currie BJ, Peacock SJ: Melioidosis. N Engl J Med 2012, 367(11):1035–1044. 10.1056/NEJMra1204699
Koh GC, Maude RR, Schreiber MF, Limmathurotsakul D, Wiersinga WJ, Wuthiekanun V, Lee SJ, Mahavanakul W, Chaowagul W, Chierakul W, White NJ, van der Poll T, Day NP, Dougan G, Peacock SJ: Glyburide is anti-inflammatory and associated with reduced mortality in melioidosis. Clin Infect Dis 2011, 52(6):717–725. 10.1093/cid/ciq192
Possession, use, and transfer of select agents and toxins: biennial review Federal Register 2012, 77: 61085–61115.
Simpson AJ: Melioidosis: a clinical model for gram-negative sepsis. J Med Microbiol 2001, 50(8):657–658.
Wiersinga WJ: Current insights in sepsis: from pathogenesis to new treatment targets. Curr Opin Crit Care 2011, 17(5):480–486. 10.1097/MCC.0b013e32834a4aeb
Achouiti A, Vogl T, Urban CF, Rohm M, Hommes TJ, van Zoelen MA, Florquin S, Roth J, van 't Veer C, de Vos AF, van der Poll T: Myeloid-related protein-14 contributes to protective immunity in gram-negative pneumonia derived sepsis. PLoS Pathog 2012, 8(10):e1002987. 10.1371/journal.ppat.1002987
Young RL, Malcolm KC, Kret JE, Caceres SM, Poch KR, Nichols DP, Taylor-Cousar JL, Saavedra MT, Randell SH, Vasil ML, Burns JL, Moskowitz SM, Nick JA: Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa : evidence of acquired resistance within the CF airway, independent of CFTR. PLoS One 2011, 6(9):e23637. 10.1371/journal.pone.0023637
Kolaczkowska E, Kubes P: Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013, 13(3):159–175. 10.1038/nri3399
Ceballos-Olvera I, Sahoo M, Miller MA, Del Barrio L, Re F: Inflammasome-dependent pyroptosis and IL-18 protect against Burkholderia pseudomallei lung infection while IL-1beta is deleterious. PLoS Pathog 2011, 7(12):e1002452. 10.1371/journal.ppat.1002452
Wiersinga WJ, Veer C, Wieland CW, Gibot S, Hooibrink B, Day NP, Peacock SJ, van der Poll T: Expression profile and function of triggering receptor expressed on myeloid cells-1 during melioidosis. J Infect Dis 2007, 196(11):1707–1716. 10.1086/522141
Easton A, Haque A, Chu K, Lukaszewski R, Bancroft GJ: A critical role for neutrophils in resistance to experimental infection with Burkholderia pseudomallei . J Infect Dis 2007, 195(1):99–107. 10.1086/509810
Suputtamongkol Y, Chaowagul W, Chetchotisakd P, Lertpatanasuwun N, Intaranongpai S, Ruchutrakool T, Budhsarawong D, Mootsikapun P, Wuthiekanun V, Teerawatasook N, Lulitanond A: Risk factors for melioidosis and bacteremic melioidosis. Clin Infect Dis 1999, 29(2):408–413. 10.1086/520223
Schuetz P, Castro P, Shapiro NI: Diabetes and sepsis: preclinical findings and clinical relevance. Diabetes Care 2011, 34(3):771–778. 10.2337/dc10-1185
Koh GC, Peacock SJ, van der Poll T, Wiersinga WJ: The impact of diabetes on the pathogenesis of sepsis. Eur J Clin Microbiol Infect Dis 2012, 31(4):379–388. 10.1007/s10096-011-1337-4
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A: Neutrophil extracellular traps kill bacteria. Science 2004, 303(5663):1532–1535. 10.1126/science.1092385
Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A: Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans . PLoS Pathog 2009, 5(10):e1000639. 10.1371/journal.ppat.1000639
Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A: Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 2010, 191(3):677–691. 10.1083/jcb.201006052
Engelmann B, Massberg S: Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol 2013, 13(1):34–45. 10.1038/nri3345
Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, Lorenz M, Bidzhekov K, Khandagale AB, Konrad I, Kennerknecht E, Reges K, Holdenrieder S, Braun S, Reinhardt C, Spannagl M, Preissner KT, Engelmann B: Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med 2010, 16(8):887–896. 10.1038/nm.2184
Kaplan MJ, Radic M: Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol 2012, 189(6):2689–2695. 10.4049/jimmunol.1201719
McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P: Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 2012, 12(3):324–333. 10.1016/j.chom.2012.06.011
Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B: An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol 2006, 16(4):401–407. 10.1016/j.cub.2006.01.056
Chan JK, Roth J, Oppenheim JJ, Tracey KJ, Vogl T, Feldmann M, Horwood N, Nanchahal J: Alarmins: awaiting a clinical response. J Clin Invest 2012, 122(8):2711–2719. 10.1172/JCI62423
Chen GY, Nunez G: Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol 2010, 10(12):826–837. 10.1038/nri2873
Pisetsky DS: The origin and properties of extracellular DNA: from PAMP to DAMP. Clin Immunol 2012, 144(1):32–40. 10.1016/j.clim.2012.04.006
Koh GC, Meijers JC, Maude RR, Limmathurotsakul D, Day NP, Peacock SJ, van der Poll T, Wiersinga WJ: Diabetes does not influence activation of coagulation, fibrinolysis or anticoagulant pathways in Gram-negative sepsis (melioidosis). Thromb Haemost 2011, 106(6):1139–1148. 10.1160/TH11-07-0504
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G: 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003, 31(4):1250–1256. 10.1097/01.CCM.0000050454.01978.3B
Koh GC, Schreiber MF, Bautista R, Maude RR, Dunachie S, Limmathurotsakul D, Day NP, Dougan G, Peacock SJ: Host responses to melioidosis and tuberculosis are both dominated by interferon-mediated signaling. PLoS One 2013, 8(1):e54961. 10.1371/journal.pone.0054961
Zeerleder S, Caliezi C, van Mierlo G, Eerenberg-Belmer A, Sulzer I, Hack CE, Wuillemin WA: Administration of C1 inhibitor reduces neutrophil activation in patients with sepsis. Clin Diagn Lab Immunol 2003, 10(4):529–535.
van Montfoort ML, Stephan F, Lauw MN, Hutten BA, Van Mierlo GJ, Solati S, Middeldorp S, Meijers JC, Zeerleder S: Circulating nucleosomes and neutrophil activation as risk factors for deep vein thrombosis. Arterioscler Thromb Vasc Biol 2013, 33(1):147–151. 10.1161/ATVBAHA.112.300498
van Nieuwenhuijze AE, van Lopik T, Smeenk RJ, Aarden LA: Time between onset of apoptosis and release of nucleosomes from apoptotic cells: putative implications for systemic lupus erythematosus. Ann Rheum Dis 2003, 62(1):10–14. 10.1136/ard.62.1.10
Zeerleder S, Zwart B, te Velthuis H, Manoe R, Bulder I, Rensink I, Aarden LA: A plasma nucleosome releasing factor (NRF) with serine protease activity is instrumental in removal of nucleosomes from secondary necrotic cells. FEBS Lett 2007, 581(28):5382–5388. 10.1016/j.febslet.2007.10.037
DeShazer D: Genomic diversity of Burkholderia pseudomallei clinical isolates: subtractive hybridization reveals a Burkholderia mallei -specific prophage in B. pseudomallei 1026b. J Bacteriol 2004, 186(12):3938–3950. 10.1128/JB.186.12.3938-3950.2004
Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FH, Surette MG, Sugai M, Bowden MG, Hussain M, Zhang K, Kubes P: A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus . J Immunol 2010, 185(12):7413–7425. 10.4049/jimmunol.1000675
Hoogendijk AJ, Roelofs JJ, Duitman J, van Lieshout MH, Blok DC, van der Poll T, Wieland CW: R-roscovitine reduces lung inflammation induced by lipoteichoic acid and Streptococcus pneumoniae . Mol Med 2012, 18: 1086–1095. 10.2119/molmed.2012.00033
de Boer JH, Hack CE, Verhoeven AJ, Baarsma GS, de Jong PT, Rademakers AJ, de Vries-Knoppert WA, Rothova A, Kijlstra A: Chemoattractant and neutrophil degranulation activities related to interleukin-8 in vitreous fluid in uveitis and vitreoretinal disorders. Invest Ophthalmol Vis Sci 1993, 34(12):3376–3385.
Zeerleder S, Zwart B, Wuillemin WA, Aarden LA, Groeneveld AB, Caliezi C, van Nieuwenhuijze AE, van Mierlo GJ, Eerenberg AJ, Lammle B, Hack CE: Elevated nucleosome levels in systemic inflammation and sepsis. Crit Care Med 2003, 31(7):1947–1951. 10.1097/01.CCM.0000074719.40109.95
Wiersinga WJ, de Vos AF, Wieland CW, Leendertse M, Roelofs JJ, van der Poll T: CD14 impairs host defense against gram-negative sepsis caused by Burkholderia pseudomallei in mice. J Infect Dis 2008, 198(9):1388–1397. 10.1086/592220
Wiersinga WJ, de Vos AF, de Beer R, Wieland CW, Roelofs JJ, Woods DE, van der Poll T: Inflammation patterns induced by different Burkholderia species in mice. Cell Microbiol 2008, 10(1):81–87.
Wiersinga WJ, Wieland CW, Dessing MC, Chantratita N, Cheng AC, Limmathurotsakul D, Chierakul W, Leendertse M, Florquin S, de Vos AF, White N, Dondorp AM, Day NP, Peacock SJ, van der Poll T: Toll-like receptor 2 impairs host defense in gram-negative sepsis caused by Burkholderia pseudomallei (melioidosis). PLoS Med 2007, 4(7):e248. 10.1371/journal.pmed.0040248
Wiersinga WJ, Wieland CW, Roelofs JJ, van der Poll T: MyD88 dependent signaling contributes to protective host defense against Burkholderia pseudomallei . PLoS One 2008, 3(10):e3494. 10.1371/journal.pone.0003494
Thomas GM, Carbo C, Curtis BR, Martinod K, Mazo IB, Schatzberg D, Cifuni SM, Fuchs TA, von Andrian UH, Hartwig JH, Aster RH, Wagner DD: Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood 2012, 119(26):6335–6343. 10.1182/blood-2012-01-405183
Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD, Pittman K, Asaduzzaman M, Wu K, Meijndert HC, Malawista SE, de Boisfleury Chevance A, Zhang K, Conly J, Kubes P: Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med 2012, 18(9):1386–1393. 10.1038/nm.2847
Strieter RM, Belperio JA, Keane MP: Cytokines in innate host defense in the lung. J Clin Invest 2002, 109(6):699–705. 10.1172/JCI0215277
Riyapa D, Buddhisa S, Korbsrisate S, Cuccui J, Wren BW, Stevens MP, Ato M, Lertmemongkolchai G: Neutrophil extracellular traps exhibit antibacterial activity against Burkholderia pseudomallei and are influenced by bacterial and host factors. Infect Immun 2012, 80(11):3921–3929. 10.1128/IAI.00806-12
Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT: Extracellular histones are major mediators of death in sepsis. Nat Med 2009, 15(11):1318–1321. 10.1038/nm.2053
Kager LM, Wiersinga WJ, Roelofs JJ, Meijers JC, Zeerleder SS, Esmon CT, van't Veer C, van der Poll T: Endogenous protein C has a protective role during Gram-negative pneumosepsis (melioidosis). J Thromb Haemost 2013, 11(2):282–292. 10.1111/jth.12094
Zeerleder S, Stephan F, Emonts M, de Kleijn ED, Esmon CT, Varadi K, Hack CE, Hazelzet JA: Circulating nucleosomes and severity of illness in children suffering from meningococcal sepsis treated with protein C. Crit Care Med 2012, 40(12):3224–3229. 10.1097/CCM.0b013e318265695f
Juneau RA, Pang B, Weimer KE, Armbruster CE, Swords WE: Nontypeable Haemophilus influenzae initiates formation of neutrophil extracellular traps. Infect Immun 2011, 79(1):431–438. 10.1128/IAI.00660-10
Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ: Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei . Nat Rev Microbiol 2006, 4(4):272–282. 10.1038/nrmicro1385
Thammavongsa V, Missiakas DM, Schneewind O: Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science 2013, 342(6160):863–866. 10.1126/science.1242255
Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P: Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 2007, 13(4):463–469. 10.1038/nm1565
Chanchamroen S, Kewcharoenwong C, Susaengrat W, Ato M, Lertmemongkolchai G: Human polymorphonuclear neutrophil responses to Burkholderia pseudomallei in healthy and diabetic subjects. Infect Immun 2009, 77(1):456–463. 10.1128/IAI.00503-08
van Zoelen MA, Vogl T, Foell D, Van Veen SQ, van Till JW, Florquin S, Tanck MW, Wittebole X, Laterre PF, Boermeester MA, Roth J, van der Poll T: Expression and role of myeloid-related protein-14 in clinical and experimental sepsis. Am J Respir Crit Care Med 2009, 180(11):1098–1106. 10.1164/rccm.200810-1552OC
Acknowledgements
We are grateful to the valuable contributions of past and present group members for their work contributing to the understanding of neutrophil extracellular traps and bacteria. Furthermore, we would like to thank Tom van der Poll for critically reading the manuscript. We would also like to thank Gerard van Mierlo, Shabnam Solati, Marieke van den Brink, and Joost Daalhuisen for their expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
HKdJ received unrestricted funding for this project from the Academic Medical Center (AMC PhD Scholarship), GCK from Wellcome Trust of Great Britain (086532/Z/08/Z), and WJW from The Netherlands Organisation for Scientific Research (NWO; VENI grant number 91610008) and The Netherlands Organisation for Health Research and Development (ZonMw; Clinical Fellowship grant number 90700424). The other authors have no competing interests.
Authors’ contributions
HKJ, GCK, AA, IB, NPD, SJP, SZ, and WJW conceived and designed the experiments. HKJ, GCK, AA, and AJM performed the experiments. HKJ, GCK, AA, AJM, IB, FS, JJR, SZ, and WJW analyzed the data. NPD, SJP, and SZ contributed reagents/materials/analysis tools. HKJ, GCK, and WJW wrote the first draft. HKJ, GCK, AA, AJM, IB, FS, JJR, NPD, SJP, SZ, and WJW contributed to the writing of the manuscript and agreed with the final manuscript results and conclusions. GCK enrolled patients. All authors read and approved the final manuscript.
Electronic supplementary material
40635_2014_21_MOESM2_ESM.doc
Additional file 2: Table S2.: Proteins that localize to NETs in peripheral leucocytes from patients with melioidosis. (DOC 58 KB)
40635_2014_21_MOESM3_ESM.tiff
Additional file 3: Figure S1.:B. pseudomallei is as potent as S. aureus in the induction of NETosis. Isolated human neutrophils were stimulated with either Burkholderia pseudomallei (Bps) or Staphylococcus aureus (SA) for 4 h after which elastase (A) and extracellular DNA (B) release were measured. For both bacterial strains, equal MOIs (101) were used. Medium (HBSS−/−) served as negative control and PMA, a known inducer of NETs, as positive control. Extracellular DNA was measured using a Picogreen dsDNA kit. Mean and SDs are shown. P value comparing Bps, SA, and PMA to medium control was determined via unpaired t test after log transformation of the data. Blood from three to six different healthy human volunteers were used. P values *< 0.05, **< 0.01, ***< 0.001 are shown. (TIFF 127 KB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
de Jong, H.K., Koh, G.C., Achouiti, A. et al. Neutrophil extracellular traps in the host defense against sepsis induced by Burkholderia pseudomallei (melioidosis). ICMx 2, 21 (2014). https://doi.org/10.1186/s40635-014-0021-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40635-014-0021-2